Relationship between the Dietary Inflammatory Index Score and Cytokine Levels in Chinese Pregnant Women during the Second and Third Trimesters
Abstract
1. Introduction
2. Materials and Methods
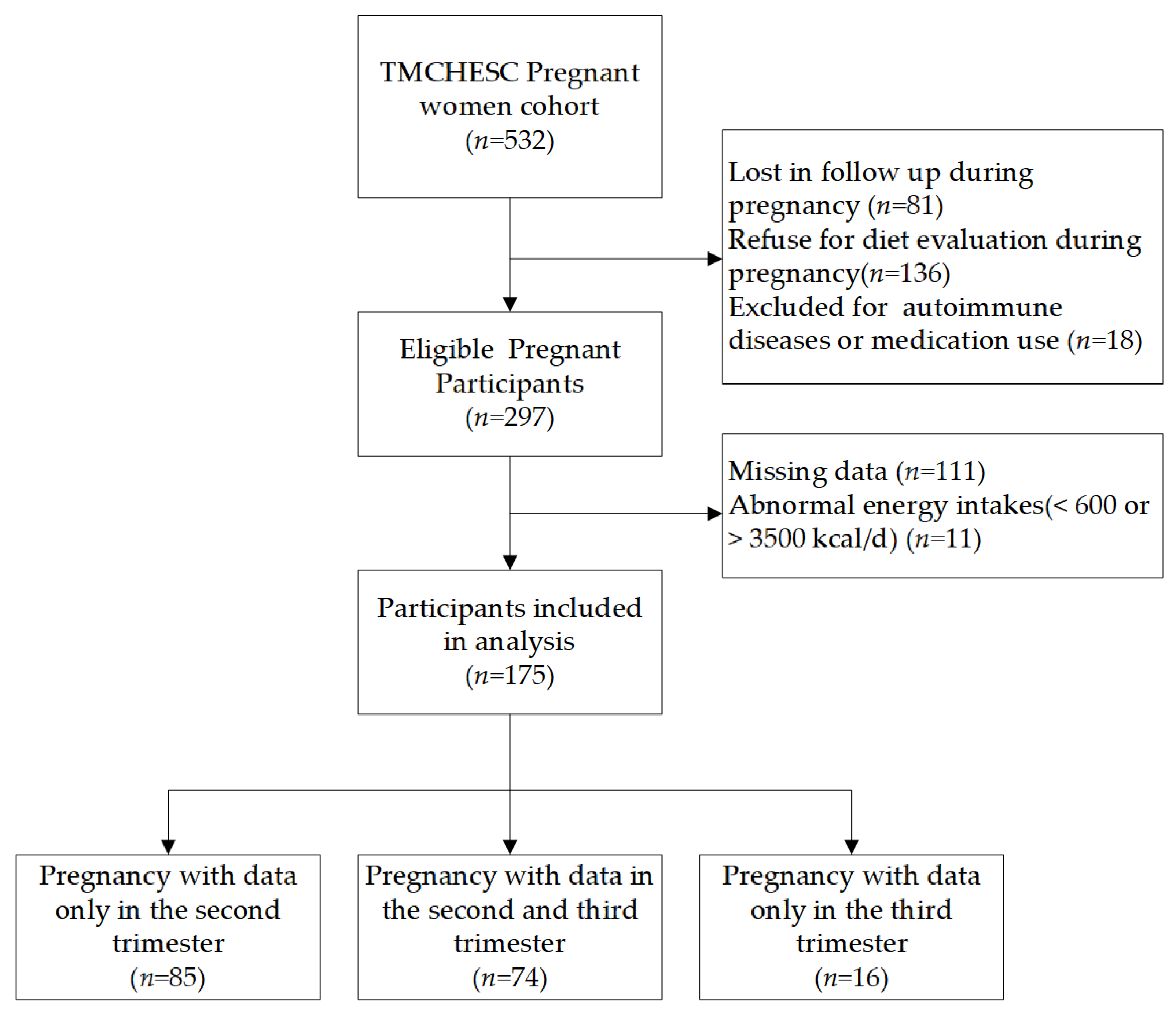
2.1. Study Design and Participants
2.2. Questionnaires
2.3. Dietary Assessment
2.4. DII Assessment
2.5. Blood Samples
2.6. Biochemical Measurements
2.7. Statistical Analysis
3. Results
3.1. Participant Baseline Characteristics
3.2. Effect of Basic Characteristics on DII Scores
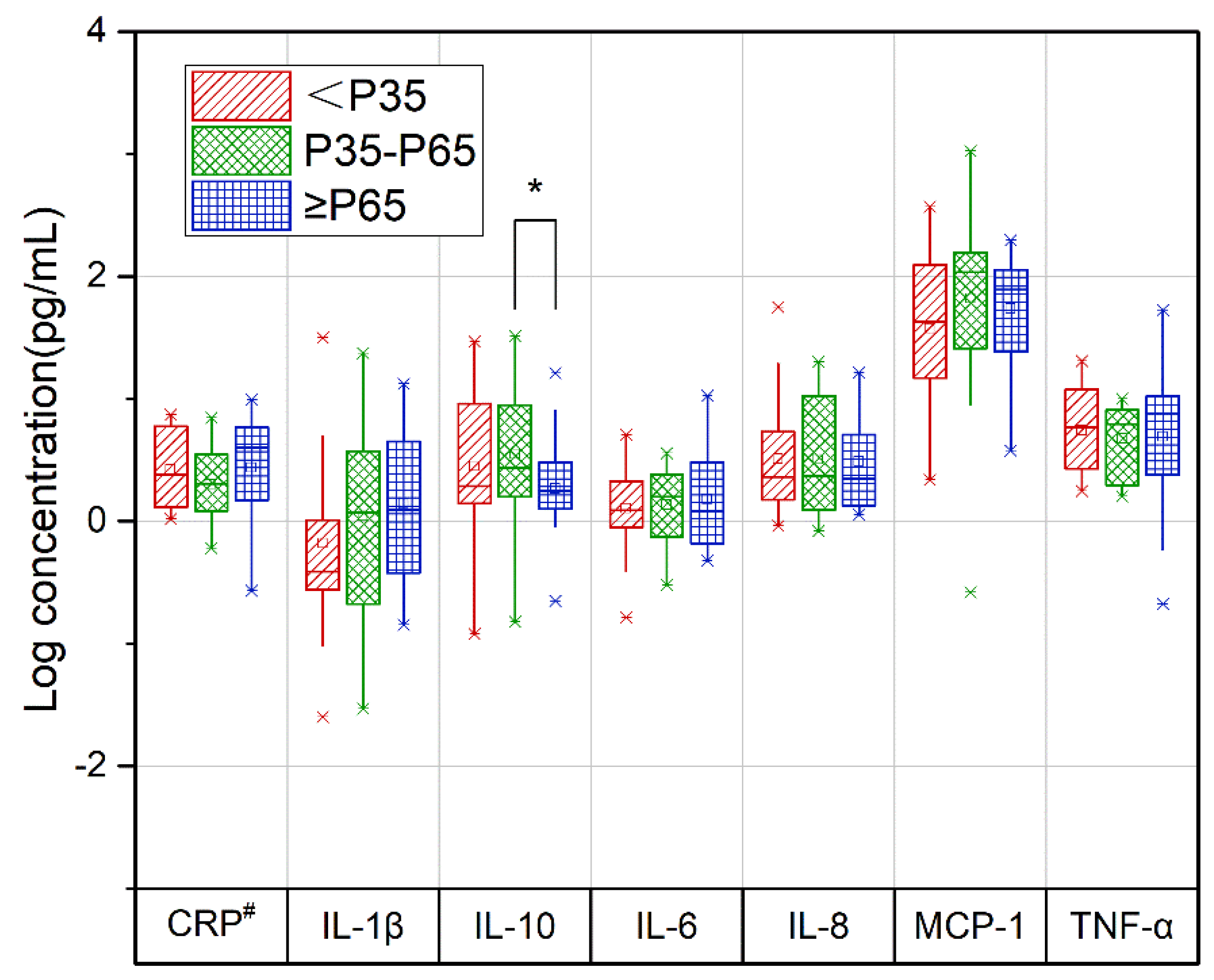
3.3. Relationship between the DII Score and Cytokine Levels during Pregnancy
3.4. Relationship between Nutrients/Food Components and Cytokine Levels during Pregnancy
3.5. Cytokine Levels in Women with Repeated Trimester Measures
3.6. Changes in DII Scores and Cytokine Levels during Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarmund, A.H.; Giskeodegard, G.F.; Ryssdal, M.; Steinkjer, B.; Stokkeland, L.M.T.; Madssen, T.S.; Stafne, S.N.; Stridsklev, S.; Moholdt, T.; Heimstad, R.; et al. Cytokine Patterns in Maternal Serum from First Trimester to Term and Beyond. Front. Immunol. 2021, 12, 752660. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Basu, A.; Fu, D.; Wu, M.; Centola, M.; Jenkins, A.J.; Hanssen, K.F.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum inflammatory markers and preeclampsia in type 1 diabetes: A prospective study. Diabetes Care 2013, 36, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Choi, G.B.; Huh, J.R. Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol. 2022, 43, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cheng, Y.J.; Beckles, G.L. Inflammation among women with a history of gestational diabetes mellitus and diagnosed diabetes in the Third National Health and Nutrition Examination Survey. Diabetes Care 2008, 31, 1386–1388. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, S.; Waghdhare, S.; Goel, C.; Panda, M.; Soneja, H.; Sundar, J.; Banerjee, M.; Jha, S.; Dubey, S. Augmentation of IL-6 production contributes to development of gestational diabetes mellitus: An Indian study. Diabetes Metab. Syndr. 2019, 13, 895–899. [Google Scholar] [CrossRef]
- Yu, N.; Cui, H.; Chen, X.; Chang, Y. Changes of serum pentraxin-3 and hypersensitive CRP levels during pregnancy and their relationship with gestational diabetes mellitus. PLoS ONE 2019, 14, e0224739. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Cos, P.; Briede, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am. J. Reprod. Immunol. 2007, 58, 21–30. [Google Scholar] [CrossRef]
- Yang, Y.; Kan, H.; Yu, X.; Yang, Y.; Li, L.; Zhao, M. Relationship between dietary inflammatory index, hs-CRP level in the second trimester and neonatal birth weight: A cohort study. J. Clin. Biochem. Nutr. 2020, 66, 163–167. [Google Scholar] [CrossRef]
- Moghaddam Banaem, L.; Mohamadi, B.; Asghari Jaafarabadi, M.; Aliyan Moghadam, N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J. Obstet. Gynaecol. Res. 2012, 38, 780–786. [Google Scholar] [CrossRef]
- Ehsani, V.; Mortazavi, M.; Ghorban, K.; Dadmanesh, M.; Bahramabadi, R.; Rezayati, M.T.; Javadi-Moghadam, E.; Rezaei, Z.; Sabzali, Z.; Fatemi, I.; et al. Role of maternal interleukin-8 (IL-8) in normal-term birth in the human. Reprod. Fertil. Dev. 2019, 31, 1049–1056. [Google Scholar] [CrossRef]
