Diagnostic Overshadowing and the Unseen Spectrum: A Narrative Review of Rare Complications in Sickle Cell Disease
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Synthesis Approach
3. Results
3.1. Neurological Complications
3.1.1. Spontaneous Epidural and Subdural Hematoma
- Definition: non-traumatic bleeding into the epidural or subdural space surrounding the spinal cord or brain;
- Key case findings: Several case reports describe non-traumatic presentations of spontaneous epidural hematoma in young adults and children with SCD, frequently amidst sickle crises, causing sudden onset of back pain, radicular pain, or neurological deficits [8]. Spontaneous subdural hemorrhage has also been reported as mimicking dural sinus thrombosis in an adolescent [9] and presenting as chronic bilateral subdural hematoma in a young child [10]. Unilateral papilledema has revealed a subdural hematoma in an adult patient [11];
- Diagnostic challenges: A sudden onset of back pain, radicular pain, or neurological deficits (weakness, sensory changes, bowel/bladder dysfunction) can be attributed solely to VOC, which can delay appropriate imaging [7,8]. Subdural bleeds can also present with non-specific headaches or neurological changes that might be overshadowed by concurrent pain or anemia symptoms;
- Pathophysiologic rationale: Hypothesized mechanisms of this symptom include skull or vertebral bone infarction, altered skull/vertebral anatomy from extramedullary hematopoiesis (EMH), and venous congestion during crises [7,8]. Subdural bleeds may also relate to vascular fragility or altered coagulation in SCD [9];
- Recommended diagnostic tests: Urgent neuroimaging is essential. An MRI of the spine or brain should be obtained immediately (ideally within hours of symptom onset) to confirm an epidural or subdural hematoma and delineate its extent. If intracranial pathology is suspected, brain MRI is preferred; for isolated spinal symptoms, spinal MRI is indicated (in acute settings where MRI is not instantly available, CT can be a first step, but MRI provides superior detail of spinal/soft tissue bleeding) [8].
- Management strategies and outcomes: Prompt neurosurgical evaluation for decompression is often required. Laminectomy or craniotomy to evacuate the hematoma can lead to good neurological recovery if performed early. Supportive care (hydration, transfusion to reduce sickling, blood pressure control) is also important. The outcomes are favorable when intervention is timely, but delays can result in permanent paralysis or other deficits [7,8,9,10,11];
- Prevention: There are no specific measures to prevent spontaneous hemorrhages, but optimal SCD management (e.g., maintaining hemoglobin levels, avoiding extreme anemia or dehydration) might reduce precipitating factors such as severe bone infarction. Additionally, clinicians should promptly investigate any atypical neurological pain in SCD rather than attributing it solely to VOC;
- Clinical takeaway: Unexplained or disproportionately severe spinal pain or neurological deficits in a patient with SCD mandate urgent neuroimaging to rule out epidural hematoma. Atypical headaches or neurological signs should prompt consideration of subdural hemorrhage.
3.1.2. Brain and Spinal Infarcts (Atypical Presentation) and Cerebral Arteriovenous Malformations
- Definition: ischemic injury to the brain or spinal cord tissue due to vascular occlusion, presenting in unusual locations or contexts beyond typical stroke, or rare vascular malformations;
- Key case findings: A retrospective review of the spinal pathology in SCD highlights spinal cord infarction as a significant complication [12]. A case report details an autopsy-confirmed spinal cord infarction in a patient with sickle cell anemia [13]. Another case describes an anterior spinal infarct in a 19-year-old man [14]. In one dramatic case, a 32-year-old woman with otherwise stable SCD developed septic shock and acute multiorgan failure (MOF) during a severe crisis, with rapid neurological decline and death; it was postulated that atypical patterns of cerebral infarction occurred in the context of MOF [15]. The development of de novo cerebral arteriovenous malformation (AVM) in a child with SCD and moyamoya arteriopathy has also been reported, with the rarity and lack of an established correlation between cerebral AVMs and SCD being noted [16];
- Diagnostic challenges: Neurological impairment may be overshadowed by systemic symptoms of severe crisis or MOF [15]. Recognizing infarcts in unusual locations (e.g., spinal cord, atypical brain regions) or concurrently with widespread organ failure is challenging. Cerebral AVMs may be asymptomatic until they hemorrhage or cause seizures, and in SCD these symptoms might initially be thought to result from more common causes (stroke or meningitis);
- Pathophysiologic rationale: Vaso-occlusion, potentially exacerbated by systemic inflammation, infection (septic shock), or hypercoagulability (e.g., associated antiphospholipid antibodies), contributes to vessel occlusion and tissue infarction [15]. The development of AVMs in SCD is poorly understood but may relate to chronic vascular remodeling or angiogenesis in response to ischemia, particularly in the setting of moyamoya [16];
- Recommended diagnostic tests: Any severe or atypical neurological deficit in SCD warrants thorough imaging. Brain MRI (with vascular sequences) and spinal MRI should be performed to localize infarcts. If an AVM is suspected or MRI suggests an abnormal tangle of vessels, cerebral angiography is the gold standard for diagnosis. In patients with unusual presentations (e.g., during sepsis or MOF), also evaluate for precipitants: blood cultures, inflammatory markers, and coagulation studies including antiphospholipid antibodies may be informative;
- Management strategies and outcomes: Management of infarcts involves acute stroke protocols (exchange transfusion to reduce the HbS < 30%, hydration, oxygen, treatment of any infection) and supportive ICU care for MOF. Blood exchange was attempted in one severe MOF case but the patient succumbed [15]. Maintaining the HbS below 30% through exchange transfusion is suggested to reduce thrombotic complications. Management of AVMs is complex and may involve surgical resection, endovascular embolization, or radiosurgery [16];
- Prevention: Standard SCD stroke prevention strategies (e.g., transcranial Doppler screening in children, prophylactic transfusions for high-risk patients) help prevent common strokes and may incidentally prevent some atypical infarcts. Keeping the HbS percentage low during high-risk periods (e.g., perioperatively or during severe illness) might reduce the infarct risk [17]. There is no known prevention for AVMs, but controlling the severity of SCD over the long term might mitigate the chronic vascular stress that potentially contributes to such anomalies;
- Clinical takeaway: Severe or atypical neurological deficits in a patient with SCD, even in the context of systemic illness, require imaging to assess for infarction. AVMs are rare but important to consider in those with seizures or hemorrhage. Associated conditions like infection or hypercoagulability should be considered.
