Beneficial Effect of Platelet-Rich Fibrin as an Adjunct to Nonsurgical Therapy After Subgingival Professional Mechanical Plaque Removal for Periodontitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Search Strategies
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
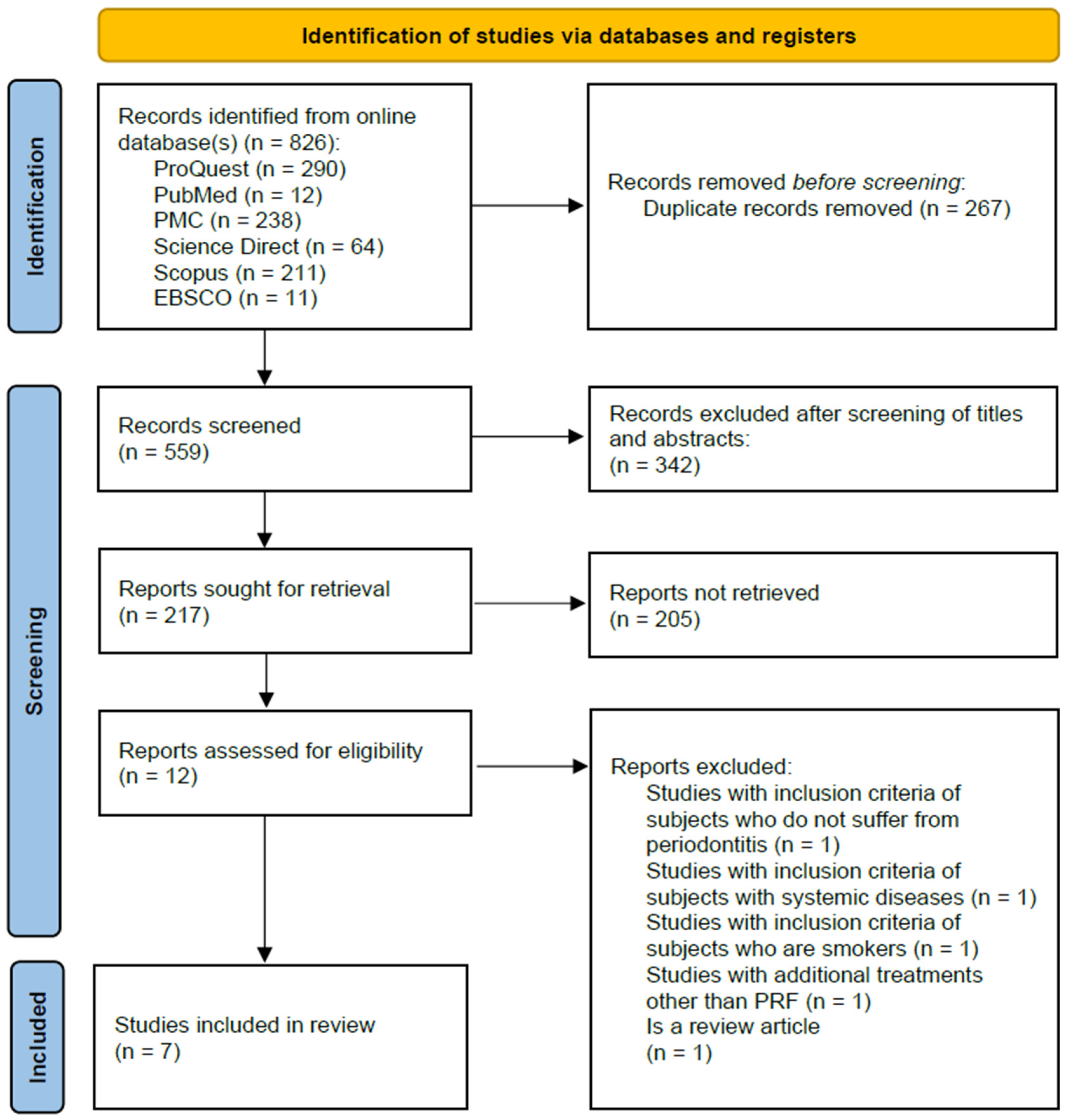
3. Results
3.1. Characteristics of the Included Studies
3.2. Risk of Bias
3.3. Assessment of Certainty
3.4. Qualitative Analysis
3.5. Quantitative Analysis
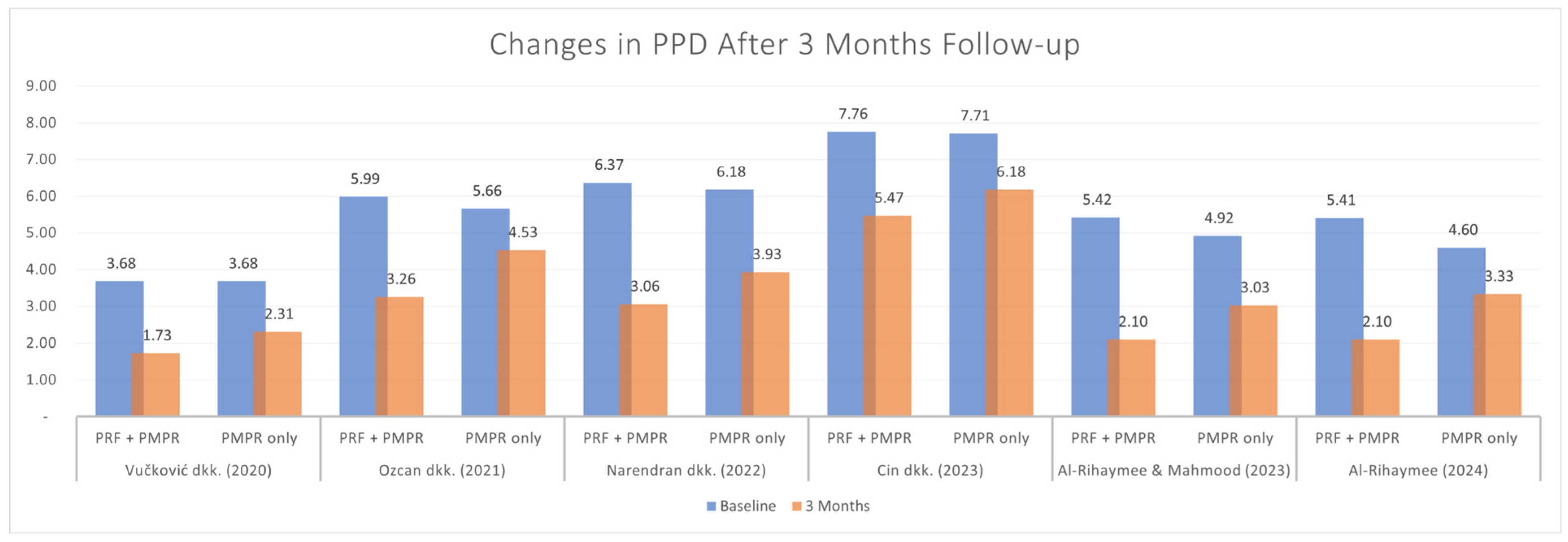
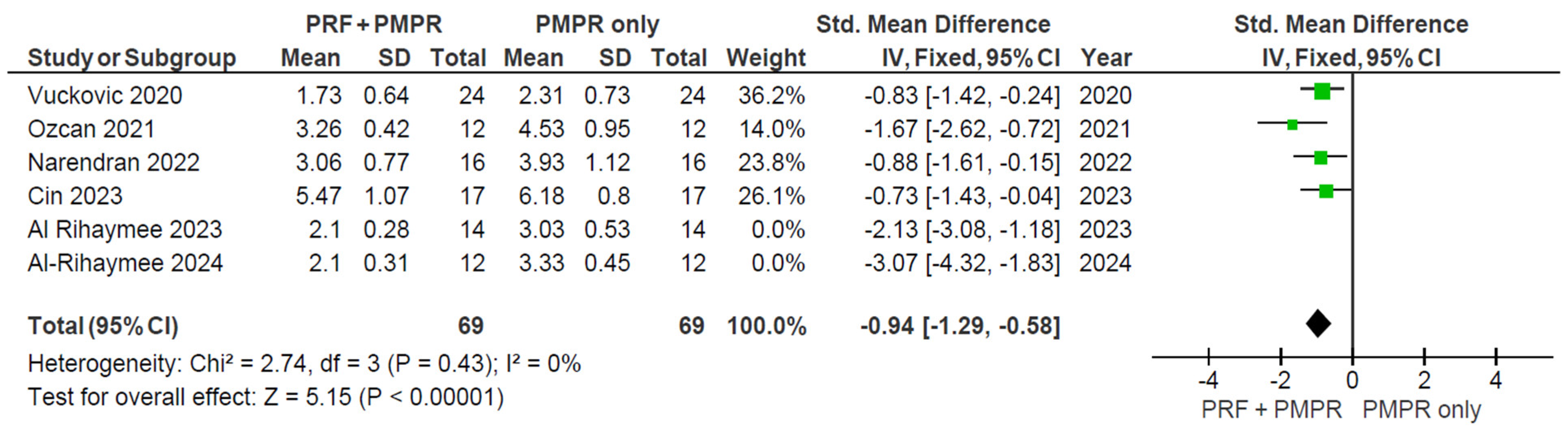
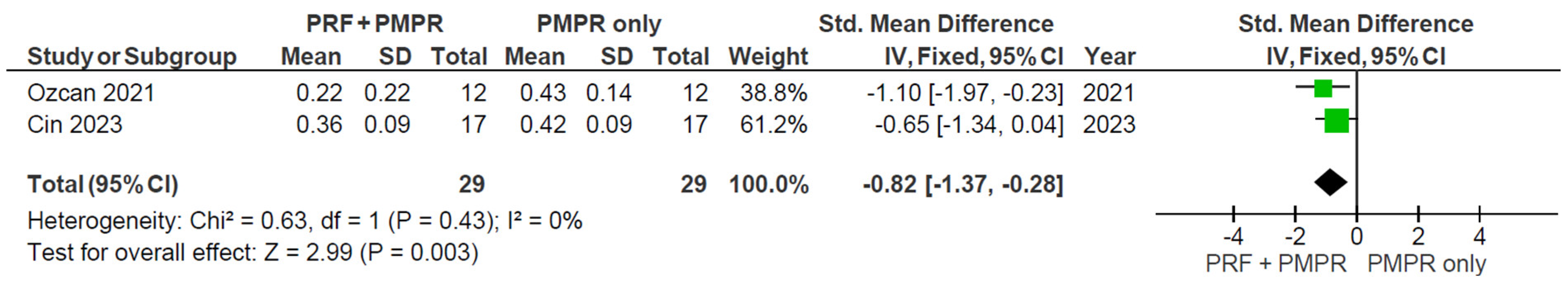
3.5.1. Probing Pocket Depth (PPD)
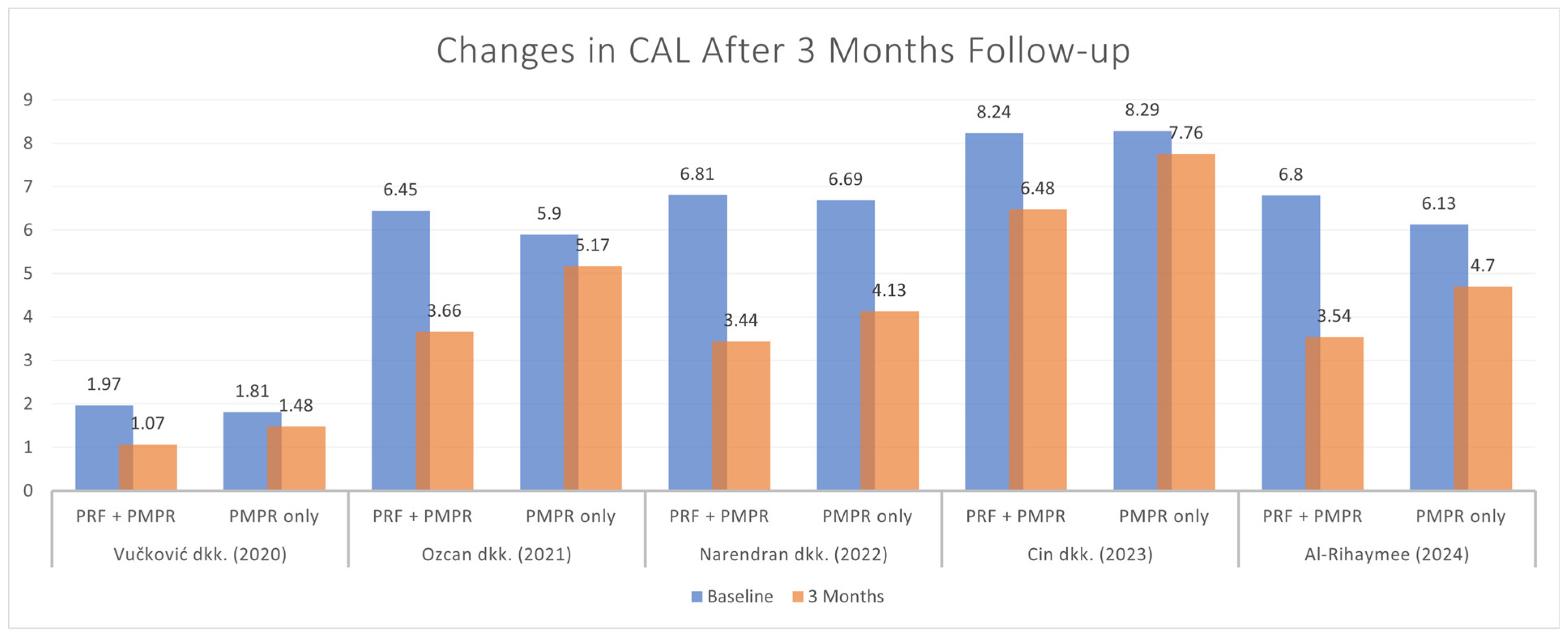
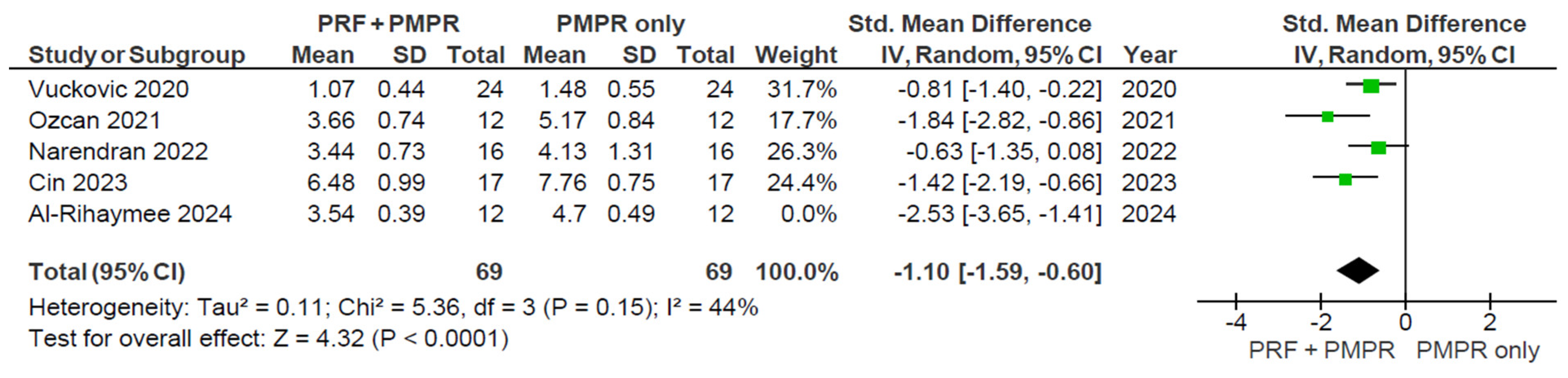
3.5.2. Clinical Attachment Loss (CAL)
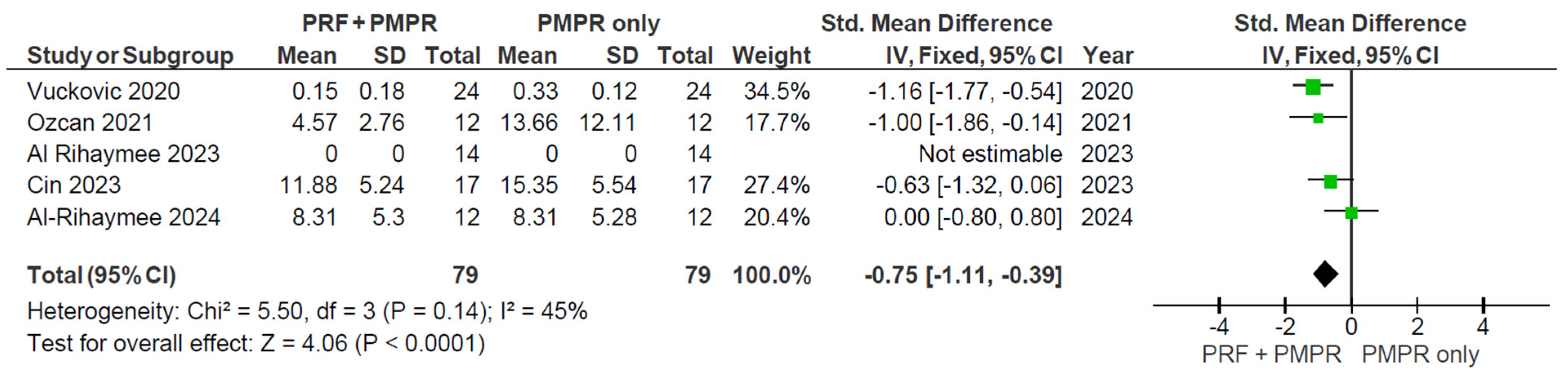
3.5.3. Bleeding on Probing (BoP)
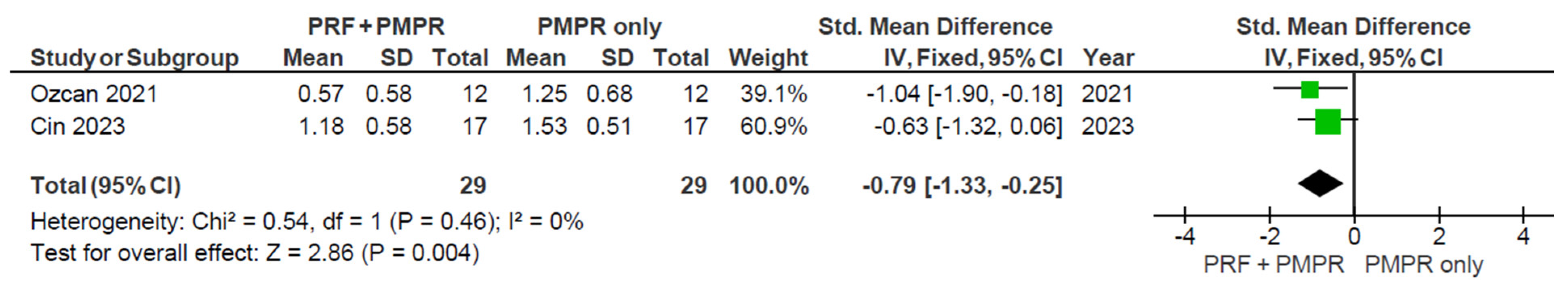
3.5.4. Gingival Recession (GR)
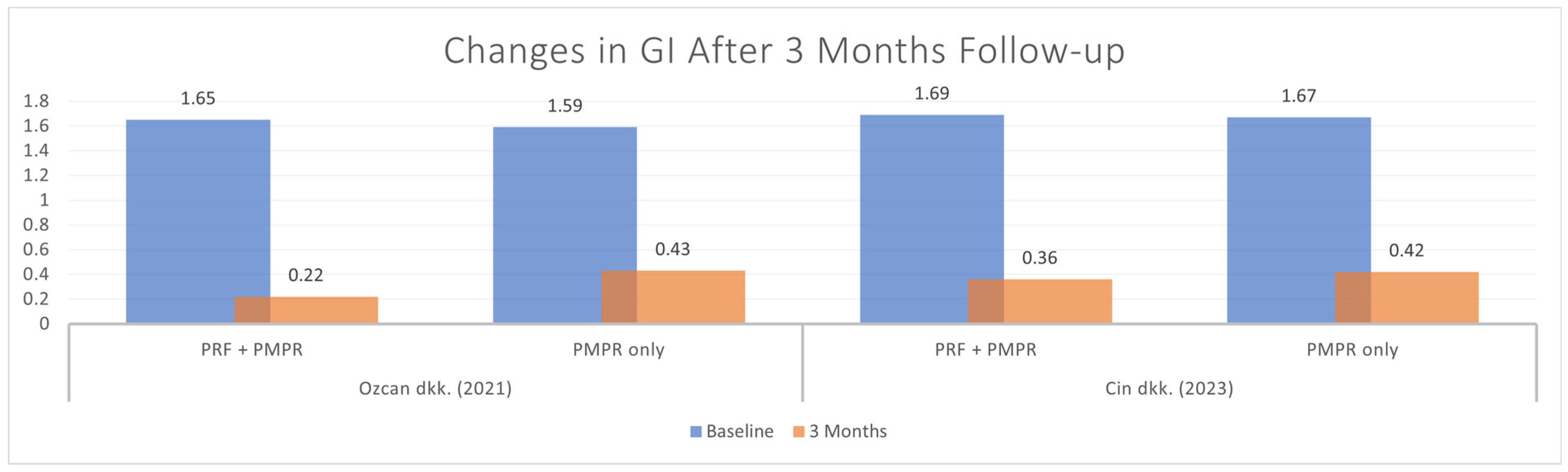
3.5.5. Gingival Index (GI)
3.5.6. Plaque Index (PlI)
4. Discussion
4.1. Beneficial Effect on Clinical Parameters
4.2. Beneficial Effect on Periodontal Stability
4.3. Beneficial Effect on Immunological Parameters
4.4. Limitations, Implications, and Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRF | Platelet-rich fibrin |
| PMPR | Professional mechanical plaque removal |
| SRP | Scaling and root planing |
| NSPT | Non-surgical periodontal therapy |
| PPD | Probing pocket depth |
| CAL | Clinical attachment loss |
| BoP | Bleeding on probing |
| GR | Gingival recession |
| GI | Gingival index |
| PlI | Plaque index |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
| TGF-β | Transforming growth factor-β |
| PDGF | Platelet-derived growth factor |
| GCF | Gingival crevicular fluid |
References
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Uzun Saylan, B.C.; Yılmaz, B.; Öztürk, V.Ö.; Atmaca, H.; Emingil, G. Evaluation of Annexin A1, Carbonic Anhydrase 1, and Elongation Factor 1-Gamma Levels in Periodontal Diseases. BMC Oral Health 2025, 25, 676. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar]
- World Health Organization. Oral Health; World Health Organization: Geneva, Switzerland, 2023; Available online: www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 11 September 2024).