- Instanes, J.T.; Halmoy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsoyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers with Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Kim, E.; Finander, B.; Duffy, E.E.; Kim, H.; Gilman, C.K.; Yim, Y.S.; Tong, L.; Kaufman, R.J.; Griffith, E.C.; et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 2021, 24, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Weber-Stadlbauer, U.; Richetto, J.; Rothmond, D.A.; Labouesse, M.A.; Polesel, M.; Robinson, K.; Shannon Weickert, C.; Meyer, U. Increased levels of midbrain immune-related transcripts in schizophrenia and in murine offspring after maternal immune activation. Mol. Psychiatry 2021, 26, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Money, K.M.; Barke, T.L.; Serezani, A.; Gannon, M.; Garbett, K.A.; Aronoff, D.M.; Mirnics, K. Gestational diabetes exacerbates maternal immune activation effects in the developing brain. Mol. Psychiatry 2018, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Huang, W.; Qian, H.; Li, Y. TNF-alpha inhibitors with anti-oxidative stress activity from natural products. Curr. Top. Med. Chem. 2012, 12, 1408–1421. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Santos, S.; Oliveira, A.; Lopes, C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr. Res. 2013, 33, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes Dos Santos, C.; Pinto, P.; Re, R.; Remond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Li, S.X.; Hodge, A.M.; MacInnis, R.J.; Bassett, J.K.; Ueland, P.M.; Midttun, O.; Ulvik, A.; Rinaldi, S.; Meyer, K.; Navionis, A.S.; et al. Inflammation-Related Marker Profiling of Dietary Patterns and All-cause Mortality in the Melbourne Collaborative Cohort Study. J. Nutr. 2021, 151, 2908–2916. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Geesey, M.E. Relation of dietary fat and fiber to elevation of C-reactive protein. Am. J. Cardiol. 2003, 92, 1335–1339. [Google Scholar] [CrossRef]
- Wood, A.D.; Strachan, A.A.; Thies, F.; Aucott, L.S.; Reid, D.M.; Hardcastle, A.C.; Mavroeidi, A.; Simpson, W.G.; Duthie, G.G.; Macdonald, H.M. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br. J. Nutr. 2014, 112, 1341–1352. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Yang, Y.; Hozawa, A.; Kogure, M.; Narita, A.; Hirata, T.; Nakamura, T.; Tsuchiya, N.; Nakaya, N.; Ninomiya, T.; Okuda, N.; et al. Dietary Inflammatory Index Positively Associated with High-Sensitivity C-Reactive Protein Level in Japanese from NIPPON DATA2010. J. Epidemiol. 2020, 30, 98–107. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Brann, L.; Shivappa, N.; Hebert, J.R. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition 2019, 63–64, 155–161. [Google Scholar] [CrossRef]
- Shivappa, N.; Wirth, M.D.; Hurley, T.G.; Hebert, J.R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Wirth, M.D.; Murphy, E.A.; Hurley, T.G.; Hebert, J.R. Association between the Dietary Inflammatory Index (DII) and urinary enterolignans and C-reactive protein from the National Health and Nutrition Examination Survey-2003–2008. Eur. J. Nutr. 2019, 58, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Aminianfar, A.; Vahid, F.; Shayanfar, M.; Davoodi, S.H.; Mohammad-Shirazi, M.; Shivappa, N.; Sharifi, G.; Hebert, J.R.; Surkan, P.J.; Faghfoori, Z.; et al. The association between the dietary inflammatory index and glioma: A case-control study. Clin. Nutr. 2020, 39, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.M.; Ibiebele, T.; Shivappa, N.; Hebert, J.R.; Spurdle, A.B.; Webb, P.M.; Australian National Endometrial Cancer Study, G. Dietary inflammatory index, risk and survival among women with endometrial cancer. Cancer Causes Control 2020, 31, 203–207. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Xiang, J. The Dietary Inflammatory Index Is Associated with Diabetes Severity. J. Am. Board Fam. Med. 2019, 32, 801–806. [Google Scholar] [CrossRef]