3.1.3. Central Retinal Artery Occlusion (CRAO)
- Definition: acute occlusion of the central retinal artery (or its branches), leading to sudden, painless monocular vision loss;
- Key case findings: While proliferative sickle retinopathy and peripheral vascular occlusions are common, CRAO is a rare and devastating complication, often presenting with sudden, painless loss of vision. A case report details concurrent bilateral CRAO during an SCD crisis that was managed with erythrocytapheresis [18];
- Diagnostic challenges: In a patient with SCD, acute vision loss might initially be attributed to more common causes like vitreous hemorrhage, retinal detachment, or even an ocular manifestation of severe anemia. Distinguishing CRAO requires prompt ophthalmologic examination, which can be delayed if the patient’s focus (and the clinicians’) is on systemic pain or other crisis symptoms. Additionally, if only one eye is affected, patients in severe pain may not immediately report vision changes;
- Pathophysiologic rationale: Sickled erythrocytes can cause thrombotic occlusion of the central retinal artery or its branches, especially under conditions of severe hypoxemia or acidosis during crises. This leads to retinal ischemia. Unlike peripheral sickle retinopathy, CRAO involves a large vessel supplying the inner retina, analogous to a stroke in the eye;
- Recommended diagnostic tests: Immediate ophthalmologic evaluation is critical. Fundoscopic examination classically shows a pale retina with a “cherry-red spot” at the macula in CRAO. Optical coherence tomography (OCT) can confirm retinal swelling, and fluorescein angiography can localize the occlusion;
- Management strategies and outcomes: CRAO is an ophthalmologic emergency. Interventions (though often of limited success) aim to restore retinal blood flow: ocular massage, anterior chamber paracentesis, and medications to lower intraocular pressure can be tried [18]. Systemic thrombolysis has also been reported in a case treated with IV alteplase and exchange transfusion [19];
- Prevention: The primary method of preventing CRAO in SCD is maintaining overall good control of the disease to prevent extreme sickling episodes. Regular ophthalmologic screening in SCD can detect early proliferative changes but will not predict CRAO. Avoidance of risk factors like uncontrolled hypertension or hyperviscosity (if on transfusions) may theoretically help [20];
- Clinical Takeaway: Sudden, painless vision loss in patient with SCD is a medical emergency requiring urgent ophthalmologic consultation to rule out CRAO.
3.1.4. Posterior Reversible Encephalopathy Syndrome (PRES)
- Definition: a neuro-radiological syndrome characterized by vasogenic edema, predominantly in the posterior white matter, and often associated with hypertension.
- Key case findings: PRES has been increasingly recognized as a rare complication in SCD, particularly in the setting of hypertension during crisis, although it can also occur normotensively. Published cases include both pediatric and adult patients with SCD developing PRES [21]. A case series and literature review specifically address PRES in adult patients with SCD [22]. PRES has also been reported in sickle-beta-thalassemia [23]. Common features in reported cases are headaches, seizures, visual disturbances, and encephalopathy during or following a VOC or hemolytic crisis;
- Diagnostic challenges: Symptoms of PRES (headache, visual disturbances, seizures, altered mental status) overlap with other neurological complications or metabolic derangements in SCD crisis. Distinguishing PRES requires neuroimaging; however, if clinicians focus only on SCD-related causes (e.g., stroke), they might overlook the pattern that is characteristic of PRES. In patients with SCD, pain and distress can elevate blood pressure, potentially precipitating PRES, but the link may not be immediately recognized. Diagnostic overshadowing can occur if providers assume seizures or visual changes are due to known stroke risk without considering PRES, which has a different treatment focus (blood pressure and symptom control);
- Pathophysiologic rationale: The exact mechanism of PRES involves failure of cerebral autoregulation, often due to acute hypertension or endothelial dysfunction, leading to hyperperfusion and leakage of fluid into the brain’s interstitium (vasogenic edema). In SCD, severe anemia, high circulating cell-free hemoglobin, and inflammation may contribute to endothelial injury. Rapid swings in blood pressure during crises, or severe systemic inflammation (even without hypertension), can precipitate PRES. Notably, patients with SCD may have baseline endothelial activation, which possibly lowers the threshold for PRES [21,22];
- Recommended diagnostic tests: Brain MRI is the diagnostic modality of choice for PRES. It classically shows T2/FLAIR hyperintensities (edema) in the posterior occipital and parietal lobes, which are often symmetrical. Diffusion-weighted imaging helps distinguish vasogenic edema (PRES) from cytotoxic edema (infarction). In suspected PRES, blood pressure measurement and control are imperative; labs should include renal function (to check for hypertensive injury) and consideration of other causes (e.g., eclampsia in pregnant patients). Importantly, in any patient with SCD with neurologic symptoms, an MRI should be obtained as soon as possible, ideally within 24 h, to differentiate PRES from acute ischemic stroke or hemorrhage, as their management differs;
- Prevention: For patients with SCD who are at risk (e.g., known history of PRES or severe hypertension episodes), careful blood pressure monitoring and control during acute pain episodes is advisable. Avoiding an excessive transfusion volume (to prevent hypertension) and treating pain adequately (to mitigate adrenergic surges) may help. There is no specific prophylactic medication for PRES, but general measures to maintain vascular health (e.g., hydroxyurea to reduce hemolysis and nitric oxide consumption) might theoretically lower the risk [24];
- Clinical takeaway: New-onset severe headache, visual disturbances, seizures, or altered consciousness in a patient with SCD, especially if accompanied by elevated blood pressure, should prompt consideration of PRES. Immediate neuroimaging and blood pressure evaluation are critical. Recognizing PRES is important because, unlike an infarct, the changes can be reversed with timely treatment, which can prevent permanent neurological damage.
3.1.5. Acute Soft Head Syndrome
- Definition: an exceedingly rare neurological manifestation in SCD characterized by skull osteolysis and swelling [25];
- Key Case Findings: Mentioned as an exceedingly rare manifestation;
- Diagnostic Challenges: The diagnostic challenges regarding this syndrome are likely due to its rarity and potentially non-specific initial symptoms (headache, skull tenderness).
- Pathophysiologic rationale: Acute soft head syndrome is thought to be related to bone infarction in the skull;
- Recommended diagnostic tests: Skull imaging (X-ray, CT, MRI) to demonstrate osteolytic lesions is recommended;
- Management Strategies and Outcomes: Management for this syndrome is likely supportive, potentially involving pain control and addressing underlying crisis (limited specific guidance is available in the provided literature);
- Clinical takeaway: Acute soft head syndrome is an extremely rare neurological event in SCD; clinicians should be aware of its existence although specific guidance is limited in the provided literature.
3.2. Hematologic Complications
3.2.1. Acute Leukemia (Particularly AML)
- (Note: Acute leukemia is not a direct complication of SCD per se, but patients with SCD can develop leukemia, and there is debate about whether hydroxyurea or chronic marrow stress increases this risk. We include it here due to its rarity and diagnostic complexity in the SCD setting.)