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Period. 2021, 48, 1165–1188. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Zhao, L.; Ren, Z.; Hu, C. Global, Regional, and National Burden Of Periodontitis from 1990 to 2019: Results from the Global Burden of Disease Study 2019. J. Periodontol. 2022, 93, 1445–1454. [Google Scholar] [CrossRef]
- Health Research and Development Ministry of Health of Indonesia. National Report of Basic Health Research 2018; Publishing Agency of Health Research and Development: Jakarta, Indonesia, 2018; p. 204. [Google Scholar]
- Al-Bitar, K.M.; Garcia, J.M.; Han, S.; Guentsch, A. Association Between Periodontal Health Status and Quality of Life: A Cross-sectional Study. Front. Oral Health 2024, 5, 1346814. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Period. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Plessas, A. Nonsurgical Periodontal Treatment: Review of the Evidence. Oral Health Dent. Manag. 2014, 13, 71–80. [Google Scholar]
- West, N.; Chapple, I.; Claydon, N.; D’aIuto, F.; Donos, N.; Ide, M.; Needleman, I.; Kebschull, M. BBSP Implementation of European S3-Level Evidence-Based Treatment Guidelines for Stage I-III Periodontitis in UK Clinical Practice. J. Dent. 2021, 106, 103562. [Google Scholar] [CrossRef]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New Tendencies in Non-surgical Periodontal Therapy. Braz. Oral Res. 2021, 35, e095. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V. Antiseptics as Adjuncts to Scaling and Root Planing in the Treatment of Periodontitis: A Systematic Literature Review. BMC Oral Health 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The Immune Nature of Platelets Revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Torge, D.; Rinaldi, F.; Piattelli, M.; Bernardi, S.; Varvara, G. Platelets’ Role in Dentistry: From Oral Pathology to Regenerative Potential. Biomedicines 2022, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Mohan, S.; Jaishangar, N.; Devy, S.; Narayanan, A.; Cherian, D.; Madhavan, S. Platelet-rich Plasma and Platelet-rich Fibrin in Periodontal Regeneration: A Review. J. Pharm. Bioallied Sci. 2019, 11, S126–S130. