1. Introduction
Lipedema is a chronic disease of the subcutaneous adipose tissue that is still poorly understood by health professionals. For this reason, it may be difficult to diagnose and, consequently, difficult to treat due to the lack of scientific data and training of the many professionals who should be involved in the care of patients. The great lack of scientific support is not justified by the rarity of the disease, as is unfortunately often the case with many lesser-known diseases, given that prevalence estimates range from 6.5% in children in the United States to 6–8% in women in Germany and 15–19% in clinics specializing in vascular diseases [
1]. While experiences with conservative treatments, such as physiotherapy, diet and/or surgery, are beginning to be shared and obtain a scientific basis, the same cannot yet be said for a possible pharmacological approach. In fact, there is currently no data available, not even on pain control, which is one of the main symptoms of the disease.
In a recent publication of ours, we noted the presence of numerous comorbidities in women with lipedema, many of which involve the endocrine system [
2]. Among the most common comorbidities we found were obesity, alterations in glucose metabolism, dyslipidemia, thyroid disorders and polycystic ovary syndrome. If it is true that there is no defined therapy for lipedema, it is also true that for these comorbidities, there is a long history of scientific experience and proven efficacy. Our data suggests that at least one third of women with lipedema are affected by insulin resistance; this means that at least one third of lipedema patients could benefit from targeted pharmacological treatment. This percentage becomes over 50% in women with both lipedema and obesity, while it rises to 75% if BMI is greater than 35 kg/m
2 [
2].
Insulin resistance, due to its implications on metabolism, adipocytes and inflammation, could reasonably be implicated in the pathogenesis and progression of adipose tissue diseases such as lipedema. Therefore, based on this scientific evidence, a treatment aimed at improving this condition could potentially have many favorable effects [
2,
3,
4,
5,
6,
7,
8]. The results obtained with some nutritional regimens, such as the ketogenic diet or a low-carb and anti-inflammatory diet, which have an impact on glucose metabolism, have already proven to be effective in treating lipedema [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18].
Metformin remains one of the cornerstone treatments for insulin resistance, even in other endocrinopathies such as polycystic ovary syndrome, where its pleiotropic effects and its role in the treatment of this syndrome are well known [
19,
20,
21]. Treatment with metformin is also a valid option for patients with lipedema and insulin resistance and is, in fact, part of our daily clinical practice regarding this metabolic condition.
But Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAs) may represent an even more interesting pharmacological option for women with lipedema and insulin resistance. In fact, to treat insulin resistance in these patients, for the past year, we have also been using one of the first drugs of the GLP-1 RA family that was introduced for the treatment of diabetes about 20 years ago in Italy; at the time, it was considered an innovative therapy with promising properties because improvements in glucose control were often accompanied by a reduction in bodyweight [
22,
23].
Type 2 diabetes mellitus is currently the only indication authorized for the prescription of exenatide, but there is a lot of data in the literature demonstrating the drug’s ability to improve hepatic, adipose and whole-body insulin sensitivity while having positive effects on body weight reduction, both in diabetic and non-diabetic patients [
24,
25]. In preclinical and clinical studies, exenatide has been demonstrated to improve glycemic control through the enhancement of glucose-dependent insulin secretion, the suppression of inappropriately elevated postprandial glucagon secretion, the slowing of gastric emptying and the reduction in food intake [
26,
27,
28,
29].
Although pharmacological intervention on insulin resistance and weight loss in these patients may be extremely important, there are other demonstrated actions of this pharmacological class that are well beyond the treatment of the metabolic disorder. More and more studies have emphasized the therapeutic potential of GLP-1RAs in a wide variety of diseases, such as neurodegenerative disorder and inflammatory bowel disease, shedding light on their anti-inflammatory effects [
30]. Exenatide, specifically, was found to reduce oxidative stress and the expression of numerous pro-inflammatory cytokines, such as, for example, monocyte chemoattractant protein 1 (MCP-1), Tumor Necrosis Factor alpha (TNF-alpha), and Interleukin-1 beta (IL-1 beta), in obese patients [
31,
32,
33]. The anti-inflammatory effect at the cellular and molecular levels was independent of weight loss [
33]. Other studies have shown the effect of exenatide on reducing inflammation and hypoxia in adipose tissue by improving angiogenesis and microcirculation [
34]. These effects, which are fundamental for the treatment of simple obesity, take on an even more potentially interesting importance when considered for the treatment of a disease such as lipedema, whose main characteristic seems to be the presence of inflamed, hypo-oxygenated and dysfunctional adipose tissue with fibrosis, extracellular matrix remodeling and lymphatic and vasculature dysfunction [
1,
35].