- Definition: malignant proliferation of myeloid blasts;
- Key case findings: An extensive review and case report published in 2023 identified over 50 published cases of acute leukemia in patients with SCD since 2000, often presenting with myelodysplastic changes and genetic abnormalities (e.g., chromosome 5/7 aberrations, TP53 mutations) [26]. The risk appears elevated, particularly in adults with severe disease or those exposed to certain therapies like hydroxyurea (though this association remains debated);
- Diagnostic challenges: The presentation of acute leukemia in patient with SCD can be mistaken for an aplastic crisis or severe sequestration crisis, since both can cause abrupt cytopenias. Fever and bone pain may be attributed to VOC or osteomyelitis. Diagnostic overshadowing occurs if clinicians assume all hematologic abnormalities are “just SCD complications.” A bone marrow biopsy (definitive test for leukemia) might be delayed if one is treating presumed parvovirus aplastic crisis, for example;
- Pathophysiologic rationale: Chronic inflammation and hematopoietic stem cell stress secondary to lifelong SCD pathology and prior treatments are thought to contribute to leukemogenic risk;
- Recommended diagnostic tests: Peripheral blood smear review, flow cytometry, bone marrow aspiration, and biopsy with cytogenetics and molecular testing are necessary for diagnosis of acute leukemia;
- Management strategies and outcomes: Management of acute leukemia follows standard leukemia protocols, but outcomes may be influenced by the underlying SCD and prior organ damage. Patients post-transplant may be at higher risk. The prognosis for AML in SCD is generally considered poor [26];
- Prevention: There is no established method of prevention for leukemia. While hydroxyurea’s leukemogenic risk is debated, the current evidence suggests it is low; nevertheless, regular blood-count monitoring in patients with SCD is standard and may catch early blast cells;
- Clinical takeaway: Acute leukemia is an emerging, rare complication in SCD; persistent or unexplained cytopenias warrant investigation, especially in those with severe disease or prior treatment exposures.
3.2.2. Extramedullary Hematopoiesis (EMH)
- Definition: hematopoietic tissue expanding outside the bone marrow, often in response to chronic marrow dysfunction.
- Key case findings: EMH is a rarely described complication in adult patients with SCD. A 2024 case series and review identified varied localizations including localizations that were paravertebral, peri-articular in the hip, adrenal, hepatic, and splenic [27]. EMH was reported in a patient who presented with right-sided thoracic pain and was subsequently found to have a right adrenal mass [28];
- Diagnostic challenges: The symptoms depend on the localization (e.g., spinal cord compression from paravertebral masses, pain from peri-articular involvement, adrenal insufficiency, hepatic dysfunction, mass effect). These can be mistaken for VOC or other organ-specific complications;
- Pathophysiologic rationale: Chronic ineffective erythropoiesis in SCD drives increased hematopoietic activity, leading to tissue expansion in extramedullary sites [28];
- Recommended diagnostic tests: Diagnosis of EMH is established by histology and/or magnetic resonance imaging (MRI);
- Management strategies and outcomes: Management of EMH is noted as non-consensual, which reflects the rarity and varied presentations. Approaches to its management may include hydroxyurea optimization, transfusion therapy to suppress erythropoiesis, or radiation depending on the site and symptoms [27]. Surgical decompression may be needed for symptomatic spinal EMH;
- Clinical takeaway: EMH is a rare, location-variable complication in adult SCD; imaging (MRI) and, potentially, biopsy are key to diagnosis in patients with unexplained masses or symptoms referable to potential EMH sites.
3.2.3. Hemophagocytic Lymphohistiocytosis (HLH)
- Definition: a life-threatening syndrome of immune dysregulation characterized by uncontrolled inflammation and tissue infiltration by activated lymphocytes and macrophages;
- Key case findings: HLH is a rare but severe complication in SCD, often triggered by infections (particularly viral). Its diagnosis can be challenging due to overlapping features with severe SCD crisis and infection [29]. A case report describes secondary HLH following coinfection with hepatitis A and E viruses in a child with sickle cell anemia [30];
- Diagnostic challenges: Distinguishing HLH from an acute SCD crisis with infection is challenging. Both can cause fever, organomegaly, cytopenias, high ferritin, and elevated liver enzymes. Physicians may treat presumed sepsis or acute chest syndrome while the HLH diagnosis is missed. A high ferritin level can be a clue, but sickle cell by itself (especially with liver involvement) can raise ferritin levels significantly. Notably, HLH typically involves extreme hyperferritinemia. While the HLH diagnostic criterion is ferritin > 500 ng/mL, in practice, HLH often shows ferritin levels in the many thousands; values > 10,000 ng/mL are highly suggestive of HLH [29,31]. Diagnostic overshadowing can occur if these signs are blamed on a sickle cell liver/splenic sequestration or severe infection alone, delaying the critical immunologic treatment for HLH;
- Pathophysiologic rationale: Triggering events like infection or severe crisis lead to uncontrolled activation of cytotoxic T cells and macrophages, resulting in a cytokine storm and organ damage;
- Management strategies and outcomes: Treatment of HLH involves addressing the underlying trigger (e.g., treating infection) and immune suppression using chemotherapy regimens developed for HLH (e.g., etoposide-containing protocols) [29]. The outcomes depend on timely diagnosis and treatment of the trigger and HLH itself;
- Prevention: Preventing HLH in SCD is not straightforward, as it often depends on unpredictable infections. However, one can argue that preventing severe infections through vaccination, penicillin prophylaxis (in children), and prompt treatment of infections could reduce the trigger risk. Also, rapidly controlling severe sickle crises and avoiding transfusional iron overload (iron can fuel macrophages) might theoretically help. Clinicians should maintain a low threshold for screening for HLH in any patient with SCD with atypical severity of illness (e.g., lab signs of hyperinflammation), which in effect is secondary prevention (catching early);
- Clinical takeaway: Unexplained fever, worsening cytopenias, organ dysfunction, and significantly elevated ferritin in a patient with severe SCD or infection should prompt evaluation for HLH; timely diagnosis and immune-modulatory therapy are critical.