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A Second-Generation Platelet Concentrate. Part I: Technological Concepts and Evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow Release of Growth Factors and Thrombospondin-1 in Choukroun’s Platelet-rich Fibrin (PRF): A Gold Standard to Achieve for All Surgical Platelet Concentrates Technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative Potential of Leucocyte- and Platelet-rich Fibrin. Part A: Intra-bony Defects, Furcation Defects and Periodontal Plastic Surgery. A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef]
- Rock, L. Potential of Platelet-rich Fibrin in Regenerative Periodontal Therapy: Literature Review. Can. J. Dent. Hyg. 2013, 47, 33–37. [Google Scholar]
- Shah, M.; Deshpande, N.; Bharwani, A.; Nadig, P.; Doshi, V.; Dave, D. Effectiveness of Autologous Platelet-rich Fibrin in the Treatment of Intra-bony Defects: A Systematic Review and Meta-analysis. J. Indian Soc. Periodontol. 2014, 18, 698–704. [Google Scholar]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative Potential of Leucocyte- and Platelet-rich Fibrin. Part B: Sinus Floor Elevation, Alveolar Ridge Preservation, and Implant Therapy. A Systematic Review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef]
- Balice, G.; Paolantonio, M.; De Ninis, P.; Rexhepi, I.; Serroni, M.; Frisone, A.; Romano, L.; Sinjari, B.; Murmura, G.; Femminella, B. Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study. Medicina 2024, 60, 1091. [Google Scholar] [CrossRef]
- Jepsen, K.; Sculean, A.; Jepsen, S. Complications and Treatment Errors Related to Regenerative Periodontal Surgery. Periodontology 2023, 92, 120–134. [Google Scholar] [CrossRef]
- Jamjoom, A.G. From Healing to Regeneration: A Comprehensive Review of the Efficacy of Platelet-Rich Fibrin in Periodontal Plastic Surgery Procedures. Cureus 2024, 16, e69287. [Google Scholar] [CrossRef]
- Parwani, S.R.; Thakare, K.S.; Kawadkar, K.P.; Soni, N.J.; Parwani, R. Platelet-Rich Fibrin in Non-Surgical Periodontal Therapy: A Split-Mouth Randomized Controlled Clinical Trial. Dent. J. 2024, 12, 135. [Google Scholar] [CrossRef]