The aim of this study, with these assumptions, is to present the first promising evidence of the efficacy of exenatide Long-Acting Release (exenatide LAR) in treating the signs and symptoms of five women affected by lipedema and insulin resistance.
2. Materials and Methods
Five cases of patients with lipedema treated for 3 to 6 months with exenatide LAR 2 mg per week in association or not with lifestyle changes (diet or physical activity) are described. During the study period, no other conservative treatments were performed, such as the use of bandages, lymphatic drainage or elastic compression garments (except for case 5, as described below).
The inclusion criteria were the presence of lipedema, female sex, age between 18 and 50 years and the presence of insulin resistance.
The exclusion criteria were the presence of diabetes mellitus, serious chronic diseases, the intake of drugs with any effect on body weight, glucose metabolism, pain and edema. Patients with a recent reduction in body weight (greater than 10% of the weight in the previous 6 months) or diet modification or patients previously treated for lipedema (including methods such as subcutaneous adipose tissue massage, lymphatic drainage, bandaging or elastic compression stocking) were also excluded. Case report 5 is an exception as the patient had undergone previous surgical therapy and was using elastic compression only for this reason: this treatment was not modified during the study and in the 6 months before the study. Informed consent was obtained from all subjects for the use of off-label exenatide therapy and for the publication of the results.
The diagnosis, staging and phenotyping of lipedema was based on clinical findings of characteristic symptoms and signs of the disease, as described in our previous work [
2,
36,
37].
The presence of insulin resistance was assessed both in fasting and after the ingestion of 75 g of glucose. All patients performed the Oral Glucose Tolerance Test (OGTT), with blood glucose and insulin levels being measured before and 30, 60, 90, 120 and 180 min after glucose ingestion. The fasting insulin resistance status was determined by calculating the HOMA-IR index (HOMA-IR = (glucose mg/dL × insulin mIU/L)/22.5) [
38]; a value greater than or equal to 2.29 was considered diagnostic for the presence of fasting insulin resistance [
39].
As a second method for the diagnosis of insulin resistance, the surrogate index called the Matsuda Index was used [
40]; a value of less than 3.5 was considered diagnostic for insulin resistance [
41].
Diagnostic criteria for the diagnosis of prediabetes were as follows: fasting glucose of 100–125 mg/dL, 120 min OGTT glucose of 140–190 mg/dL or HbA1c 5.7–6.4% [
42].
The presence of diabetes was excluded according to Italian and international guidelines [
43,
44].
No specific questionnaires were used to evaluate diet and physical activity, which were assessed by nutritionists and doctors during the visits. The recommended diet, in case reports from a retrospective observational study, could be different, as described in individual cases. Generally, during the pharmacological treatment phase, moderate aerobic physical activity is recommended, such as walking or gym exercises for 30–60 min a day at least three times a week.
No assessment of body composition was performed, although patients were evaluated using anthropometric measurements (such as body weight, BMI and waist and hip circumference), a specific questionnaire for lipedema symptoms, a Progressive Pain Check for lipoalgia and an ultrasound assessment of the adipose tissue.
2.1. Evaluation of Lipedema Symptoms
For the evaluation of symptoms we used a specific questionnaire, as described in our previous work [
36]. The questionnaire was used to evaluate the severity of symptoms before beginning therapy and after therapy and consists of 17 questions that investigate the presence and extent of the main symptoms of the disease. To quantify the extent of symptoms, a 6-point Likert-type scale was used (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = strong and 5 = very strong) [
45].
Symptoms can be assessed individually, and a final score can be calculated from the sum of individual scores, ranging from 0 to 85 [
36].
2.2. Evaluation of Lipoalgia
The presence and extent of pain from the subcutaneous fat fold, or provoked lipoalgia, were assessed using a method called Progressive Pain Check (PPC), described in our previous work [
36]. The clinical examination involves the assessment of pain evoked by the subcutaneous fat fold at 8 points on the lower body (7 points at the level of the lower limb and 1 point at the level of the lower abdomen) and at 3 points on the upper body (dorsal region, arm and forearm). Pain is quantified with the VRS, which is represented by a 5-point Likert-type scale with 5 verbal descriptors: no pain, mild pain, moderate pain, severe pain and very severe pain [
36].