- Fu, W.; Pei, H.; Shivappa, N.; Hebert, J.R.; Luo, T.; Tian, T.; Alimu, D.; Zhang, Z.; Dai, J. Association between Dietary Inflammatory Index and Type 2 diabetes mellitus in Xinjiang Uyghur autonomous region, China. PeerJ 2021, 9, e11159. [Google Scholar] [CrossRef]
- Vissers, L.E.T.; Waller, M.; van der Schouw, Y.T.; Hebert, J.R.; Shivappa, N.; Schoenaker, D.; Mishra, G.D. A pro-inflammatory diet is associated with increased risk of developing hypertension among middle-aged women. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 564–570. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Association between dietary inflammatory index and psychological profile in adults. Clin. Nutr. 2019, 38, 2360–2368. [Google Scholar] [CrossRef]
- Canto-Osorio, F.; Denova-Gutierrez, E.; Sanchez-Romero, L.M.; Salmeron, J.; Barrientos-Gutierrez, T. Dietary Inflammatory Index and metabolic syndrome in Mexican adult population. Am. J. Clin. Nutr. 2020, 112, 373–380. [Google Scholar] [CrossRef]
- Ghorabi, S.; Esteghamati, A.; Azam, K.; Daneshzad, E.; Sadeghi, O.; Salari-Moghaddam, A.; Azadbakht, L.; Djafarian, K. Association between dietary inflammatory index and components of metabolic syndrome. J. Cardiovasc. Thorac. Res. 2020, 12, 27–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Zhong, C.; Zhou, X.; Liu, C.; Li, Q.; Chen, R.; Gao, Q.; Li, X.; Zhang, H.; et al. Association between dietary inflammatory index and gestational diabetes mellitus risk in a prospective birth cohort study. Nutrition 2021, 87–88, 111193. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Lu, Q.; Huang, J.; Chen, Y.; Mao, L. Association between the dietary inflammatory index, interleukin-6 of late pregnant women and birth weight. Wei Sheng Yan Jiu 2021, 50, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Faleschini, S.; Rifas-Shiman, S.L.; Monthe-Dreze, C.; Switkowski, K.M.; Gingras, V.; Perng, W.; Oken, E.; Hivert, M.F.; Tiemeier, H. Maternal Dietary Inflammatory Index in Pregnancy and Offspring Behavioral Problems in Mid-Childhood and Early Adolescence. Biol. Psychiatry 2021, 90, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Lertxundi, N.; Molinuevo, A.; Valvi, D.; Gorostiaga, A.; Balluerka, N.; Shivappa, N.; Hebert, J.; Navarrete-Munoz, E.M.; Vioque, J.; Tardon, A.; et al. Dietary inflammatory index of mothers during pregnancy and Attention Deficit-Hyperactivity Disorder symptoms in the child at preschool age: A prospective investigation in the INMA and RHEA cohorts. Eur. Child Adolesc. Psychiatry 2021, 31, 615–624. [Google Scholar] [CrossRef]
- Polanska, K.; Kaluzny, P.; Aubert, A.M.; Bernard, J.Y.; Duijts, L.; El Marroun, H.; Hanke, W.; Hebert, J.R.; Heude, B.; Jankowska, A.; et al. Dietary Quality and Dietary Inflammatory Potential During Pregnancy and Offspring Emotional and Behavioral Symptoms in Childhood: An Individual Participant Data Meta-analysis of Four European Cohorts. Biol. Psychiatry 2021, 89, 550–559. [Google Scholar] [CrossRef]