- Hala Albonni Alaa, A.; Hamwi, A.; Al-Hamoui, W.B.; Sawaf, H. Clinical Effectiveness of a Topical Subgingival Application of Injectable Platelet-Rich Fibrin as Adjunctive Therapy to Scaling and Root Planing: A Double-Blind, Split-Mouth, Randomized, Prospective, Comparative Controlled Trial. Quint. Int. 2021, 52, 676–685. [Google Scholar] [CrossRef]
- Vučković, M.; Nikolić, N.; Milašin, J.; Đorđević, V.; Milinkovic, I.; Asotic, J.; Jezdic, Z.; Jankovic, S.; Aleksic, Z. The Effect of Injectable Platelet-Rich Fibrin Use in The Initial Treatment of Chronic Periodontitis. Srpski arhiv za Celokupno Lekarstvo 2020, 148, 280–285. [Google Scholar] [CrossRef]
- Özcan, E.; Saygun, I.; Kantarcı, A.; Özarslantürk, S.; Serdar, M.A.; Özgürtaş, T. The Effects of a Novel Non-invasive Application Of Platelet-rich Fibrin on Periodontal Clinical Parameters and Gingival Crevicular Fluid Transforming Growth Factor-β and Collagen-1 Levels: A Randomized, Controlled, Clinical Study. J. Periodontol. 2021, 92, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Anegundi, R.V.; Shenoy, S.B.; Chandran, T. Autologous Platelet-Rich Fibrin as an Adjunct to Non-Surgical Periodontal Therapy—A Follow-Up Clinical Pilot Study. Wound Repair. Regen. 2022, 30, 140–145. [Google Scholar] [CrossRef]
- Al-Rihaymee, S.; Mahmood, M.S.; Abdulbaqi, H.R.; Majeed, Z.N. Platelet-Rich Fibrin as an Adjunct to Scaling and Root Planing in Treatment of Shallow Periodontal Pockets: A Randomized Clinical Trial. J. Oral Biosci. 2024, 66, 612–618. [Google Scholar] [CrossRef]
- Al-Rihaymee, S.; Sh Mahmood, M. The Efficacy of Non-surgical Platelet-rich Fibrin Application on Clinical Periodontal Parameters and Periostin Level in Periodontitis: Clinical Trial. J. Cell. Mol. Med. 2023, 27, 529–537. [Google Scholar] [CrossRef]
- Cin, G.T.; Lektemur Alpan, A.; Çevik, Ö. Efficacy of Injectable Platelet-Rich Fibrin on Clinical and Biochemical Parameters in Non-Surgical Periodontal Treatment: A 2014Split-Mouth Randomized Controlled Trial. Clin. Oral Investig. 2023, 28, 46. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet-Rich Fibrin (I-PRF): Opportunities in Regenerative Dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Pullishery, F.; Alattas, M.H.; Abdelrasoul, M.R.; Hassan, A.F.; Derbala, D.A.A.; Hashir, S. Effectiveness of i-PRF in Periodontal Regeneration—A Systematic Review and Meta-Analysis. Saudi Dent. J. 2024, 36, 214–221. [Google Scholar] [CrossRef]