3.3. Cardiopulmonary and Vaso-Occlusive Complications (Severe Forms)
Fat Embolism Syndrome (FES) and Severe Bone Marrow Necrosis
- Key case findings: FES and severe bone marrow necrosis are among the most severe and rare SCD complications. The case reports and reviews that discuss FES in SCD discuss ~80+ reported cases of FES in SCD. Notably, FES in SCD, paradoxically, appears more common in patients with milder genotypes (HbSC or HbSβ+ thalassemia) who have had relatively few prior crises [32,34]. Its clinical presentation typically involves a seeming severe pain crisis followed within 1–3 days by acute respiratory failure, neurological changes (ranging from confusion to coma), fever, and, often, a new petechial rash. Laboratory clues include a sudden drop in levels of hemoglobin and platelets. Some patients have evidence of recent parvovirus B19 infection as a trigger for marrow necrosis. One unusual presentation of previously undiagnosed sickle-β+ thalassemia involved FES [35]. Bone marrow necrosis is associated with high morbidity and mortality [36]. Cerebral fat embolism syndrome without myonecrosis has also been reported [37];
- Diagnostic challenges: Clinical features of FES (neurological changes, respiratory distress, petechial rash) can overlap with severe acute chest syndrome, stroke, or MOF [32]. Its diagnosis requires a high index of suspicion in the context of severe bone pain or evidence of bone marrow infarction. Characteristic MRI findings like the “starfield” pattern in the brain can support the diagnosis of cerebral fat embolism [38]. Diagnostic overshadowing is a big risk here, as it result from attributing the multi-system deterioration to “severe sickle cell crisis” or sepsis rather than recognizing fat embolism. A clue can be the degree of anemia and reticulocytopenia being out of proportion to that of usual VOC, which suggests bone marrow necrosis;
- Pathophysiologic rationale: Extensive sickling in the bone marrow causes infarction and necrosis of marrow tissue. This necrotic marrow releases fat globules (and pro-inflammatory substances) into venous blood. These fat emboli lodge in pulmonary capillaries (causing acute respiratory distress similar to ARDS) and can traverse to systemic circulation (especially if there is a right-to-left shunt or via overwhelmed pulmonary capillaries), causing cerebral emboli and other organ damage. Having an inflammatory response to the fat leads to the fever and systemic inflammatory response [32];
- Recommended diagnostic tests: Imaging of bones may show infarction. Pulmonary function tests and imaging (CT scan) may show evidence of lung injury. Neurological assessment and imaging (MRI, particularly with diffusion-weighted sequences for a “starfield” pattern) are important. Detection of fat globules in urine, sputum, or bronchoalveolar lavage can support the diagnosis but is not always sensitive or specific [32];
- Management strategies and outcomes: Immediate ICU care is required. Supportive care for FES includes high-flow oxygen or mechanical ventilation for respiratory failure, and often invasive monitoring. Exchange transfusion is widely recommended as soon as FES is suspected, to rapidly reduce the proportion of sickled cells and halt ongoing infarction. Evidence suggests that prompt red cell exchange improves survival [32];
- Prevention: Preventing FES is challenging because it often strikes unexpectedly in a patient who was not previously considered high-risk. However, one suggestion is to ensure aggressive management of any very severe pain crisis (some have advocated for early exchange transfusion in an unusually severe VOC to preempt marrow necrosis) [39];
- Clinical takeaway: Severe bone pain followed by rapid neurological or respiratory deterioration should prompt consideration of bone marrow necrosis and FES. This is a medical emergency—intensive supportive care and urgent exchange transfusion can be life-saving. Even with optimal care, FES carries a high mortality rate, so maintaining vigilance for this complication in any unusually severe SCD crisis is essential;
- Acute chest syndrome (ACS) vs. FES—a brief note: It is worth noting that FES can be mistaken for ACS (and ACS is common in SCD). The presence of neurological symptoms or severe cytopenias can help differentiate the two. Some FES cases were likely labeled as “atypical ACS” historically. In practice, when a patient with SCD presents with ACS but has unusually severe systemic findings, clinicians should consider FES on the differential and manage accordingly (with exchange transfusion and broader supportive care).
3.4. Abdominal Complications
Hepatic Complications: Sequestration, Cholestasis, and Liver Transplantation
- Definition: a spectrum of liver dysfunction in SCD, beyond typical sickling hepatopathy, including acute hepatic sequestration (sudden trapping of red cells in the liver), sickle cell intrahepatic cholestasis (severe cholestasis due to sinusoid occlusion), and chronic liver damage, that sometimes requires transplantation;
- Key case findings: Sickle cell hepatopathy is a well-recognized complication, but acute hepatic sequestration crisis is considered rare [40,41]. Sickle cell intrahepatic cholestasis is extremely rare but often fatal [42]. Reviews cover the liver in SCD and management of complications [43]. Liver transplantation has been performed in patients with SCD, sometimes after hematopoietic stem cell transplant (HSCT) for the underlying SCD [44];
- Diagnostic challenges: Acute abdominal pain, jaundice, and elevated liver enzymes are common in various SCD crises. Distinguishing acute hepatic sequestration (rapid drop in hemoglobin, tender hepatomegaly) from a vaso-occlusive crisis or cholecystitis/cholangitis requires careful assessment [41]. Intrahepatic cholestasis presents with profound jaundice, coagulopathy, and synthetic dysfunction [42];
- Recommended diagnostic tests: The recommended tests for hepatic complications include liver function tests (ALT, AST, bilirubin, alkaline phosphatase), a coagulation panel, and ultrasound/CT of the abdomen to assess the liver size and look for other causes (gallstones, common bile duct dilation). Liver biopsy may be needed for unclear cases or suspected cholestasis [40,41,42,43];
- Management strategies and outcomes: Management of hepatic complications depends on the specific complication. Acute hepatic sequestration may require exchange transfusion [41]. Intrahepatic cholestasis is medically challenging; supportive care and exchange transfusion are options, but their prognosis is poor [42]. Liver transplantation is a consideration for end-stage liver disease, potentially combined with or following HSCT [44];
- Prevention: To prevent hepatic sequestration or cholestasis, one can only generalize: avoid dehydration and hypoxia, which precipitate sickling, and treat the sickle cell disease aggressively to reduce crisis frequency (hydroxyurea, transfusions for severe phenotype). Prompt transfusion in the case of any acute hepatic involvement might prevent progression to full cholestatic syndrome. Closely monitoring patients with known liver iron overload or hepatitis during crises is warranted since they may tolerate less hepatic stress;
- Clinical takeaway: Severe or rapidly worsening liver function abnormalities, significant hepatomegaly, or profound jaundice in patient with SCD warrants investigation for acute hepatic sequestration or intrahepatic cholestasis; these are serious complications that require specialized management.