- Pieczynska, J.; Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Sozanski, R.; Grajeta, H. Association of Dietary Inflammatory Index with Serum IL-6, IL-10, and CRP Concentration during Pregnancy. Nutrients 2020, 12, 2789. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tang, L.; Hu, M.; Xiang, Z.; Hu, Y. Changes of serum interleukin-6 in healthy pregnant women and establishment of relevant reference intervals. Clin. Chim. Acta 2020, 502, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Tagoma, A.; Haller-Kikkatalo, K.; Roos, K.; Oras, A.; Kirss, A.; Ilonen, J.; Uibo, R. Interleukin-7, T helper 1, and regulatory T-cell activity-related cytokines are increased during the second trimester of healthy pregnancy compared to non-pregnant women. Am. J. Reprod. Immunol. 2019, 82, e13188. [Google Scholar] [CrossRef] [PubMed]
- Kalva-Borato, D.C.; Ribas, J.T.; Parabocz, G.C.; Borba, L.M.; Maciel, M.A.S.; Santos, F.A.D.; Vellosa, J.C.R. Biomarkers in Non-Complicated Pregnancy: Insights About Serum Myeloperoxidase and Ultrasensitive C-Reactive Protein. Exp. Clin. Endocrinol. Diabetes 2019, 127, 585–589. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Shivappa, N.; Prioreschi, A.; Hebert, J.R.; Norris, S.A. Anti-inflammatory diets reduce the risk of excessive gestational weight gain in urban South Africans from the Soweto First 1000-Day Study (S1000). Eur. J. Nutr. 2022, 61, 3929–3941. [Google Scholar] [CrossRef]
- Camerota, M.; Wylie, A.C.; Goldblum, J.; Wideman, L.; Cheatham, C.L.; Propper, C.B. Testing a cascade model linking prenatal inflammation to child executive function. Behav. Brain Res. 2022, 431, 113959. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lu, X.; Xie, Z.; Jiang, T.; Song, C.; Wang, Z. Evaluation of a Novel WeChat Applet for Image-Based Dietary Assessment among Pregnant Women in China. Nutrients 2021, 13, 3158. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, Y.; Li, F.; Shao, Y.; Sun, Z.; Zhong, C.; Fan, P.; Li, Z.; Zhang, M.; Li, X.; et al. Development and validation of a photographic atlas of food portions for accurate quantification of dietary intakes in China. J. Hum. Nutr. Diet. 2021, 34, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. China Food Composition Tables, 6th ed.; Peking University Medical Press: Beijing, China, 2019; p. 429. [Google Scholar]
- Lee, A.C.; Cherkerzian, S.; Olson, I.E.; Ahmed, S.; Chowdhury, N.H.; Khanam, R.; Rahman, S.; Andrews, C.; Baqui, A.H.; Fawzi, W.; et al. Maternal Diet, Infection, and Risk of Cord Blood Inflammation in the Bangladesh Projahnmo Pregnancy Cohort. Nutrients 2021, 13, 3792. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, M.; Karlsson, L.; Paavonen, E.J.; Polo-Kantola, P.; Pelto, J.; Nousiainen, N.; Scheinin, N.M.; Maksimow, M.; Salmi, M.; Karlsson, H. Maternal tiredness and cytokine concentrations in mid-pregnancy. J. Psychosom. Res. 2019, 127, 109843. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Pham, N.M.; Lee, A.H.; Shivappa, N.; Hebert, J.R.; Zhao, J.; Su, D.; Binns, C.W.; Li, C. Dietary Inflammatory Index and Epithelial Ovarian Cancer in Southern Chinese Women: A Case-Control Study. Cancer Control 2020, 27, 1073274820977203. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Zhang, M.; Li, Y.; Liu, W.; Huang, H.; Xu, Y. Association between dietary inflammatory index and bone density in lactating women at 6 months postpartum: A longitudinal study. BMC Public Health 2019, 19, 1076. [Google Scholar] [CrossRef]
- de Castro Mendes, F.; Thawer, S.; Shivappa, N.; Hebert, J.R.; Burney, P.G.; Garcia-Larsen, V. Dietary Inflammatory Index (DII®) and Lung Function in Adults from Ten European Countries–Evidence from the GA2LEN Follow-Up Survey. Curr. Dev. Nutr. 2020, 4, 1393. [Google Scholar] [CrossRef]