- Soeroso, Y.; Akase, T.; Sunarto, H.; Kemal, Y.; Salim, R.; Octavia, M.; Viandita, A.; Setiawan, J.; Bachtiar, B. The Risk Reduction of Recurrent Periodontal Pathogens of Local Application Minocycline HCl 2% Gel, Used as an Adjunct to Scaling and Root Planing for Chronic Periodontitis Treatment. Ther. Clin. Risk Manag. 2017, 13, 307–314. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Jati, A.S.; Furquim, L.Z.; Consolaro, A. Gingival Recession: Its Causes and Types, and the Importance of Orthodontic Treatment. Dent. Press. J. Orthod. 2016, 21, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Emingil, G.; Gürkan, A.; Atilla, G.; Kantarci, A. Subantimicrobial-dose Doxycycline and Cytokine-Chemokine Levels in Gingival Crevicular Fluid. J. Periodontol. 2011, 82, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Passoja, A.; Puijola, I.; Knuuttila, M.; Niemelä, O.; Karttunen, R.; Raunio, T.; Tervonen, T. Serum Levels of Interleukin-10 and Tumour Necrosis Factor-A in Chronic Periodontitis. J. Clin. Periodontol. 2010, 37, 881–887. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, C.; Zhao, Q.; Zhao, Z.; Wang, J.; Miron, R.J.; Zhang, Y. Anti-Inflammation Effects of Injectable Platelet-Rich Fibrin via Macrophages and Dendritic Cells. J. Biomed. Mater. Res. A 2020, 108, 61–68. [Google Scholar] [CrossRef]
- Niklander, S.; Bordagaray, M.J.; Fernández, A.; Hernández, M. Vascular Endothelial Growth Factor: A Translational View in Oral Non-Communicable Diseases. Biomolecules 2021, 11, 85. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, Z.; Wei, X.; Lin, L.; Chen, L.; Wang, H.; Fu, X.; Zhang, J.; Yu, C. Effects of Transforming Growth Factor-Beta1 and Vascular Endothelial Growth Factor 165 Gene Transfer on Achilles Tendon Healing. Matrix Biol. J. Int. Soc. Matrix Biol. 2009, 28, 324–335. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 Inhibits Bone Resorption: A Potential Therapeutic Strategy in Periodontitis and Other Bone Loss Diseases. BioMed Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef]