3.5. Musculoskeletal Complications
Deep Compartment Syndrome Without Myonecrosis
- Definition: increased pressure within a muscle compartment, compromising circulation and tissue function, but without the extensive muscle tissue death typical of myonecrosis;
- Key case findings: Deep compartment syndrome is a rare complication of SCD, often occurring in extremities. A case report describes deep compartment syndrome without myonecrosis in patient with SCD [45];
- Diagnostic challenges: Pain and swelling in an extremity can mimic typical vaso-occlusive crisis. Diagnosis of this syndrome requires clinical suspicion based on the severity and location of pain, the tenseness of the compartment, and, potentially, the measurement of compartment pressures;
- Pathophysiologic rationale: The pathophysiologic rationale includes vaso-occlusion leading to tissue swelling within a closed fascial compartment. While bone infarction is common, severe soft tissue edema leading to compartment syndrome is less frequent;
- Recommended diagnostic tests: Clinical examination is a recommended test for the diagnosis of this syndrome. Measurement of compartment pressures can confirm the diagnosis. MRI may show muscle edema;
- Management strategies and outcomes: Urgent fasciotomy may be required to relieve pressure and preserve the tissue’s viability [45]. Prompt intervention is key to preventing permanent damage;
- Clinical takeaway: Severe, disproportionate, and localized pain and swelling in an extremity with signs of tense compartment in patient with SCD should raise suspicion for compartment syndrome, even without apparent muscle death; urgent surgical consultation is needed.
3.6. Dermatologic Complications
- Leg Ulcers: Often occurring around the ankles, these chronic, refractory ulcers are believed to result from microvascular occlusion, local trauma, and perhaps impaired wound healing due to hemolysis-related endothelial dysfunction. They can be very painful and lead to recurrent infections. Although not “rare” in the global SCD population, they are underrepresented in the literature relative to their impact. Their management is challenging (wound care, skin grafts, transfusions, and maybe hyperbaric oxygen) [47]. Their prevention revolves around avoiding trauma, using compression/support stockings to improve circulation, and using hydroxyurea, which has been associated with promoting ulcer healing in some cases;
- Hydroxyurea-related skin ulcers: A subset of patients on hydroxyurea develop painful leg ulcers as a side effect of the drug. This is relatively uncommon but important to distinguish from SCD-related ulcers, as the management may involve reducing the dose or switching to a different therapy [48];
- Livedoid vasculopathy: Recent reports describe patients with SCD with lesions consistent with livedoid vasculopathy (a thrombotic skin condition that causes painful ulcers on the legs) [47];
- Infections: Patients with SCD can get unusual skin infections due to their immune-compromised state (asplenia) and frequent hospital exposures. Chronic ulcers can be colonized with atypical organisms (including pseudomonas, atypical mycobacteria) and fungal infections have been noted anecdotally [49];
- Others: There is evidence that patients with SCD have a lower incidence of cutaneous malignancies (like melanoma and non-melanoma skin cancer) than expected, possibly due to their shorter lifespan historically or protective factors of hemolysis (this is an evolving area) [50]. Also noted are pigmentary changes (hyperpigmentation of skin and nails) in children on chronic transfusions or hydroxyurea [51].
4. Discussion
- Maintaining a high index of suspicion: Clinicians must consciously consider rare possibilities when a patient’s symptoms are severe, persistent, or atypical for common SCD events, especially when symptoms do not respond to standard management;
- Structured differential diagnosis: Develop and utilize differential diagnosis frameworks that explicitly include rare SCD complications alongside common ones based on symptom clusters. The emergency department represents a critical juncture where atypical presentations must be promptly recognized [53];
- Prompt and appropriate investigations: Do not hesitate to order specific diagnostic tests (e.g., urgent MRI for neurological/spinal symptoms, bone marrow biopsy for unexplained cytopenia or suspected HLH, cross-sectional imaging for unexplained masses or specific abdominal complaints, urgent ophthalmologic evaluation for vision changes, compartment pressure measurement for suspected compartment syndrome when initial clinical assessment raises suspicion for a rare entity, even in the setting of a typical-appearing sickle crisis);
- Leveraging multidisciplinary expertise: Engage specialists (e.g., neurology, neurosurgery, oncology, radiology, transfusion medicine, gastroenterology, ophthalmology, orthopedics) early when a rare complication is suspected;
- Education and awareness: Continually educate healthcare providers across all levels of care, particularly those in emergency departments and general hospital wards, about the spectrum of rare SCD complications and the pitfalls of diagnostic overshadowing.
- Centralized reporting systems: Establishing a dedicated national or international platform for reporting rare SCD complications beyond individual case reports would create a larger dataset for analysis;
- SCD registries: Expanding existing SCD registries to include detailed documentation of rare complications would allow for better estimation of incidence, identification of risk factors, evaluation of management strategies, and assessment of outcomes;
- App-based decision support tools: Developing digital tools or algorithms integrated into electronic health records or standalone applications could prompt clinicians to consider specific rare diagnoses based on present symptom constellations, guiding appropriate investigations and initial management.
