Abstract
Background/Objectives: Renal cell cancer is a rare occurrence in patients with ulcerative colitis (UC), with no clearly demonstrated association between UC and an increased risk of renal malignancies. In this article, a case report concerning this relationship is presented. Methods: Our research group presented a case of clear cell renal carcinoma in a 56-year-old male with UC who had previously undergone ileorectal anastomosis and subtotal colectomy. Results: The patient developed a complex renal cyst that progressed to malignancy within one year while on immunosuppressive therapy with infliximab and then filgotinib. Previous ultrasound examinations of the kidney highlighted only simple cysts in the contralateral kidney in previous years. The neoplasm was promptly examined using contrast-enhanced ultrasound, confirming the diagnosis of a Bosniak IV cyst, which was corroborated by a subsequent computed tomography study. Conclusions: The patient underwent a nephrectomy and is currently scheduled for therapy with vedolizumab. Given the increasing use of biologics and small molecules in UC management, periodic ultrasound screening may be a valuable tool for the long-term monitoring of these patients.
1. Introduction
Ulcerative colitis (UC) belongs to the group of inflammatory bowel diseases (IBDs) and induces a chronic, self-perpetuating inflammatory condition affecting the colorectal tract, starting from the rectum, and this condition exposes patients to both short- and long-term complications, including colectomy and colorectal cancer [1]. In addition to colorectal cancer, which has a prevalence of nearly 4% among UC patients and for which they face a significantly higher risk compared to the general population, other forms of neoplastic associations have also been described, including renal cancer [2].
In this regard, Feng et al. [3], in a pooled analysis of population-based studies, demonstrated an increased risk of renal cancer in patients with IBD (pooled standardised incidence ratio of 1.53), particularly in those with Crohn’s disease (pooled standardised incidence ratio of 1.95). However, no increased risk was observed in UC patients compared to the general population [3]. This risk profile was further confirmed by a subsequent reanalysis of meta-analyses conducted by Piovani et al. [4], which ruled out any defined associations between renal cancer and UC. Conversely, data from Mendelian randomization genetic studies have failed to demonstrate a potential causal association between IBD and renal cancer [5].
Advanced immunosuppressive therapies for IBD, including biologics and small molecules, have demonstrated—albeit with a low level of evidence and considerable heterogeneity among the limited studies conducted—a variable association with different malignancies [6]. Cases of renal cell carcinoma in patients with UC are rare [7,8,9] and tend to occur at a younger age, with better survival outcomes irrespective of immunosuppressive therapy use [10].
This report highlights the rare onset, in the context of UC, of renal cancer arising from a simple renal cyst background, developing from a complex cyst in the short-term following immunosuppressive treatment with a biologic agent.
2. Case Presentation
Our research group reports the case of a UC 56-year-old male patient (diagnosis in 2011) who underwent colectomy with ileorectal anastomosis. In December 2022, a complete abdominal ultrasound revealed only bilateral simple renal cysts (maximum diameter of 18 mm in the left kidney) and fatty liver.
Additionally, the patient’s medical history included osteoporosis complicated by vertebral collapse, as well as grade B oesophagitis according to the Los Angeles classification, which was managed with acid-suppressive therapy.
Following surgery, the patient was placed on oral maintenance therapy with mesalamine at a dosage of 2.4 g/day, combined with intermittent cycles of topical mesalamine suppositories. However, adherence to topical therapy was inconsistent due to suboptimal patient compliance. The subsequent development of steroid dependency necessitated consideration of the introduction of biological therapy.
In March 2023, he began treatment with infliximab (at a dose of 5 mg/kg using the standard regimen for UC) due to severe endoscopic activity (Mayo endoscopic score 3) and lack of compliance with further surgery. Before the introduction of infliximab, the patient underwent infectious disease screening, which was negative (i.e., TORCH complex, QuantiFERON for tuberculosis), as well as a complete abdominal ultrasound. The ultrasound revealed a bright liver with a homogeneous hepatic structure and mild splenomegaly and confirmed the presence of simple bilateral renal cysts with a stable overtime maximum diameter of 18 mm in the left kidney.