- Fujii, S.; Maeda, H.; Tomokiyo, A.; Monnouchi, S.; Hori, K.; Wada, N.; Akamine, A. Effects of TGF-β1 on the Proliferation and Differentiation of Human Periodontal Ligament Cells and a Human Periodontal Ligament Stem/Progenitor Cell Line. Cell Tissue Res. 2010, 342, 233–242. [Google Scholar] [CrossRef]
- Shashank, B.; Bhushan, M. Injectable Platelet-Rich Fibrin (PRF): The Newest Biomaterial and Its Use in Various Dermatological Conditions in Our Practice: A Case Series. J. Cosmet. Dermatol. 2021, 20, 1421–1426. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced Regenerative Technologies for Periodontal Tissue Repair. Periodontology 2012, 59, 185. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Population (P) | Adult patients (>18 years) with periodontitis |
| Intervention (I) | Subgingival PMPR + PRF as an adjunctive NSPT for periodontitis |
| Comparison (C) | Subgingival PMPR alone as a control measure |
| Outcome (O) | Outcome measures were clinical parameters (PPD/CAL/BoP/GR/GI/PlI) and/or immunological parameters (IL/TNF-α/VEGF/TGF-β/Periostin/Collagen type I) |
| Study design (S) | Clinical studies (RCT, pilot study, comparative study, case report) |
| Author (Year) | Samples Characteristics | Definition/ Diagnosis of Periodontitis | Intervention | Parameters | Follow-Up Periods | |
|---|---|---|---|---|---|---|
| Size | Age (Mean ± SD) | |||||
| Vučković et al., 2020 [34] | 24; 10 males, 14 females | 22–64 (37.29 ± 10.23) | Chronic periodontitis (minimum two teeth in each quadrant with PPD ≥ 5 mm; BoP ≥ 40%; no furcation involvement) | Subgingival PMPR + i-PRF | Clinical: PPD, CAL, BoP, GML, PlI | Baseline 3 months |
| Özcan et al., 2021 [35] | 12; 6 males, 6 females | 30–57 (43.33 ± 8.34) | Stage III grade B periodontitis (PPD ≥ 6 mm, CAL ≥ 5 mm, radiographic bone loss extending to the middle or apical third of the root, and tooth loss due to periodontitis ≤ 4 in different quadrants) | Subgingival PMPR + PRF | Clinical: PPD, CAL, BoP, GR, GI, PlI Immunological: TGF-β Col-1 | Clinical: Baseline 3 months 6 months Immunological: Baseline 3 days 7 days 14 days |