The quantification of pain severity was achieved through the assignment of numerical values to the VRS items, ranging from 0 to 4. These values are defined as follows: 0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain and 4 = very severe pain. Subsequently, three scores were calculated: (the Lower Body Pain Score (LBPS), the Upper Body Pain Score (UBPS) and the Total body pain score or Ricolfi–Patton Score (RPS), as described in our previous work [
36].
2.3. Ultrasound Measurement
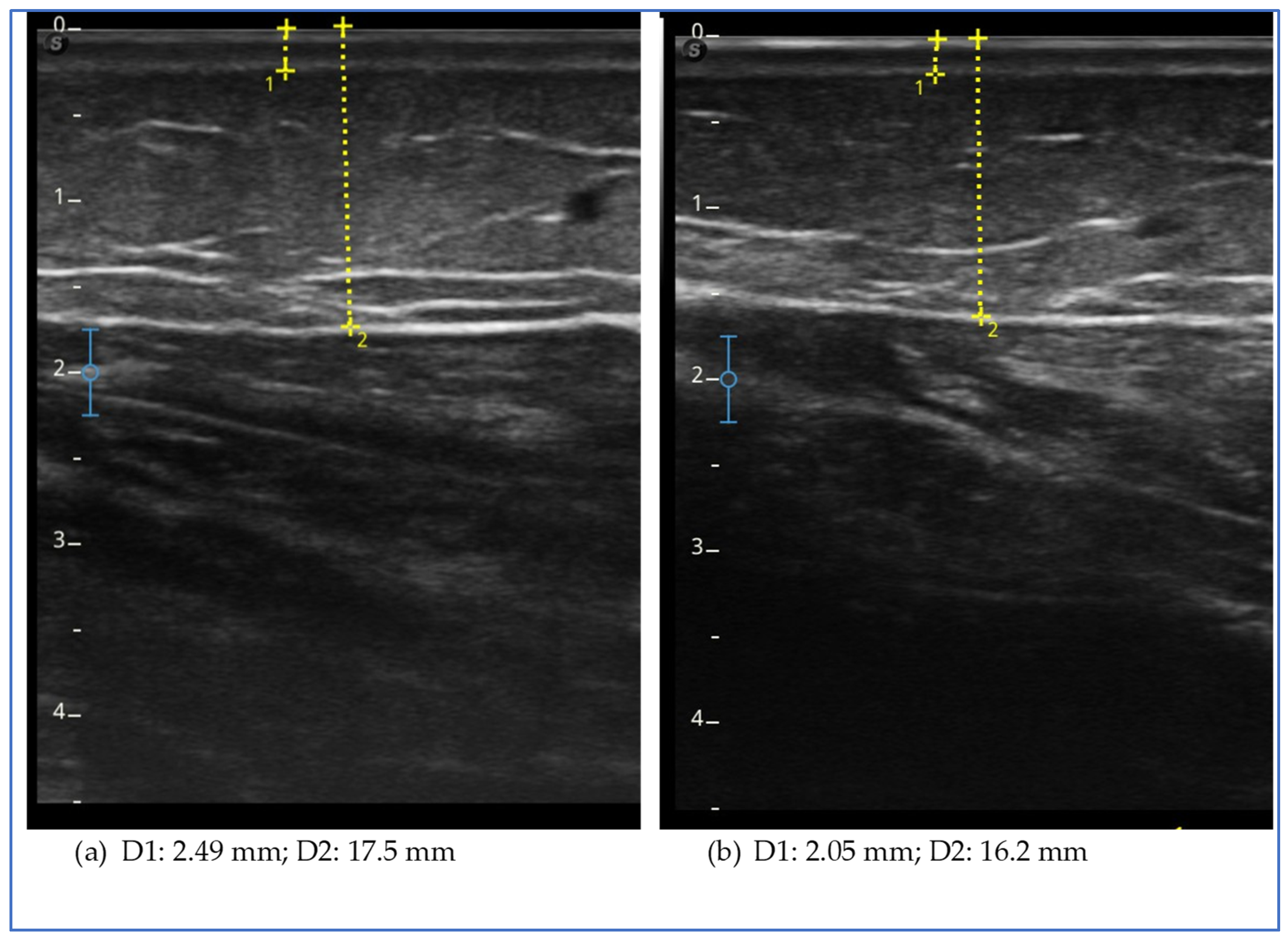
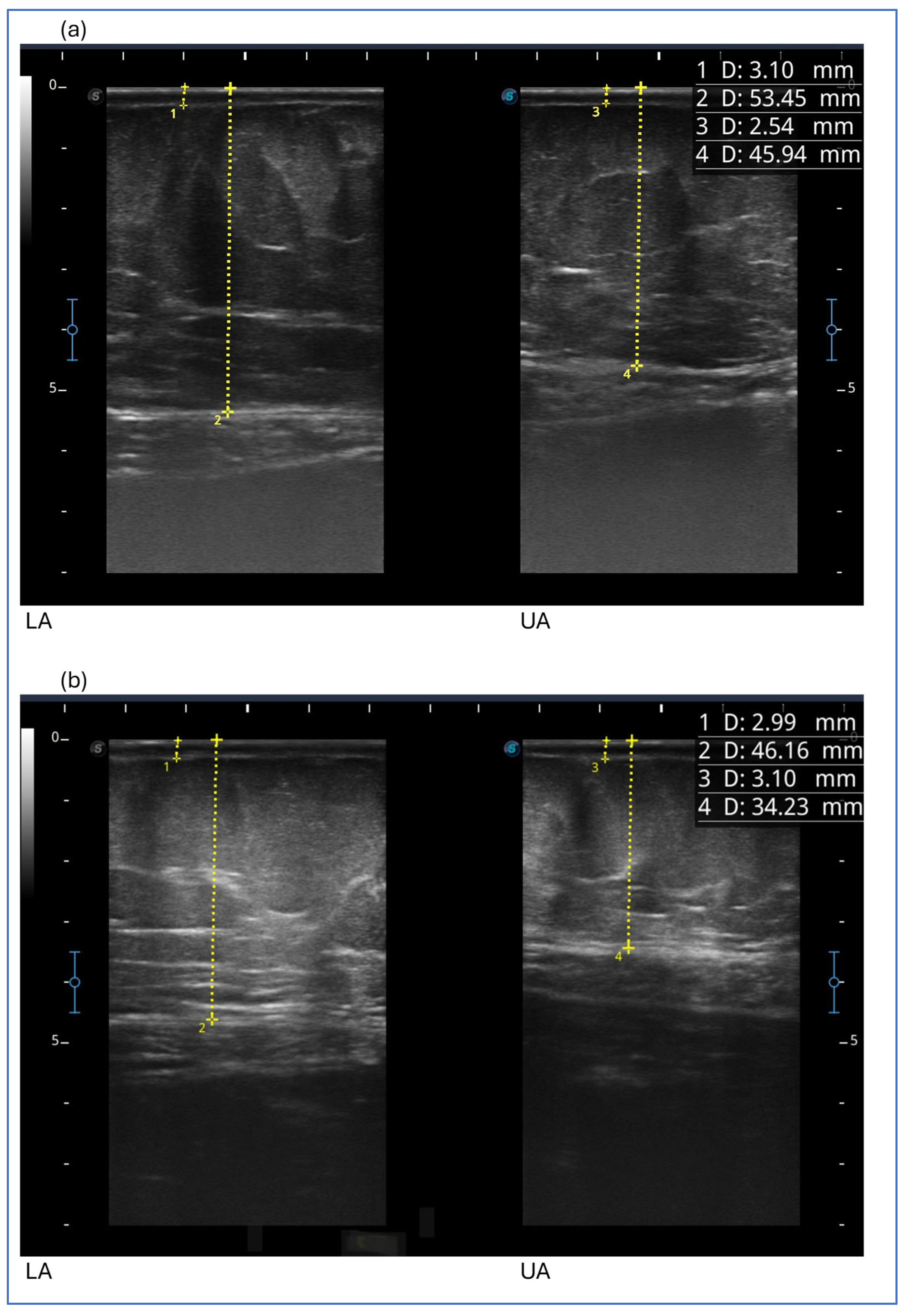
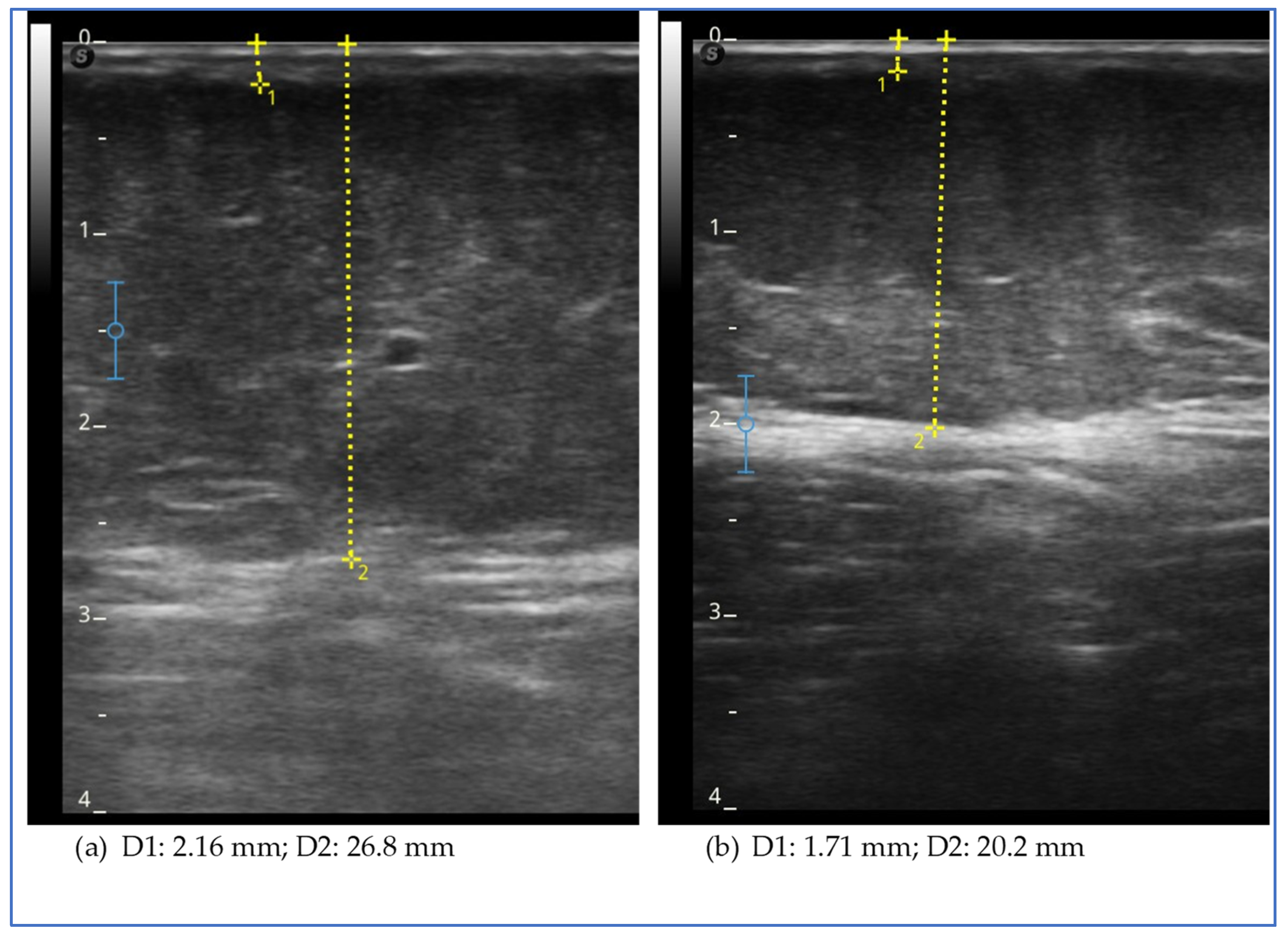
Ultrasound measurement of the adipose tissue was conducted using a high-frequency linear probe with a frequency range of 8–14 MHz (SonoScape X3, SonoScape Medical Corp., Shenzhen, China). The probe was maintained in a perpendicular position relative to the skin and no pressure was applied to the underlying tissue.