Seven months later, the patient switched to filgotinib due to a failure to maintain clinical remission (i.e., secondary loss of response).
In April 2024, the patient underwent an endoscopic reassessment for persistent clinical disease activity, which confirmed rectal endoscopic activity (as per Mayo endoscopic score 3). Additional medical tests were conducted during the general reassessment of the patient, summarised in Table A1. The patient also underwent an essential cardiological evaluation with an electrocardiogram, demonstrating a sinus rhythm at a heart rate of 78 bpm (PR interval within normal limits, QRS electrical axis normally oriented, QRS within normal limits, no significant abnormalities in ventricular repolarisation). In addition, the patient underwent a microbiological rectal and pharyngeal-tonsillar swab, both of which resulted negative.
During the same evaluation, an abdominal ultrasound revealed a 45 mm cystic lesion with septa at the lower pole of the right kidney. Upon power Doppler and microvascular blood imaging ultrasound evaluation, the septa appeared vascularised. CEUS evaluation demonstrated hyperenhancement, raising suspicion for cystic renal neoplasia. Further investigation with contrast-enhanced CT of the abdomen (Figure 1) confirmed the presence of a Bosniak IV complex renal cyst.
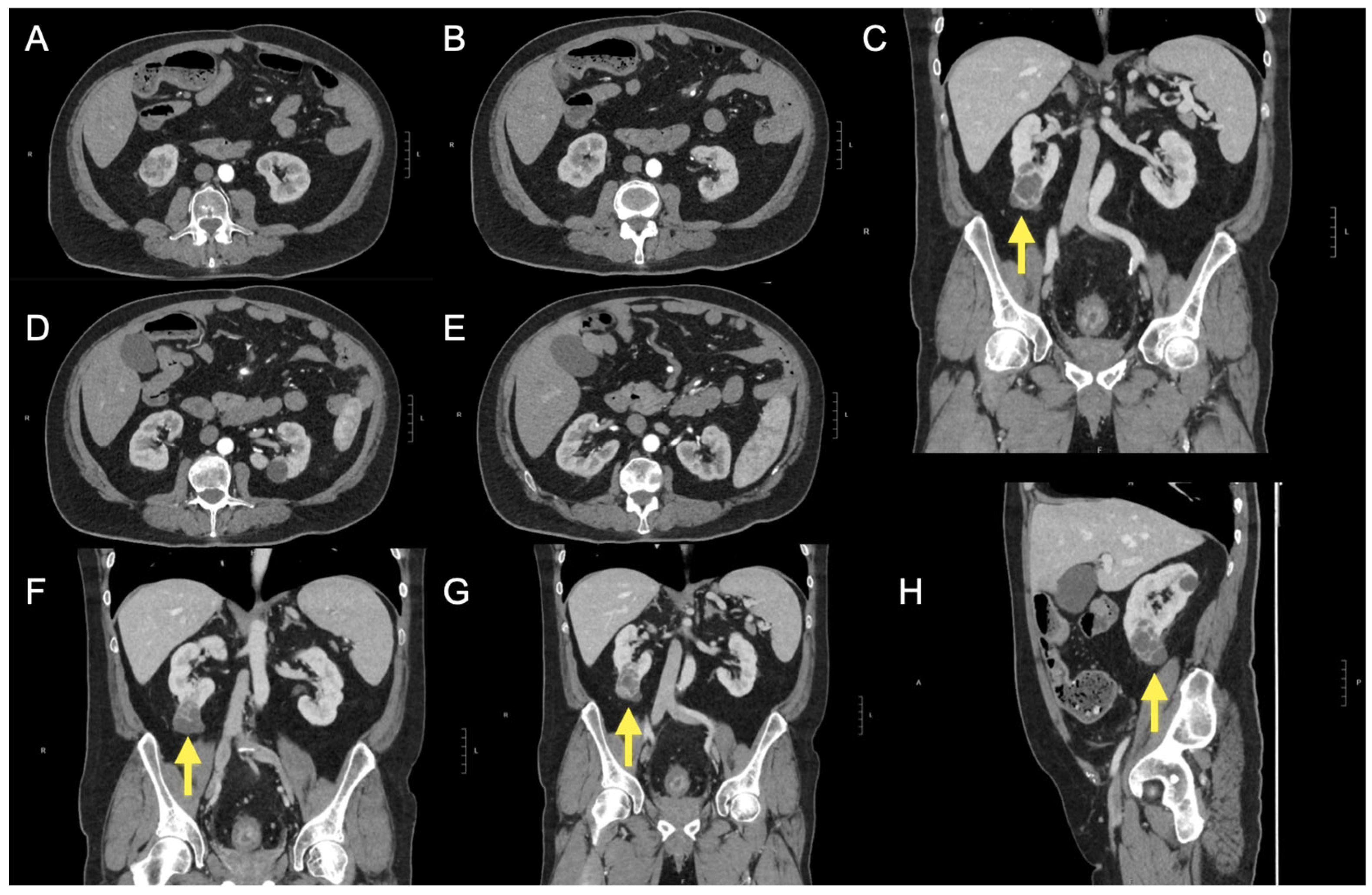
Figure 1.