5. Implications for Practice
6. Future Directions and Research
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Smith-Whitley, K. Complications in pregnant women with sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 359–366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ochocinski, D.; Dalal, M.; Black, L.V.; Carr, S.; Lew, J.; Sullivan, K.; Kissoon, N. Life-Threatening Infectious Complications in Sickle Cell Disease: A Concise Narrative Review. Front. Pediatr. 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raval, D.M.; Rathod, V.M. A rare presentation of sickle cell disease diagnosed for the first time in a 60-year-old female: A misdiagnosis or missed diagnosis. Med. India 2023, 2, 7. [Google Scholar] [CrossRef]
- Se, B.; Frisch, A.; Hwang, M.W.; Polani, F.; Bade, N. Fat Embolism Syndrome Mimicking Thrombotic Thrombocytopenic Purpura in a Patient With Hemoglobin S/Beta-Thalassemia. J. Hematol. 2024, 13, 104–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perimbeti, S.P.; Hou, K.Y.; Ramanathan, S.; Woodard, A.; Kyung, D.; Wang, Q.; Crilley, P.A.; Ward, K.; Styler, M. The Effect of Health Care Disparities on Complications and Mortality in Sickle Cell Disease. Blood 2018, 132 (Suppl. S1), 5886. [Google Scholar] [CrossRef]
- Joy, J.; Vasnaik, M.A.; Bhat, V.; Anandram, S.; George, A. Spontaneous Epidural Hematoma in Sickle Cell Crisis: A Case Report. Cureus 2022, 14, e24492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takroni, S.Y.; Nasiri, A.M.; Ahmed, E.; Alkharras, R.A. Spontaneous epidural hematoma: A case report of rare crisis of sickle cell disease. J. Fam. Med. Prim. Care 2021, 10, 4286–4289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chipongo, H.; Sarkar, A.; Bosco, K.; Sangey, E. Massive spontaneous subdural hemorrhage mimicking dural venous thrombosis in a sickle cell adolescent, a rare case report. Int. J. Surg. Case Rep. 2024, 115, 109255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miraulle, T.Y.S.; Synèse, B.J.; D’assise, M.N.; Charles, R.E.; Clément, A.; Rabarijaona, M.; Willy, R. A Rare Complications of Sickle Cell Disease: Empyema and Chronic Subdural Hematoma. Eur. J. Clin. Med. 2021, 2, 23–25. [Google Scholar] [CrossRef]
- Laminou, L.; T, H.A.B.; Adam, N.; Y, A.K.; Abdou, A. Unilateral Papilledema Revealing a Subdural Hematoma in a Sickle Cell Patient. East Afr. Sch. J. Med. Surg. 2022, 4, 4. [Google Scholar] [CrossRef]
- Rudy, H.L.; Yang, D.; Nam, A.D.; Cho, W. Review of Sickle Cell Disease and Spinal Pathology. Glob. Spine J. 2019, 9, 761–766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rothman, S.M.; Nelson, J.S. Spinal cord infarction in a patient with sickle cell anemia. Neurology 1980, 30, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Clay, E.L.; Jewells, V.; Adams, S.; Crawford, R.D.; Redding-Lallinger, R. A 19-year-old man with sickle cell disease presenting with spinal infarction: A case report. J. Med. Case Rep. 2013, 7, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibrahim, R.; Fadel, A.; Sawli, N.; Mecheik, A. A Challenging Case of Severe Sickle Cell Crisis With Multiorgan Involvement: A Case Report. Cureus 2023, 15, e42437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Shaughnessy, B.A.; DiPatri, A.J.; Jr Parkinson, R.J.; Batjer, H.H. Development of a de novo cerebral arteriovenous malformation in a child with sickle cell disease and moyamoya arteriopathy. Case report. J. Neurosurg. 2005, 102 (Suppl. S2), 238–243. [Google Scholar] [CrossRef] [PubMed]
- Enninful-Eghan, H.; Moore, R.H.; Ichord, R.; Smith-Whitley, K.; Kwiatkowski, J.L. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J. Pediatr. 2010, 157, 479–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renganathan, G.; Natarajan, P.; Ruck, L.; Prieto, R.; Prakash, B.V.; Thangarasu, S. Concurrent Bilateral Central Retinal Artery Occlusion Secondary to Sickle Cell Crisis. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211028392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaac, E.; Saherwal, A.A.; Alam, S. Systemic thrombolysis for acute central retinal artery occlusion in sickle cell disease: Case report. J. Natl. Med. Assoc. 2022, 114, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Peycheva, M.V.; Aghayan-Ugurluoglu, R.; Ganser, B.; Trinka, E. Central retinal artery occlusion in a patient with sickle cell disease treated with recombinant tissue plasminogen activator. Folia Med. 2022, 64, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, V.; Jain, G. Newly diagnosed PRES in a sickle cell diseased patient: A case report. Ann. Med. Surg. 2023, 85, 1975–1977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vargas, A.; Testai, F.D. Posterior Reversible Encephalopathy Syndrome in adult sickle-cell patients: Case series and literature review. J. Clin. Neurosci. 2019, 70, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Kausha, M.; Vishal, S.; Kondekar Alpana, S. Posterior reversible encephalopathy syndrome in a known case of sickle-beta-thalassemia: A case presentation. Egypt. J. Intern. Med. 2022, 34, 31. [Google Scholar] [CrossRef]
- Liem, R.I.; Lanzkron, S.; Coates, T.D.; DeCastro, L.; Desai, A.A.; Ataga, K.I.; Cohen, R.T.; Haynes, J.J.; Osunkwo, I.; Lebensburger, J.D.; et al. American Society of Hematology 2019 guidelines for sickle cell disease: Cardiopulmonary and kidney disease. Blood Adv. 2019, 3, 3867–3897. [Google Scholar] [CrossRef]