- Park, S.Y.; Boushey, C.J.; Shvetsov, Y.B.; Wirth, M.D.; Shivappa, N.; Hebert, J.R.; Haiman, C.A.; Wilkens, L.R.; Le Marchand, L. Diet Quality and Risk of Lung Cancer in the Multiethnic Cohort Study. Nutrients 2021, 13, 1614. [Google Scholar] [CrossRef]
- Vahid, F.; Shivappa, N.; Hekmatdoost, A.; Hebert, J.R.; Davoodi, S.H.; Sadeghi, M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: Case-control study. Appl. Physiol. Nutr. Metab. 2017, 42, 511–516. [Google Scholar] [CrossRef]
- Kendel Jovanovic, G.; Mrakovcic-Sutic, I.; Pavicic Zezelj, S.; Susa, B.; Rahelic, D.; Klobucar Majanovic, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef] [PubMed]
- Shin, P.K.; Park, S.J.; Kim, M.S.; Kwon, D.Y.; Kim, M.J.; Kim, K.; Chun, S.; Lee, H.J.; Choi, S.W. A Traditional Korean Diet with a Low Dietary Inflammatory Index Increases Anti-Inflammatory IL-10 and Decreases Pro-Inflammatory NF-kappaB in a Small Dietary Intervention Study. Nutrients 2020, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.M.; Wirth, M.D.; Shivappa, N.; Dunn, C.G.; Crimarco, A.; Hurley, T.G.; West, D.S.; Hussey, J.R.; Hebert, J.R. Impact of a 12-month Inflammation Management Intervention on the Dietary Inflammatory Index, inflammation, and lipids. Clin. Nutr. ESPEN 2019, 30, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Sharma, S. Interleukin-10: A pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef]
- Thaker, R.; Oza, H.; Verma, V.; Gor, M.; Kumar, S. The Association of Circulatory Cytokines (IL-6 and IL-10) Level with Spontaneous Abortion-a Preliminary Observation. Reprod. Sci. 2021, 28, 857–864. [Google Scholar] [CrossRef]
- Danaii, S.; Ghorbani, F.; Ahmadi, M.; Abbaszadeh, H.; Koushaeian, L.; Soltani-Zangbar, M.S.; Mehdizadeh, A.; Hojjat-Farsangi, M.; Kafil, H.S.; Aghebati-Maleki, L.; et al. IL-10-producing B cells play important role in the pathogenesis of recurrent pregnancy loss. Int. Immunopharmacol. 2020, 87, 106806. [Google Scholar] [CrossRef]
- Ghodoosi, N.; Mirzababaei, A.; Rashidbeygi, E.; Badrooj, N.; Sajjadi, S.F.; Setayesh, L.; Yekaninejad, M.S.; Keshavarz, S.A.; Shiraseb, F.; Mirzaei, K. Associations of dietary inflammatory index, serum levels of MCP-1 and body composition in Iranian overweight and obese women: A cross-sectional study. BMC Res. Notes 2020, 13, 544. [Google Scholar] [CrossRef]
- Corley, J.; Shivappa, N.; Hebert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef]
- Suzuki, K.; Shivappa, N.; Kawado, M.; Yamada, H.; Hashimoto, S.; Wakai, K.; Iso, H.; Okada, E.; Fujii, R.; Hebert, J.R.; et al. Association between dietary inflammatory index and serum C-reactive protein concentrations in the Japan Collaborative Cohort Study. Nagoya J. Med. Sci. 2020, 82, 237–249. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Urpi-Sarda, M.; Sacanella, E.; Arranz, S.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Lapetra, J.; Portillo, M.P.; et al. Anti-Inflammatory Effects of the Mediterranean Diet in the Early and Late Stages of Atheroma Plaque Development. Mediat. Inflamm. 2017, 2017, 3674390. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Plante, A.S.; Gagnon, M.; Leblanc, N.; Veilleux, A.; Tchernof, A.; Morisset, A.S. Longitudinal changes in circulating concentrations of inflammatory markers throughout pregnancy: Are there associations with diet and weight status? Appl. Physiol. Nutr. Metab. 