After the ultrasound findings, a computed tomography (CT) scan was performed (A–H) with and without intravenous contrast administration (iopromide 370 mg/mL). At the lower pole of the right kidney, the scan revealed a complex cystic lesion characterised by multiple thick septa and several small nodular projections (C,F,G,H), which demonstrated contrast enhancement. The lesion measured approximately 38 × 32 × 55 mm (anteroposterior, transverse, and craniocaudal diameters). These findings are consistent with a Bosniak category IV cyst. The lesion did not infiltrate vascular structures or the renal sinus. The left kidney was unremarkable. The excretory pathways were not dilated. Additional simple cortical cysts were noted bilaterally in the kidneys. No significant abnormalities were detected in other abdominal structures, except for two small hypodense lesions located subcapsularly in the liver, at the fifth and anterior fourth segments, likely cystic. The CT scan also demonstrated diffuse and concentric thickening of the rectal walls, indicative of chronic inflammatory changes and a few sub-centimetric mesorectal lymph nodes. The yellow arrow further helps to identify the renal lesion described.
The multidisciplinary evaluation recommended a laparoscopic right nephrectomy, which the patient underwent in June 2024 with an uneventful postoperative course. A histopathological examination revealed a clear cell renal carcinoma with a maximum lesion diameter of 15 mm, graded as G2 according to the WHO/ISUP system. There was no infiltration of the renal sinus fat or renal capsule, corresponding to a pT1a stage according to the 8th edition of the AJCC/TNM classification. Renal function indices were consistently within normal limits before the diagnosis of the complex cyst, and in the reports from our institution since 2022, there has been no flag indicating renal function impairment or alterations in the related haematochemical parameters.
We will evaluate with the oncologist, surgeon, and patient the continuation of therapy for UC, considering surgery or, if declined, continuing with biologics with a top safety profile (e.g., vedolizumab).
3. Discussion
3.1. What are the Possible Considerations in This Case Report?
This clinical case reported the rare onset of a renal neoplasm based on a complex renal cyst that developed over approximately one year in a patient with a previously documented absence of related lesions. The patient had undergone prior treatment with infliximab and was receiving active treatment with filgotinib for ulcerative proctitis with severe endoscopic activity, steroid-dependent, following ileorectal anastomosis and subtotal colectomy. In other terms, this case highlights the potential oncogenic risk of chronic immunosuppression in immune-mediated diseases. It also underscores the value of a non-invasive investigation such as the US, which identified this complex cyst (harbouring clear cell renal carcinoma) that evolved significantly in size and characteristics over a year (on a background of bilateral simple renal cysts).
As previously mentioned, the reported and well-documented cases of renal cancer in patients undergoing immunosuppressive treatment for UC are not numerous. In a series from the 1990s, Satsangi et al. [8] described three cases of renal cancer in patients with an oncological diagnosis occurring between seven and fourteen years after the diagnosis of UC, all of whom had been treated with azathioprine.
Casellas et al. [7] instead described two cases of renal cancer, one in a young 37-year-old patient treated only with oral sulfasalazine and topical steroids and another in a 53-year-old patient treated with steroids and oral sulfasalazine but with a history of chronic renal failure as an underlying condition.