At the level of the lower limbs, as described in our previous studies [
2,
36], the measurement of the subcutaneous adipose tissue was taken from the surface of the skin to the muscle fascia. The measurements were performed at the levels of the lower limb, lower abdomen, arm and forearm. At the lower-limb level, the measurements were taken at these points:
- -
The medial and lateral lower third of the leg (about 5 cm above the medial and the lateral malleolus);
- -
The medial and lateral upper third of the leg (about 5 cm below the prominence of the tibial tuberosity and an equivalent point on the lateral upper third);
- -
The medial and lateral lower third of the thigh (about 5 cm above the upper edge of the patella);
- -
The medial upper third of the tight (about 5 cm below the inguinal crease).
- -
The midpoint of the thigh on the anterior side, corresponding to the point where the skin fold is generally detected [
46,
47].
Measurements were also taken at the level of the abdomen and upper limb.
At the level of the abdomen (anterior surface and with the patient supine), measurements were taken using the navel as a reference point:
- -
At the point 5 cm lateral and inferior to the umbilicus (lower abdomen)
- -
At the point 5 cm lateral and superior to the umbilicus (upper abdomen)
Measurements at the upper-limb level were taken with the patient lying down and the arm raised above the head: in this position, we took two measurements of adipose tissue thickness, namely at the midpoint of the upper arm and at the midpoint of the forearm (midpoint upper arm and midpoint forearm).
4. Discussion
Insulin resistance is defined as a condition in which there is an inability of insulin-targeted tissue to respond to normal insulin levels, and thus, higher-than-normal levels of insulin are required to maintain the normal functions of insulin [
3]. In recent years, the opposite hypothesis has also been postulated. It states that insulin resistance is a secondary phenomenon, an adaptive defense mechanism to a primary condition of hyperinsulinemia [
4]. Both insulin resistance and hyperinsulinemia per se could be implicated in the case of lipedema, and both conditions could be either the consequence or the cause of a dysfunctional alteration in the adipose tissue [
3,
4,
5,
6]. The close physiological relationship between insulin and adipose tissue is an increasingly studied phenomenon because of its implications on human health, both in physiological and pathological conditions in which the biological cross talk is interfered by an unbalanced metabolic system or by dysfunctional adipose tissue. These phenomena, if persistent over time, tend to worsen and self-maintain. They involve adipose tissue as a whole and in all its functions, both as an endocrine organ and as an immune organ, determining local and systemic effects in a pro-inflammatory sense [
3,
4,
5,
6,
7,
8]. These phenomena are studied in the case of obesity but much less in the presence of lipedema, although, based on actual knowledge, they seem to be completely involved.
We presented five cases of patients with lipedema and insulin resistance who we treated with exenatide alone or in combination with modifications of diet and physical activity. We chose the cases of patients not treated with decongestive therapy because of possible greater interference in the results obtained.
We must acknowledge that it is not possible to know whether the observed outcomes can be attributed solely to pharmacological treatment because it is possible that the lifestyle changes implemented by the patients had a direct influence or favored all of the results described.
It is not possible with these few cases and without an adequate study protocol to exclude this aspect. To better comprehend this aspect, a more appropriate study (like a crossover study) could be conducted in the future.
At the time we started the pharmacological treatment of these patients, affected by both insulin resistance and obesity, no other GLP-1 RA or agonist of both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) with an indication for obesity treatment were available in Italy. The choice of exenatide was strongly supported by the data reported in the literature that showed, in addition to a good safety profile and good tolerability, positive effects on insulin resistance, body weight reduction, vascular and anti-inflammatory effects that were demonstrated both in patients with and without diabetes and with other chronic diseases [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35]. We used once-weekly exenatide or exenatide long-acting release (LAR), available in this formulation in Italy for about 10 years. The long-acting formulation has proven to be well accepted by patients and easy to use with a maintained good safety profile while showing efficacy regarding metabolism and body weight control. Weekly dosing resulted in steady-state plasma exenatide concentrations after 6–7 weeks [
48,
49]. Our daily clinical experience, not limited to these five described cases, suggests a close relationship with the pharmacological profile: we are used to seeing a positive effect on exenatide therapy within the first 2–3 months of treatment. In our clinical practice, if no response is seen within these terms, continuation does not lead to important results, so we generally modify the therapeutic plan.