- Zadeh, C.; Rameh, V.; Atweh, L.A. Acute soft head syndrome in a sickle cell disease patient. J. Radiol. Case Rep. 2021, 15, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cannas, G.; Poutrel, S.; Heiblig, M.; Labussière, H.; Larcher, M.-V.; Thomas, X.; Hot, A. Sickle cell disease and acute leukemia: One case report and an extensive review. Ann Hematol. 2023, 102, 1657–1667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boccadifuoco, U.; Cheminet, G.; Morino, B.; Arlet, J.B. Extramedullary hematopoiesis, a rare complication of sickle cell disease: A six-case series and literature review. Rev. Med. Interne. 2025, 46, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, F.; Nali, M.S.; Ali, R.; Patel, A.; Shaaban, H. Extra-Medullary Hematopoiesis in Sickle Cell Disease Presenting as a Right Adrenal Mass. Cureus 2022, 14, e21334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahu, S.K.; Agrawal, A.; Das, P. The Dilemma of Diagnosing Hemophagocytic Lymphohistiocytosis in Sickle Cell Disease. Cureus 2020, 12, e12255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choudhary, G.; Mirza, N.; Patel, S. Secondary hemophagocytic lymphohistiocytosis in a child with Sickle Cell Anemia and Hepatitis A and Hepatitis E co-infection: A case report. Pediatr. Hematol. Oncol. J. 2024, 9, 28–31. [Google Scholar] [CrossRef]
- Allen, C.E.; Yu, X.; Kozinetz, C.A.; McClain, K.L. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Tsitsikas, D.A.; Vize, J.; Abukar, J. Fat Embolism Syndrome in Sickle Cell Disease. J. Clin. Med. 2020, 9, 3601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsafwani, S.A.; Al-Saeed, A.; Bukhamsin, R. Extensive Bone Marrow Necrosis: Initial Presentation in Sickle Cell Anemia—A Case Report and Review of the Literature. Case Rep. Hematol. 2017, 2017, 7185604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almatar, A.M.; Kawther, K. Fat embolism in sickle-cell disease: A case report with literature review. Casp. J. Intern. Med. 2023, 14, 143–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangani, V.; Pokal, M.; Balla, M.; Merugu, G.P.; Khokher, W.; Gayam, V.; Konala, V.M. Fat Embolism Syndrome in Sickle Cell β-Thalassemia Patient With Osteonecrosis: An Uncommon Presentation in a Young Adult. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211012266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsitsikas, D.A.; Gallinella, G.; Patel, S.; Seligman, H.; Greaves, P.; Amos, R.J. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: Increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev. 2014, 28, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.L.; Aamodt, W.W.; Yalamarti, T.; Dogon, C.; Kinniry, P. Cerebral fat embolism syndrome in sickle cell disease without evidence of shunt. eNeurologicalSci 2019, 14, 19–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, J.H.; Hargett, C.W.; Sevilis, T.; Luedke, M. Sickle cell disease, fat embolism syndrome, and “starfield” pattern on MRI. Neurol. Clin. Pract. 2018, 8, 162–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakraborty, S.; Danewa, A.; Arora, S.; Bansal, S.; Singh, P.P.; Bhargava, R.; Dua, V. Fat embolism syndrome in a child with sickle cell disease. Pediatr. Hematol. Oncol. J. 2024, 9, 189–192. [Google Scholar] [CrossRef]
- Mohamed, K.A.; Nephew, L.D.; Kaur, H. Sickle cell hepatopathy: An underrecognized and undertreated cause of chronic liver disease. Clin. Liver Dis. 2023, 22, 229–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitmer, B.; Gamarra, F.; Stawick, L. Acute Hepatic Sequestration Crisis: A Rare Complication of Sickle Cell Disease: 798. Am. J. Gastroenterol. 2010, 105, S289–S290. [Google Scholar] [CrossRef]
- Khan, A.; Nashed, B.; Issa, M.; Khan, M.Z. Sickle Cell Intrahepatic Cholestasis: Extremely Rare but Fatal Complication of Sickle Cell Disease. Cureus 2022, 14, e22050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suddle, A.R. Management of liver complications in sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 345–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sousa Arantes Ferreira, G.; Ferreira, C.A.; Watanabe, A.L.C.; Trevizoli, N.C.; Murta, M.C.B.; Figueira, A.V.F.; de Fatima Couto, C. Liver Transplantation After Hematopoietic Stem Cell Transplant for the Treatment of Sickle Cell Disease: A Case Report. Transpl. Proc. 2022, 54, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.O.; Hankin, A.; Horn, J.; Pedersen, T.H.; Borgersen, R.; Frøen, H.M. Deep Compartment Syndrome Without Myonecrosis: A Case Report on a Rare Complication of Sickle Cell Disease. Cureus 2022, 14, e29164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minniti, C.P.; Eckman, J.; Sebastiani, P.; Steinberg, M.H.; Ballas, S.K. Leg ulcers in sickle cell disease. Am. J. Hematol. 2010, 85, 831–833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dick, A.; Schwartzman, G.; Khachemoune, A. Cutaneous manifestations of sickle cell disease: An updated review. Arch. Dermatol. Res. 2023, 315, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dudhe, M.S.; Singh, A.; Ganguly, S.; Singh, C. Hydroxyurea-Induced Cutaneous Ulcers in a Sickle Cell Disease Patient. Indian Dermatol. Online J. 2024, 15, 521–522. [Google Scholar] [CrossRef]
- Rabodonirina, M.; Piens, M.A.; Monier, M.F.; Guého, E.; Fière, D.; Mojon, M. Fusarium infections in immunocompromised patients: Case reports and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 152–161. [Google Scholar] [CrossRef]
- Soutou, B.; Senet, P.; Lionnet, F.; Habibi, A.; Aractingi, S. Sickle cell disease induces resistance to cutaneous carcinogenesis. Orphanet J. Rare Dis. 2020, 15, 66. [Google Scholar] [CrossRef]
- O’Branski, E.E.; Ware, R.E.; Prose, N.S.; Kinney, T.R. Skin and nail changes in children with sickle cell anemia receiving hydroxyurea therapy. J. Am. Acad. Dermatol. 2001, 44, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Mamede, S.; Schmidt, H.G. The twin traps of overtreatment and therapeutic nihilism in clinical practice. Med. Educ. 2014, 48, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Brandow, A.M.; Liem, R. “Sickle Cell Disease in the Emergency Department: Atypical Complications and Management”. Clin. Pediatr. Emerg. Med. 2011, 12, 202–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
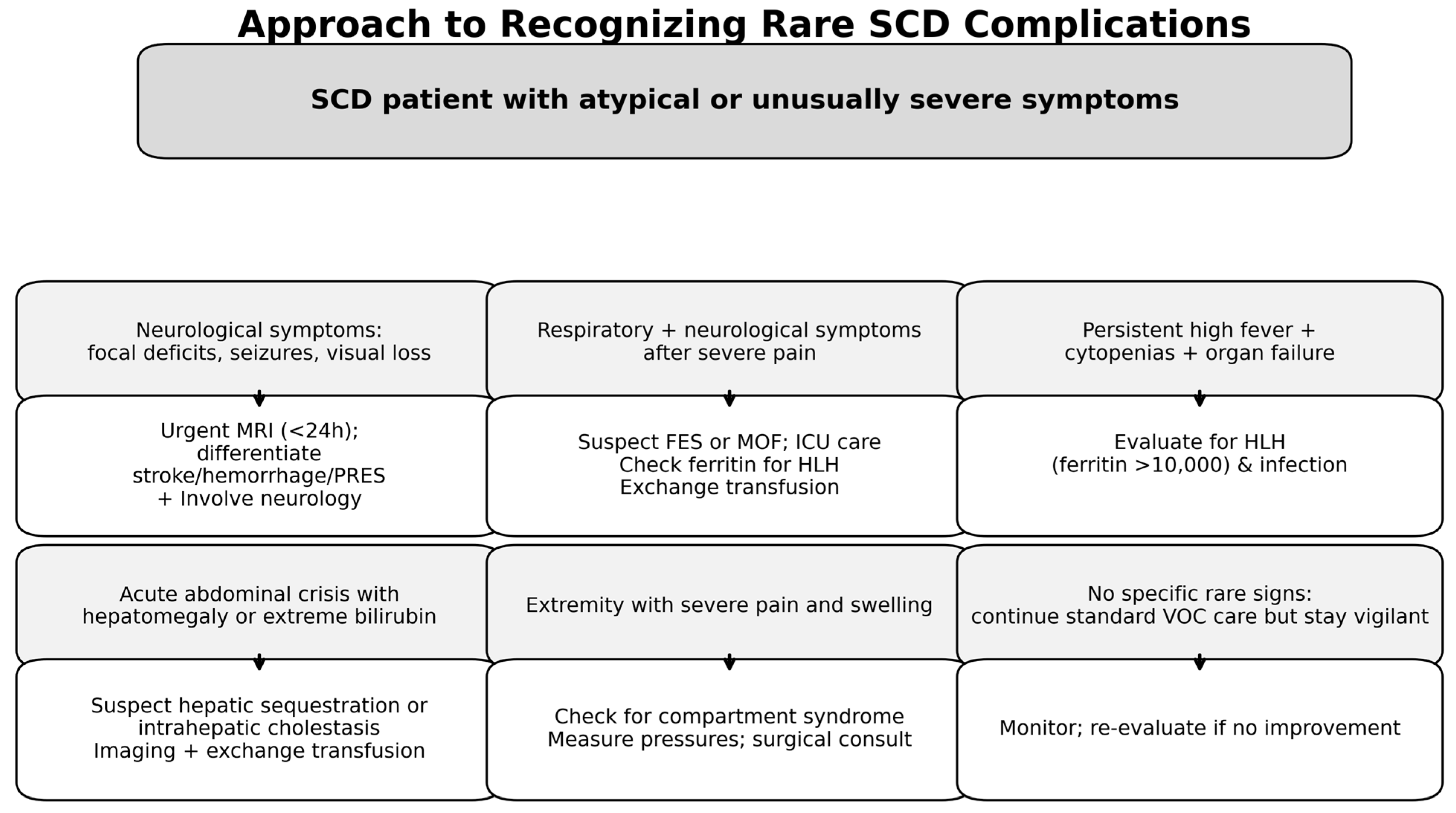
| Rare Complication | Clinical Presentation | Diagnostic Clue | Recommended Action | Prognostic Implications |
|---|---|---|---|---|
| Neurological Complications | ||||