Moreover, cases of acute severe UC have also been described, induced by the concurrent presence of renal cancer, triggering a condition similar to toxic megacolon due to mass effect, as in the report by Kukulska et al. [11]. However, whether the patient described had previously undergone immunosuppressive therapy is not specified.
In addition, beyond UC, Zarur et al. [12] described two cases of renal cancer arising during immunosuppressive therapy for psoriasis. One case involved a 66-year-old patient treated with methotrexate and etanercept who was diagnosed with papillary renal cancer approximately seven months after starting treatment. The other case involved a 41-year-old man treated with methotrexate and infliximab, who was diagnosed with renal cancer about four months after initiating therapy.
3.2. The Risk of Renal Cancer During Immunosuppressive Therapy in IBD
As previously stated, the current evidence does not explicitly demonstrate an increased baseline risk of renal cancer in patients with UC [3,4]. It is therefore plausible that factors related to chronic therapeutic immunomodulation may modify this risk.
Nevertheless, a large meta-analysis of randomised controlled trials has already demonstrated that the use of anti-TNF agents in patients with IBD showed a prevalence of neoplasia of 0.39% among the more than four thousand patients analysed, with a relative risk of cancer compared to placebo of 0.77 (95% CI 0.37–1.59), which was not statistically significant [13]. This finding indicates no association with an increased risk of malignancy, albeit within the limitation of a follow-up period generally confined to approximately one year of treatment [13]. On the other hand, for the filgotinib class (i.e., JAK inhibitors), Olivera et al. [14] conducted a meta-analysis of trials, confirming the absence of an increased cancer risk for this treatment class. This finding was further confirmed in a subsequent meta-analysis [15]. Strong real-world practice data are also available for infliximab, which has been used for decades compared to JAK inhibitors. These data, distinct from those of randomised controlled trials, have confirmed the absence of a clear association between these biological agents and cancer risk [16].
In addition, this report highlights how the diagnosis of neoplasia can occur even after short-term use of immunosuppressants, whereas in other cases, tumours have been reported only after several years of use [17,18]. However, albeit rare, cases have also been documented after just a few months [19].
Some evidence can also be extrapolated from settings not explicitly related to IBD. Ilham et al. [20] also conducted a large retrospective cohort study on over thirteen thousand patients, including recipients of solid organ transplants or hematopoietic stem cell transplants, patients diagnosed with primary or secondary immunodeficiency disorders, and those receiving anti-TNF agents (the latter constituting 24% of the sample). In this large cohort, they identified a cumulative cancer incidence of 11.5% (specifically 8.8% in the group treated with anti-TNF agents). Interestingly, patients with renal cysts (HR 2.54) had an increased risk of developing cancer in general compared to those without. Moreover, having a renal cyst can increase the overall risk of renal cell carcinoma [21,22]. Nonetheless, when examining cohort studies in populations of solid organ transplant recipients, such as the US Scientific Registry of Transplant Recipients (1987–2008) involving over 170,000 patients, a cancer incidence of 1375 per 100,000 person-years was identified, with a specific incidence for kidney cancer of 97 per 100,000 person-years [23].
3.3. Case Report Limitations
By its very nature, this report is limited by the description of a single case, which is a consequence of the condition’s rarity, making it challenging to organise larger-scale studies with a greater sample size. Furthermore, no specific molecular assessments were conducted on the tumour regarding the signalling pathways related to filgotinib and infliximab. However, this is understandable given the observational nature of the report and the fact that the procedures performed were those pertaining to standard clinical practice. Clearly, this report cannot demonstrate a direct causal link between the administered drugs and the onset of cancer but merely describes an epidemiological event that occurred.
4. Conclusions
In conclusion, all these cumulative data demonstrate the rarity of a malignant renal neoplasm diagnosis in the context of IBD treated with biological agents or small molecules. However, although rare and isolated, cases can still occur; this underscores the importance of implementing long-term monitoring for these patients using basic, non-invasive, cost-effective, and repeatable tools, such as ultrasound examinations. These provide a broader abdominal overview, not solely focused on the gastrointestinal tract, as with endoscopy and intestinal radiology techniques. Especially in the current context, where intestinal ultrasound also provides the opportunity to assess the small intestine and colon effectively, and now, with transperineal ultrasound, even the rectum [24,25,26,27].