There are more interesting aspects that emerged from the results obtained that, in our opinion, should be considered, starting with the effect on body weight reduction. This effect, however, was not obtained in all cases: case report 1 did not achieve weight reduction. This patient was the one who had a lower degree of fasting insulin resistance (calculated with HOMA-IR) and the only one with a normal Matsuda index compared to the other cases.
This patient differs from the others in having less fasting insulin resistance and no insulin resistance, hyperinsulinemia, or glycemic abnormalities on OGTT. This suggests a possible connection between the severity of metabolic impairment and the response in terms of body weight reduction to the drug in the patient with lipedema. We cannot exclude the fact that glycemic levels influence the results; however, the most relevant metabolic alteration would appear to be insulin resistance and hyperinsulinemia. This hypothesis can also be supported by the marked weight reduction obtained in case 5, a case that differs from the others because it underwent previous surgery, especially because it is the case with greater hyperinsulinemia to OGTT.
Case report 1, however, is interesting precisely because it demonstrates an improvement in symptoms, clinical features and a reduction in adipose tissue thickness, even in the absence of weight loss. This result suggests a potential direct effect of exenatide on lipedematous tissue, also supported by the fact that the degree of insulin resistance in this patient was minimal. It is therefore possible to think that exenatide may have a role in the treatment of lipedema and that it could be mediated not only by the metabolic effect on insulin resistance, but also by other effects already reported in the literature involving the inflammatory and vascular states. As this is an inflammatory adipose tissue disorder, it is hypothesized that this effect is mediated by the reduction in systemic and tissue inflammation that GLP-1 drugs have been shown to cause in other inflammatory and chronic diseases, such as fatty liver disease and inflammatory bowel disease [
30,
31,
32,
33,
34].
Focusing on the response to pain, we can see that spontaneous pain in the lower limbs has not improved, while provoked pain has reduced. This could be explained by the fact that spontaneous pain is more variable, which is why we prefer to evaluate all the symptoms together with the questionnaire. Even if it is easier and quicker to obtain a reduction in the spontaneous pain evaluation compared to the pain detected clinically with the tissue fold, this symptom can also be influenced by numerous other factors, including the emotional state, the phase of the menstrual cycle and even incorrect lifestyle habits [
48,
49,
50].
Weight reduction was achieved in all other cases, both in association with or without an association with changes in the nutritional plan. The reduction in body weight in the other four patients reflects what has already been reported in the literature in patients with type 2 diabetes. In fact, the literature states that body weight reduction is progressive in the first 3 months and, subsequently, it continues in a more slowly way [
51,
52]. Even though there are only a few cases, the percentage reduction in weight obtained in the first 3 months of treatment, from 4.5% to 11.2%, in patients suffering from lipedema would appear to be potentially even greater than what is reported in the literature, which is approximately 2.5–3.4% in the first 3 months and 3.6% after 6 months in patients with type 2 diabetes mellitus [
51,
52]. The patient who showed the greatest percentage reduction is the one who was treated in combination with a ketogenic diet and then quickly regained weight with the reintroduction of carbohydrates (case 4). From this case, we could deduce at least two observations: The first is that exenatide could promote weight reduction following VLCD given that the patient had previously followed the same diet without obtaining any results, or simply that the effect of exenatide was enhanced by the current diet. The second observation, however, involves an alert relative to the excess weight produced and the excess speed in which it was reduced, with an immediate rebound effect upon the reintroduction of carbohydrates. Based on our experience, we observed that marked and sudden weight fluctuations with a yo-yo effect should be avoided both for the potential negative effects on adipose tissue and metabolic consequences, including hyperinsulinemia and elevated basal insulin secretion [
53,
54], but also because the goal of treating lipedema is to obtain positive results that can be maintained over time given the chronicity of the disease. However, it is also true that, in this case, the improvement in the subjective symptomatology and the clinical picture continued to improve despite the recovery of body weight. There is, however, one last observation for this case, which is more specific but must be highlighted: the exacerbation of the underlying psychiatric condition that the patient suffered during the combined treatment period and the marked reduction in weight. The reported effect was transitory and did not require changes in psychiatric therapy; however, it could be an aspect that must not be underestimated in the choice of a personalized therapeutic path.