| Narendran et al., 2022 [36] | 16 | 35–45 (40.56 ± 3.39) | Moderate periodontitis; stage III (PPD ≥ 5 mm and ≤ 7 mm) | Subgingival PMPR + PRF | Clinical: PPD, CAL | Baseline 60 days 90 days |
| Al-Rihaymee & Mahmood, 2023 [38] | 14; 12 males, 2 females | NR * | Periodontitis (two contralateral pockets with a depth of 4–6 mm) | Subgingival PMPR + PRF | Clinical: PPD, RAL, BoP, PlI Immunological: Periostin | Clinical: Baseline 1 month 3 months Immunological: Baseline 1 month 3 months |
| Cin et al., 2023 [39] | 17; 7 males, 10 females | 37.41 ± 5.84 | Stage III grade B periodontitis (PPD ≥ 6 mm, CAL ≥ 5 mm, radiographic bone loss extending to the middle or apical third of the root, and tooth loss due to periodontitis ≤ 4 in different quadrants) | Subgingival PMPR + i-PRF | Clinical: PPD, CAL, BoP, GR, GI, PlI Immunological: VEGF TNF-α IL-10 | Clinical: Baseline 1 month 3 months 6 months Immunological: Baseline 7 days 14 days |
| Parwani et al., 2024 [32] | 13; 6 males, 7 females | 30–60 (29.5) | Stage III grade A periodontitis with 5–6 mm pocket | Subgingival PMPR + PRF | Clinical: PPD, CAL, GR, GI, PlI | Baseline 6 weeks |
| Al-Rihaymee et al., 2024 [37] | 12; 9 males, 3 females | 20–40 (29.83 ± 5.7) | Unstable periodontitis with PPD 4–5 mm on both sides | Subgingival PMPR + PRF | Clinical: PPD, CAL, BoP, PlI Immunological: PDGF-BB | Clinical: Baseline 1 month 3 months Immunological: Baseline 1 month 3 months |
| Author (Year) | Pre-Intervention | PMPR Procedure | PRF Procedure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anesthesia | Method | Instrument | Type | Tools | Duration | Speed | RCF | ||
| Vučković et al., 2020 [34] | Self-performed plaque control (brushing, interdental cleaning | Yes | FMS | NR | i-PRF | Duo Centrifuge (Process for PRF) Nice, France | 3 min | 700 rpm | 60 g |
| Özcan et al., 2021 [35] | Manual scaling (Gracey curette) and ultrasonic, oral health education | NR | FMS | Standard curettes | PRF clot | Nuve, CN 180, Bench-Top Centrifuge Ankara, Turkey | 10 min | 3000 rpm | 400 g |
| Narendran et al., 2022 [36] | NR | NR | FMS | NR | PRF clot | Remi R-8c BL Centrifugation System Mumbai, India | 12 min | 2700 rpm | NR |