| Spontaneous Epidural Hematoma | Sudden onset back pain, radicular pain, neurological deficits (weakness, sensory changes, bowel/bladder dysfunction). Frequently during sickle crises. | Unexplained or disproportionately severe spinal pain or neurological deficits in a patient with SCD. Non-traumatic presentation. | Urgent MRI of the affected spine or brain. Neurosurgical intervention critical for decompression. | Favorable if diagnosed and treated promptly; delayed treatment risks permanent neurological deficits. |
| Spontaneous Subdural Hematoma | Headache, neurological changes, papilledema. Can mimic dural sinus thrombosis. | Atypical headaches or neurological signs, especially with new papilledema. | Urgent MRI or CT brain. Neurosurgical evaluation for management. | Potentially life-threatening; early diagnosis improves outcomes but morbidity can be high. |
| Brain and Spinal Infarcts | Neurological impairment in unusual locations (e.g., supra/infratentorial, anterior spinal cord) or contexts (e.g., during septic shock, MOF). | Severe or atypical neurological deficits, even with systemic illness. Recognizing infarcts in unusual locations. | Brain and spinal MRI. Evaluate for infection, antiphospholipid antibodies. Treat underlying crisis/infection. Exchange transfusion to keep HbS < 30%. Consult neurology/Nneurosurgery. | Variable; early intervention can improve neurological recovery; delayed diagnosis may result in permanent deficits. |
| Hematologic Complications | ||||
| Acute Leukemia (AML) | Atypical or persistent cytopenias; overlap with severe aplastic or sequestration crises; fever, fatigue, bleeding, infection. | Persistent atypical cytopenias in patients with SCD, especially with prior severe disease. | Peripheral blood smear, flow cytometry, bone marrow biopsy with cytogenetics and molecular testing. Oncology/hematology consult urgent. | Poor prognosis; delayed diagnosis worsens outcomes; aggressive treatment necessary. |
| Hemophagocytic Lymphohistiocytosis (HLH) | Persistent fever, worsening cytopenias, splenomegaly, hyperferritinemia, coagulopathy, organ dysfunction; overlaps with severe infection/crisis. | Unexplained fever, worsening cytopenias, very high ferritin (>500 ng/mL), immune activation signs. | Evaluate HLH criteria; labs: CBC, LFTs, coags, triglycerides, fibrinogen, ferritin, sCD25; bone marrow biopsy. Treat triggers; immunosuppression; urgent Hematology/Oncology/Critical Care consult. | High mortality if untreated; early diagnosis improves survival. |
| Cardiopulmonary and Vaso-occlusive Complications (Severe Forms) | ||||
| Acute Multiorgan Failure (MOF) Syndrome | Acute failure in ≥2 vital organs during acute VOC or progressive acute chest syndrome. Rapid progression. | Rapidly progressive multiorgan dysfunction without other MOF causes. | Organ function evaluation, imaging (CXR/CT), blood cultures, assess for bone marrow necrosis/fat embolism. Intensive supportive care; consider urgent exchange transfusion. | Very high mortality; early aggressive treatment required. |
| Fat Embolism Syndrome (FES)/Severe Bone Marrow Necrosis | Abrupt neurologic deterioration, respiratory failure, multiorgan dysfunction; petechial rash possible; may occur with unusual presentations (e.g., de novo diagnosis). | Severe bone pain followed by rapid neuro/respiratory decline; “starfield” pattern on brain MRI. | Supportive care including respiratory support and pain control; exchange transfusion; critical care and hematology consultation. Bone imaging, chest CT, brain MRI. High mortality risk. | High mortality risk; prognosis poor without timely intervention. |
| Abdominal Complications | ||||
| Hepatic Sequestration Crisis | Acute right upper quadrant pain, tender hepatomegaly, rapid hemoglobin drop. | Tender hepatomegaly with acute anemia and elevated liver enzymes. | Supportive care, pain management, consider exchange transfusion. Gastroenterology/hematology consult. | Generally favorable with prompt treatment; risk of severe anemia and shock if untreated. |
| Sickle Cell Intrahepatic Cholestasis | Profound jaundice, liver dysfunction (coagulopathy, synthetic failure). | Severe jaundice with direct hyperbilirubinemia, coagulopathy. | Intensive supportive care; may require exchange transfusion; liver biopsy for confirmation. Critical care needed. | High mortality if diagnosis delayed; early treatment essential. |
| Extramedullary Hematopoiesis (Adrenal, Hepatic, Splenic) | Symptoms depend on specific site: adrenal insufficiency, hepatic dysfunction, mass effect. | Unexplained masses or symptoms referable to these sites. | Imaging (MRI), possibly biopsy; management varies including observation, hydroxyurea, transfusion, radiation. | Variable prognosis depending on site and severity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, A.; Alshammari, M.; Alkharras, R.; Madkhali, A.; Mohammed Saleh, M.F.; Alzahrani, H. Diagnostic Overshadowing and the Unseen Spectrum: A Narrative Review of Rare Complications in Sickle Cell Disease. Clin. Pract. 2025, 15, 156. https://doi.org/10.3390/clinpract15090156
Nasiri A, Alshammari M, Alkharras R, Madkhali A, Mohammed Saleh MF, Alzahrani H. Diagnostic Overshadowing and the Unseen Spectrum: A Narrative Review of Rare Complications in Sickle Cell Disease. Clinics and Practice. 2025; 15(9):156. https://doi.org/10.3390/clinpract15090156
Chicago/Turabian StyleNasiri, Abdulrahman, Manal Alshammari, Reem Alkharras, Albaraa Madkhali, Mostafa F. Mohammed Saleh, and Hazza Alzahrani. 2025. "Diagnostic Overshadowing and the Unseen Spectrum: A Narrative Review of Rare Complications in Sickle Cell Disease" Clinics and Practice 15, no. 9: 156. https://doi.org/10.3390/clinpract15090156
APA StyleNasiri, A., Alshammari, M., Alkharras, R., Madkhali, A., Mohammed Saleh, M. F., & Alzahrani, H. (2025). Diagnostic Overshadowing and the Unseen Spectrum: A Narrative Review of Rare Complications in Sickle Cell Disease. Clinics and Practice, 15(9), 156. https://doi.org/10.3390/clinpract15090156