2022, 47, 287–295. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.E.; Miller, E.E.; Calderwood, L.E.; Shivappa, N.; Steck, S.E.; Forman, M.R.; Mendez, A.M.; Maguire, R.; Fuemmeler, B.F.; Kollins, S.H.; et al. Maternal inflammatory diet and adverse pregnancy outcomes: Circulating cytokines and genomic imprinting as potential regulators? Epigenetics 2017, 12, 688–697. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, Z.; El-Kassas, G.; Ziade, F.M.; Shivappa, N.; Hebert, J.R.; Zmerly, H.; Bissar, N. Evaluation of circulating levels of Interleukin-10 and Interleukin-16 and dietary inflammatory index in Lebanese knee osteoarthritis patients. Heliyon 2021, 7, e07551. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef]
- Finch, C.L.; Zhang, A.; Kosikova, M.; Kawano, T.; Pasetti, M.F.; Ye, Z.; Ascher, J.R.; Xie, H. Pregnancy level of estradiol attenuated virus-specific humoral immune response in H5N1-infected female mice despite inducing anti-inflammatory protection. Emerg. Microbes Infect. 2019, 8, 1146–1156. [Google Scholar] [CrossRef]
- Jamar, G.; Ribeiro, D.A.; Pisani, L.P. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit. Rev. Food Sci. Nutr. 2021, 61, 836–854. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Rees, A.; Richards, O.; Allen-Kormylo, A.; Jones, N.; Thornton, C.A. Maternal body mass index is associated with an altered immunological profile at 28 weeks of gestation. Clin. Exp. Immunol. 2022, 208, 114–128. [Google Scholar] [CrossRef]
- Yeh, K.L.; Kautz, A.; Lohse, B.; Groth, S.W. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Nazarian, B.; Sarreshtedari, M.; Mozaffari-Khosravi, H.; Maleki, V.; Alizadeh, M.; Shokri, A.; Sadeghi, O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 17234. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, O.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Bizerea, T.O.; Dezsi, S.G.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The Link Between Selenium, Oxidative Stress and Pregnancy Induced Hypertensive Disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef] [PubMed]

| Participants | |
|---|---|
| Age (years) | 30.86 ± 3.47 |
| Prepregnancy BMI (kg/m2) | 22.23 ± 3.59 |
| Education level (years) | |
| ≤12 | 19 (10.86%) |
| 12~15 | 139 (79.43%) |
| ≥15 | 17 (9.71%) |
| Annual household income (CNY) | |
| ≤120,000 | 15 (8.57%) |
| 120,000~240,000 | 132 (75.43%) |
| ≥240,000 | 28 (16.00%) |
| Smoking during the prepregnancy period | |
| No | 165 (94.29%) |
| Yes | 10 (5.71%) |
| Prepregnancy secondhand smoke exposure | |
| No | 125 (71.43%) |
| Yes | 50 (28.57%) |
| Secondhand smoke exposure in the second trimester * | |
| No | 111 (69.81%) |
| Yes | 48 (30.19%) |
| Secondhand smoke exposure in the third trimester ** | |
| No | 71 (78.89%) |
| Yes | 19 (21.11%) |
| Drinking during prepregnancy period | |
| No | 171 (97.71%) |
| Yes | 4 (2.29%) |
| Second Trimester physical activity * (MET h/day) | |
| Resting | 14.13 (10.82, 17.40) |
| Light | 9.38 (5.00, 13.00) |
| Moderate | 10.50 (7.50, 14.00) |
| Heavy | 0.00 (0.00, 0.00) |
| Third Trimester physical activity ** (MET h/day) | |
| Resting | 12.92 (10.08, 16.00) |
| Light | 10.70 (5.50, 14.19) |
| Moderate | 11.65 (7.50, 15.13) |
| Heavy | 0.00 (0.00, 0.00) |
| DII score | |
| Second Trimester * | −0.07 ± 1.65 |
| Third Trimester ** | 0.06 ± 1.65 |
| DII Score in the Second Trimester * | p1 | DII Score in the Third Trimester ** | p2 | |
|---|---|---|---|---|