| Al-Rihaymee & Mahmood, 2023 [38] | Oral hygiene education, ultrasonic scaling | NR | FMS | NR | PRF clot | Intraspin Centrifuge Boca Raton, FL, USA | 10 min | 3000 rpm | 805 g |
| Cin et al., 2023 [39] | Supragingival scaling, oral hygiene education | Yes | FMS | Gracey curettes | i-PRF | Duo Centrifuge (Process for PRF) Nice, France | 3 min | 700 rpm | 60 g |
| Parwani et al., 2024 [32] | NR | NR | FMS | Gracey Curettes, ultrasonic scaler | PRF clot | PC-02 (Process for PRF) Nice, France | 8 min | 4000 rpm | NR |
| Al-Rihaymee et al., 2024 [37] | Supragingival scaling, oral hygiene education | NR | FMS | Gracey curettes | PRF clot | Primefuge, model TG12 China | 12 min | 2700 rpm | 653 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanady, M.; Tadjoedin, F.M.; Masulili, S.L.C.; Harsas, N.A.; Widaryono, A. Beneficial Effect of Platelet-Rich Fibrin as an Adjunct to Nonsurgical Therapy After Subgingival Professional Mechanical Plaque Removal for Periodontitis: A Systematic Review and Meta-Analysis. Clin. Pract. 2025, 15, 127. https://doi.org/10.3390/clinpract15070127
Tanady M, Tadjoedin FM, Masulili SLC, Harsas NA, Widaryono A. Beneficial Effect of Platelet-Rich Fibrin as an Adjunct to Nonsurgical Therapy After Subgingival Professional Mechanical Plaque Removal for Periodontitis: A Systematic Review and Meta-Analysis. Clinics and Practice. 2025; 15(7):127. https://doi.org/10.3390/clinpract15070127
Chicago/Turabian StyleTanady, Monica, Fatimah Maria Tadjoedin, Sri Lelyati C. Masulili, Nadhia Anindhita Harsas, and Adityo Widaryono. 2025. "Beneficial Effect of Platelet-Rich Fibrin as an Adjunct to Nonsurgical Therapy After Subgingival Professional Mechanical Plaque Removal for Periodontitis: A Systematic Review and Meta-Analysis" Clinics and Practice 15, no. 7: 127. https://doi.org/10.3390/clinpract15070127
APA StyleTanady, M., Tadjoedin, F. M., Masulili, S. L. C., Harsas, N. A., & Widaryono, A. (2025). Beneficial Effect of Platelet-Rich Fibrin as an Adjunct to Nonsurgical Therapy After Subgingival Professional Mechanical Plaque Removal for Periodontitis: A Systematic Review and Meta-Analysis. Clinics and Practice, 15(7), 127. https://doi.org/10.3390/clinpract15070127