A marked reduction in body weight was also observed in another patient who did not change her lifestyle and who, unlike the others, was the only one who had previously undergone liposuction. In this case, one might also think that the effect could have been facilitated by the fact that the increase in body weight and the worsening of lipedema were more recent compared to the other cases. It can therefore be assumed that the adipose tissue may have different characteristics, perhaps with less compromise of the adipose tissue, perhaps with less inflammatory, with less vascular compromise and a fibrotic component, but this is only a hypothesis. Although these observations were made in a single case, the extent of the clinical response, not only in terms of weight reduction, suggests the continued efficacy of the therapies and the potential use of these drugs in the post-operative phase. Also, from our daily experience, after surgery, there might be a possibility of relapses, or the disease may progress in untreated body areas. In fact, although targeted scientific studies are necessary, growth of tissue outside of the areas treated with surgery was reported in over 50% of patients of women with stage 2 or 3 lipedema and lipo-lymphedema. A total of 61% of patients noted new tissue growth within the first 6 months. In the same study, tissue growth in the areas treated with surgery was reported in approximately 30% of patients [
55].
A reduction in waist circumference was obtained in all cases except for case 2, while in all cases, we observed a reduction, albeit variable, in pelvic circumference.
Focusing specifically on the symptoms reported by patients in the lower limbs, the results were consistent: in all cases, we observed a reduction in symptoms after the first 3 months of treatment independently of weight reduction or associated treatments.
The same result was obtained by evaluating the variation in pain evoked by the adipose tissue fold: in all cases, a reduction in the total score was obtained, with a reduction in both the score of the lower part of the body and of the upper part of the body. In our experience, this result is of particular clinical relevance, not always being consistent with the improvement in subjective symptoms.
In fact, in clinical practice, we have often observed that, despite a striking reduction in subjective symptoms, such as after a period of diet or halfway through decongestive treatment, clinically evoked lipoalgia remains markedly evocable [
56]. It is not said that clinical findings, such as clinically evoked lipoalgia, are more important than subjective symptoms. However, in our opinion, the pain evoked by the tissue fold is fundamental in deciding whether to continue or modify the therapeutic plan. There are no available studies that have evaluated the effect of exenatide on these parameters, neither in patients with diabetes nor in patients without diabetes. This data is specific for lipedema, and with the same method, it has only been evaluated in our previous study following the use of a stocking with a micro massage effect not associated with changes in diet and in the absence of changes in body weight; even in this case, a reduction in the score of subjective symptoms in the lower limbs and the score of evoked lipoalgia in the lower limbs was obtained. However, no significant difference in evoked pain was observed in the untreated area of the upper body [
36]. In the case of pharmacological treatment, the positive effect would appear to be systemic, i.e., more generalized than with local treatment, as expected.
The evaluation of the effect in this study is also supported by the observation of the modification of the thickness of the adipose tissue in specific points of the lower limbs, abdomen and upper limbs. This measurement, theoretically, can be more specific to study the effect on the subcutaneous tissue rather than what we can deduce on this tissue by observing the circumference of the limb or of the abdomen. However, it is not free from possible measurement errors and imprecision due to the ultrasound technique considering the difficulty in finding safe landmarks and the anatomical variation of each patient. In our opinion, it is of great help in clinical practice to evaluate the effects of all treatments, both conservative and surgical, and its use should be suggested and encouraged. In the five cases described, at the level of the lower limbs, we obtained a reduction in thickness in all patients and at all points evaluated, with the exception of three isolated points of different cases (the lateral lower third of the thigh in case 2, the lower lateral third of the leg in case 3 and the medial upper third of the leg in case 5). Case report 2 was the only one in which we did not observe a reduction in the thickness of the subcutaneous tissue at the level of the upper abdomen, while in all cases, a reduction in thickness was observed in the lower abdomen, an area often involved in lipedema. The presence of pain and the characteristics of the adipose tissue suggested that the lower abdomen was also involved in lipedema, with the exception of case 2. Therefore, the lack of tissue reduction in this case could be significant. However, further data are needed to verify the hypothesis. A reduction in upper limb thickness was variably observed in all cases described, except for case report 1. The reduction in upper limbs was particularly marked in the patient who had previously undergone liposuction of the lower limbs; it could be assumed that the tissue response to treatment may be more evident due to the recent clinical worsening. This data suggests a possible role of exenatide in patients with lipedema affected by insulin and who are undergoing surgery in the case of recurrence or clinical worsening.