Author Contributions
Conceptualization, R.P. and A.G.G.; methodology, R.P. and A.G.G.; validation, R.P., A.G.G., and A.F.; investigation, R.P., G.I., M.I., I.D.C., F.L., P.C., M.N., A.G.G., and A.F.; data curation, R.P., G.I., M.I., I.D.C., F.L., P.C., M.N., A.G.G., and A.F.; writing—original draft preparation, R.P. and A.G.G.; writing—review and editing, R.P., G.I., M.I., I.D.C., F.L., P.C., M.N., A.G.G., and A.F.; visualization, R.P. and A.G.G.; supervision, R.P., A.G.G., and A.F.; project administration, R.P., A.G.G., and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki; ethical review and approval were waived for this study, due to retrospective, deidentified data.
Informed Consent Statement
Patient consent was waived due to the procedures described in this case report are retrospective. In accordance with the Italian Medicines Agency determination of 20 March 2008, no informed consent is necessary since the data are not “experimental” but “observational”. In other words, everything described in the case report falls within standard clinical practice and does not require additional consent. In addition, all data were collected with strict anonymity, analysed in strictly aggregated form, and no data can be directly traced back to the subjects included in the study records.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Haematochemical evaluations of the patient related to the reassessment in April 2024.
Table A1.
Haematochemical evaluations of the patient related to the reassessment in April 2024.
| Parameter | Value | Normal Values | Unit of Measurement |
|---|---|---|---|
| WBC | 6.28 | 4–11 | 103/µL |
| RBC | 4.61 | 4.5–6 | 106/µL |
| RET | 0.17 | 0.025–0.075 | 106/µL |
| HGB | 7.9 | 13–17.5 | g/dL |
| HCT | 29.8 | 42–51 | % |
| MCV | 64.6 | 82–96 | fL |
| MCH | 17.1 | 27–31 | pg |
| RDW-SD | 43.7 | 36–43 | fL |
| PLT | 384 | 150–450 | 103/µL |
| PT | 10.6 | 9–13 | s |
| INR | 0.92 | 0.8–1.2 | N.A. |
| aPTT | 27.8 | 24–38 | s |
| Fibrinogen | 484 | 200–450 | mg/dL |
| Blood glucose | 92 | 70–100 | mg/dL |
| Urea | 37 | 10–50 | mg/dL |
| Creatinine | 0.84 | 0.67–1.17 | mg/dL |
| Uric acid | 4.6 | 3.4–7 | mg/dL |
| Na+ | 141 | 135–146 | mEq/L |
| K+ | 4.2 | 3.5–5.3 | mEq/L |
| Cl- | 108 | 98–111 | mEq/L |
| Mg2+ | 1.9 | 1.6–2.6 | mg/dL |
| Ca2+ | 9 | 8.6–10.2 | mg/dL |
| ALT | 15 | 5–41 | U/L |
| AST | 16 | 5–40 | U/L |
| Amylase | 94 | 28–100 | U/L |
| Lipase | 34 | 13–78 | U/L |
| Alkaline phosphatase | 58 | 40–129 | U/L |
| CPK | 51 | 60–190 | U/L |
| LDH | 153 | 120–240 | U/L |
| CRP | 1.9 | 0–1 | mg/dL |
| Cholinesterase | 8241 | 5320–12,920 | U/L |
| Total bilirubin | 0.36 | <1.2 | mg/dL |
| Conjugated bilirubin | 0.14 | <0.3 | mg/dL |
| Unconjugated bilirubin | 0.22 | <0.75 | mg/dL |
| Total proteins | 6.9 | 6.6–8.7 | g/dL |
| Albumin | 3.9 | 3.5–5.5 | g/dL |
| Serum iron | 14 | 59–158 | µg/dL |
| Ferritin | 2 | 30–400 | ng/dL |
| Transferrin | 315 | 191–337 | mg/dL |
| ESR | 34 | 2–15 | mm/h |
| IgA | 313 | 70–400 | mg/dL |
| IgG | 1210 | 700–1600 | mg/dL |
| IgM | 138 | 40–230 | mg/dL |
| Anti-endomysial IgA | Absent | Absent | - |
| IgA anti-Tg | 3.2 | <20 | U/mL |
| IgG anti-Tg | 3.2 | <9 | U/mL |
| HBsAg | 0.03 | <0.05 | IU/mL |
| HBsAb | <3 | >9 | mUI/mL |
| HBcAb | Absent | Absent | - |
| HBeAb | Absent | Absent | - |
| HCVAb | Absent | Absent | - |
| Vitamin B12 | 275 | 187–883 | pg/mL |
| HDL | 30 | >35 | mg/dL |
| LDL | 94 | 10–129 | mg/dL |