| B (95% CI) | B (95% CI) | |||
| Age (years) | −0.09 (−0.17, −0.02) | 0.014 | −0.09 (−0.18, 0.00) | 0.052 |
| Prepregnancy BMI (kg/m2) | −0.02 (−0.12, 0.09) | 0.763 | −0.09 (−0.24, 0.06) | 0.263 |
| Gestational age (weeks) | 0.01 (−0.07, 0.08) | 0.872 | −0.17 (−0.32, −0.02) | 0.022 |
| Pregnancy BMI (kg/m2) | 0.03 (−0.07, 0.12) | 0.562 | 0.13 (−0.04, 0.30) | 0.124 |
| Physical activity (MET h/day) | ||||
| Resting | −0.19 (−0.73, 0.35) | 0.486 | −0.02 (−0.30, 0.26) | 0.890 |
| Light | −0.13 (−0.47, 0.20) | 0.439 | 0.00 (−0.17, 0.17) | 0.991 |
| Moderate | −0.03 (−0.17, 0.11) | 0.627 | 0.01 (−0.09, 0.11) | 0.823 |
| Heavy | −0.33 (−0.79, 0.13) | 0.163 | 0.02 (−0.18, 0.21) | 0.861 |
| Education level (years) | ||||
| ≤12 | 1.28 (0.07, 2.49) | 0.038 | −0.14 (−0.15, 1.23) | 0.846 |
| 12~15 | 1.16 (0.24, 2.09) | 0.014 | 0.13 (−0.81, 1.08) | 0.781 |
| ≥15 | 0 | 0 | ||
| Annual household income (CNY) | ||||
| ≤120,000 | 0.18 (−0.87, 1.23) | 0.733 | 2.50 (1.09, 3.91) | 0.001 |
| 120,000~240,000 | 0.13 (−0.54, 0.81) | 0.698 | 1.11 (0.05, 2.16) | 0.039 |
| ≥240,000 | 0 | 0 | ||
| Smoking during the prepregnancy period | 0.878 | 0.882 | ||
| Yes | 0.09 (−1.01, 1.18) | −0.18 (−2.55, 2.19) | ||
| No | 0 | 0 | ||
| Prepregnancy secondhand smoke exposure | 0.715 | 0.133 | ||
| Yes | 0.11 (−0.48, 0.71) | 0.57 (−0.17, 1.32) | ||
| No | 0 | 0 | ||
| Drinking during the Prepregnancy period | 0.902 | 0.088 | ||
| Yes | 0.12 (−1.80, 2.04) | 1.80 (−0.26, 3.86) | ||
| No | 0 | 0 | ||
| Pregnancy secondhand smoke exposure | 0.738 | 0.558 | ||
| Yes | 0.10 (−0.48, 0.68) | −0.26 (−1.13, 0.61) | ||
| No | 0 | 0 |
| T2 # | T3 $ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | CRP | MCP-1 | IL-6 | IL-8 | IL-10 | TNF-α | IL-1β | CRP | MCP-1 | IL-6 | IL-8 | IL-10 | |
| β-carotenes (mg) | −0.006 | −0.063 | 0.001 | −0.040 | 0.077 * | 0.068 | −0.004 | 0.110 | 0.197 | −0.028 | 0.002 | 0.014 | −0.022 | 0.103 |
| SFAs (kg) | −0.041 | 4.003 | −5.602 | −4.043 | −2.126 | −2.659 | 5.746 | 15.615 | 3.441 | −5.808 | 3.807 | 8.895 | −57.744 * | −15.385 |
| MUFAs (kg) | −2.037 | 13.337 | −0.049 | 0.998 | 1.372 | −11.778 | 11.632 | −1.183 | −39.581 | 9.963 | −1.371 | 31.757 | 75.872 * | −6.024 |
| PUFAs (kg) | −0.384 | −7.785 | 2.179 | −2.525 | −5.815 | −1.868 | −6.117 | −17.129 | 24.640 | −3.917 | −9.323 | −19.402 | −34.231 * | 6.757 |
| Protein (kg) | −2.823 | 4.728 | −1.916 | 2.682 | −2.126 | −0.797 | 4.745 | 3.059 | 14.157 | −9.174 * | 1.361 | −4.151 | −2.407 | 8.992 |
| Total fat (kg) | 1.373 | −0.539 | 1.711 | 4.712 | −1.120 | 9.784 * | −3.679 | 3.523 | 2.921 | 1.079 | 2.379 | −5.264 | −3.503 | −3.515 |
| Carbohydrates (kg) | −0.519 | −1.418 | 0.095 | −1.037 | 0.289 | −0.750 | −0.567 | 0.074 | −0.851 | 1.018 | −1.298 | 1.335 | 2.454 * | −3.707 |
| Fiber (kg) | 5.837 | −10.707 | −2.158 | −4.822 | 3.217 | −12.347 | −9.584 | −3.216 | 2.346 | 5.420 | 24.027 | 22.867 | 31.786 * | 12.533 |
| Cholesterol (g) | −0.111 | −0.302 | 0.182 | −0.508 * | 0.191 | −0.315 | −0.049 | −0.497 | −0.313 | 0.102 | −0.472 | 0.054 | −0.081 | −0.668 |
| Vitamin A (RE 1000) | 0.043 | 0.127 | 0.012 | 0.133 * | 0.021 | −0.002 | −0.022 | −0.095 | 0.156 | −0.143 | 0.053 | 0.094 | 0.044 | 0.052 |
| Vitamin D (mg) | 1.400 | 1.781 | 1.393 | −0.374 | 0.167 | 2.793 | 2.447 | −5.432 | −16.065 | −5.561 | −8.376 | −1.197 | −9.779 | −25.791 * |
| Vitamin E (g) | 3.457 | −9.954 | −0.636 | −2.835 | 3.053 | 11.057 | 5.194 | 20.098 * | −18.074 | 1.257 | 5.287 | 19.495 * | 11.286 | −30.201 * |