| Total cholesterol | 164 | 60–200 | mg/dL |
| QuantiFERON-TB Gold plus test for Mycobacterium tuberculosis: IFN-γ after stimulation with TB-specific antigens TB1 (CD4) | 0 | <0.35 | UI/mL |
| QuantiFERON-TB Gold plus test for Mycobacterium tuberculosis: IFN-γ after stimulation with TB-specific antigens TB2 (CD4/CD8) | 0 | <0.35 | UI/mL |
| QuantiFERON-TB Gold plus test for Mycobacterium tuberculosis: IFN-γ after stimulation with mitogen | 9.9 | ≥0.5 | UI/mL |
Note: WBC: white blood cells; RBC: red blood cells; RET: reticulocytes; HGB: haemoglobin; HCT: haematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular haemoglobin; RDW-SD: red cell distribution width—standard deviation; PLT: platelets; PT: prothrombin time; INR: international normalised ratio; aPTT: activated partial thromboplastin time; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CPK: creatine phosphokinase; LDH: lactate dehydrogenase; CRP: C-reactive protein; ESR: erythrocyte sedimentation Rate; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; Tg: transglutaminase; HBsAg: hepatitis B surface antigen; HBsAb: hepatitis B surface antibody; HBcAb: hepatitis B core antibody; HBeAb: hepatitis B envelope antibody; HCVAb: hepatitis C virus antibodies; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Bai, Y.; Liu, S.; Yang, Y.; Han, P.; Wei, W. Risk of Renal Cancer in Patients with Inflammatory Bowel Disease: A Pooled Analysis of Population-Based Studies. Urol. Oncol. 2021, 39, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Hassan, C.; Repici, A.; Rimassa, L.; Carlo-Stella, C.; Nikolopoulos, G.K.; Riboli, E.; Bonovas, S. Risk of Cancer in Inflammatory Bowel Diseases: Umbrella Review and Reanalysis of Meta-Analyses. Gastroenterology 2022, 163, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zheng, S.; Yuan, X.; Xie, J.; Xu, L. Causal Association between Inflammatory Bowel Disease and 32 Site-Specific Extracolonic Cancers: A Mendelian Randomization Study. BMC Med. 2023, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Sardi, J.; Malagelada, J.R. [Renal cell carcinoma and ulcerative colitis]. Rev. Esp. Enferm. Dig. 2002, 94, 107–108. [Google Scholar] [PubMed]
- Satsangi, J.; Marshall, J.; Roskell, D.; Jewell, D. Ulcerative Colitis Complicated by Renal Cell Carcinoma: A Series of Three Patients. Gut 1996, 38, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Plaisier, P.W. Ulcerative Colitis and Renal Cell Carcinoma. Gut 1996, 38, 936. [Google Scholar] [CrossRef] [PubMed]
- Derikx, L.A.A.P.; Nissen, L.H.C.; Drenth, J.P.H.; van Herpen, C.M.; Kievit, W.; Verhoeven, R.H.A.; Mulders, P.F.A.; Hulsbergen-van de Kaa, C.A.; Boers-Sonderen, M.J.; van den Heuvel, T.R.A.; et al. Better Survival of Renal Cell Carcinoma in Patients with Inflammatory Bowel Disease. Oncotarget 2015, 6, 38336–38347. [Google Scholar] [CrossRef] [PubMed]
- Kukulska, M.; Smola, I.; Halon, A.; Paradowski, L.; Poniewierka, E.; Kempinski, R.; Annabhani, A. A Case of Severe Ulcerative Colitis with Colonic Dilatation Caused by Renal Mucinous Tubular and Spindle Cell Carcinoma. Euroasian J. Hepatogastroenterol. 2016, 6, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Zarur, F.P.; d’Almeida, L.F.V.; Mafort, M.S.P.; de Gusmão, P.R.; Avelleira, J.C.R. Two Cases of Renal Cell Cancer during Immunobiologic Therapy for Psoriasis. An. Bras. Dermatol. 