| Thiamin (mg) | −0.136 | −0.168 | −0.076 | 0.108 | 0.243 | 0.074 | −0.283 | −0.384 | −0.123 | 0.076 | −0.879 * | −0.602 * | −0.478 | 0.393 |
| Riboflavin (mg) | −0.021 | 0.064 | −0.044 | −0.034 | −0.043 | −0.067 | 0.116 * | 0.163 | −0.175 | −0.083 | 0.064 | −0.176 | −0.061 | 0.041 |
| Vitamin B6 (mg) | 0.078 | −0.085 | 0.213 | −0.174 | −0.059 | −0.065 | 0.200 | 0.532 | −0.440 | 0.669 * | −0.349 | 0.406 | 0.511 | −0.391 |
| Vitamin B12 (μg) | −0.010 | 0.070 | −0.099 | 0.047 | −0.011 | −0.020 | −0.147 | −0.508 | 0.445 | −0.362 | 0.532 | 0.061 | −0.125 | 0.207 |
| IL-6 (g) | −0.919 | 1.323 | −0.487 | 0.806 | −0.418 | −0.222 | 0.685 | 1.273 | −1.364 | 0.159 | −2.888 * | −1.794 * | −0.433 | −1.250 |
| Folic acid (mg) | 0.150 | −0.339 | 0.078 | −0.358 | −0.151 | −0.222 | −0.102 | 0.216 | −0.237 | −0.112 | 0.363 | −0.325 | −0.434 | −0.101 |
| Niacin (g) | 4.788 | 9.442 | −0.519 | 7.310 | 11.739 | −9.219 | 6.653 | −32.335 * | −19.130 | −0.544 | 12.451 | −2.881 | −6.040 | 5.186 |
| Mg (g) | 0.397 | 1.986 | 0.307 | 0.103 | −0.684 | −0.879 | 0.222 | −2.056 | −0.697 | −0.292 | −0.353 | −0.789 | −0.827 | 2.231 |
| Fe (g) | −1.462 | 2.835 | −1.609 | 2.726 | 1.523 | −1.368 | 2.683 | 15.640 * | 5.617 | 0.202 | −0.957 | 2.101 | 5.491 | 11.353 * |
| Zn (g) | 13.237 | −60.299 * | 11.816 | −26.479 | −13.029 | 26.992 | −26.969 | −30.878 | −1.211 | −8.921 | 18.601 | −45.600 | −38.110 | −69.354 * |
| Se (mg) | 1.206 | −1.458 | 0.776 | 4.212 | 1.642 | 3.717 | −3.438 | 7.687 | 4.716 | 6.643 | 6.677 | 10.970 * | 6.238 | 11.536 |
| T2 | T3 | T3 < T2 n (%) | p * | |
|---|---|---|---|---|
| DII score | −0.04 ± 1.63 | −0.15 ± 1.56 | 34 (45.95%) | 0.633 |
| TNF-α (pg/mL) | 7.41 (4.15, 9.55) | 6.66 (2.46, 9.10) | 32 (43.24%) | 0.066 |
| IL-1β (pg/mL) | 1.11 (0.37, 5.88) | 0.83 (0.31, 3.95) | 26 (35.14%) | 0.901 |
| CRP (ng/mL) | 3.10 (1.81, 4.75) | 2.49 (1.30, 4.59) | 36 (48.65%) | 0.214 |
| MCP-1 (pg/mL) | 77.24 (21.68, 146.77) | 66.04 (19.94, 133.18) | 38 (51.35%) | 0.353 |
| IL-6 (pg/mL) | 0.61 (0.35, 0.98) | 1.32 (0.70, 2.69) | 14 (18.92%) | <0.001 |
| IL-8 (pg/mL) | 1.92 (1.29, 8.90) | 2.26 (1.29, 8.37) | 26 (35.14%) | 0.940 |
| IL-10 (pg/mL) | 3.09 (1.45, 10.23) | 1.96 (1.35, 7.47) | 29 (39.19%) | 0.714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Zhang, J.; Liu, L.; Xiong, W.; Su, Y.; Han, Y.; Gao, L.; Qu, Z.; Zhang, X. Relationship between the Dietary Inflammatory Index Score and Cytokine Levels in Chinese Pregnant Women during the Second and Third Trimesters. Nutrients 2023, 15, 194. https://doi.org/10.3390/nu15010194
Cui T, Zhang J, Liu L, Xiong W, Su Y, Han Y, Gao L, Qu Z, Zhang X. Relationship between the Dietary Inflammatory Index Score and Cytokine Levels in Chinese Pregnant Women during the Second and Third Trimesters. Nutrients. 2023; 15(1):194. https://doi.org/10.3390/nu15010194
Chicago/Turabian StyleCui, Tingkai, Jingchao Zhang, Liyuan Liu, Wenjuan Xiong, Yuanyuan Su, Yu Han, Lei Gao, Zhiyi Qu, and Xin Zhang. 2023. "Relationship between the Dietary Inflammatory Index Score and Cytokine Levels in Chinese Pregnant Women during the Second and Third Trimesters" Nutrients 15, no. 1: 194. https://doi.org/10.3390/nu15010194
APA StyleCui, T., Zhang, J., Liu, L., Xiong, W., Su, Y., Han, Y., Gao, L., Qu, Z., & Zhang, X. (2023). Relationship between the Dietary Inflammatory Index Score and Cytokine Levels in Chinese Pregnant Women during the Second and Third Trimesters. Nutrients, 15(1), 194. https://doi.org/10.3390/nu15010194