2014, 89, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.M.; Peyrin-Biroulet, L.; Ford, A.C. Systematic Review with Meta-Analysis: Malignancies with Anti-Tumour Necrosis Factor-α Therapy in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2014, 39, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, C.; Ding, H.; Mei, Q. Small Molecules for Inflammatory Bowel Disease and the Risk of Infection and Malignancy: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2024, 56, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. TNF Inhibitors and Risk of Malignancy in Patients with Inflammatory Bowel Diseases: A Systematic Review. J. Crohns Colitis 2021, 15, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Baek, S.K.; Yoon, H.-J.; Kim, S.-Y.; Maeng, C.H.; Park, T.S.; Kim, H.-J.; Choi, Y.Y.; Um, Y.J. Thyroid Cancer and T Lymphoblastic Leukemia in Crohn Disease: A Case Report and Literature Review. Lab. Med. 2015, 46, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Palladino, G.; Pagliuca, F.; Lucà, S.; Federico, A.; Gravina, A.G. Cutaneous Kaposi’s Sarcoma Following Long-Term Infliximab Treatment in a Patient with HIV-Negative Antibiotic-Dependent Chronic Pouchitis: Considerations on an Exceptional Finding. Gastrointest. Disord. 2024, 6, 984–992. [Google Scholar] [CrossRef]
- Goral, V.; Unsal, B.; Nermin Sivrikoz, O. A Case of Breast Cancer Following Infliximab Treatment for Treatment-Refractory Crohn’s Disease. Euroasian J. Hepatogastroenterol. 2014, 4, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Ilham, S.; Willis, C.; Kim, K.; Chung, K.C.; Wood, B.M.; Tan, M.S.; Tan, C.J.; Nguyen, D.T.; Brixner, D.I.; Stenehjem, D.D. Cancer Incidence in Immunocompromised Patients: A Single-Center Cohort Study. BMC Cancer 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.; Vathsala, A. Native Renal Cysts and Dialysis Duration Are Risk Factors for Renal Cell Carcinoma in Renal Transplant Recipients. Am. J. Transplant. 2011, 11, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hurst, F.P.; Jindal, R.M.; Fletcher, J.J.; Dharnidharka, V.; Gorman, G.; Lechner, B.; Nee, R.; Agodoa, L.Y.; Abbott, K.C. Incidence, Predictors and Associated Outcomes of Renal Cell Carcinoma in Long-Term Dialysis Patients. Urology 2011, 77, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk among US Solid Organ Transplant Recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Ilvemark, J.F.K.F.; Hansen, T.; Goodsall, T.M.; Seidelin, J.B.; Al-Farhan, H.; Allocca, M.; Begun, J.; Bryant, R.V.; Carter, D.; Christensen, B.; et al. Defining Transabdominal Intestinal Ultrasound Treatment Response and Remission in Inflammatory Bowel Disease: Systematic Review and Expert Consensus Statement. J. Crohns Colitis 2022, 16, 554–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, W.; Wang, L.; Mao, X.; Ye, Z.; Zhang, H. Intestinal Ultrasound for Differentiating Fibrotic or Inflammatory Stenosis in Crohn’s Disease: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2022, 16, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Sagami, S.; Kobayashi, T.; Aihara, K.; Umeda, M.; Morikubo, H.; Matsubayashi, M.; Kiyohara, H.; Nakano, M.; Ohbu, M.; Hibi, T. Transperineal Ultrasound Predicts Endoscopic and Histological Healing in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2020, 51, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.; Burr, N.E.; Mohammed, N.; Subramanian, V. Colonic Lesion Characterization in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2018, 24, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).