Surgical Site Infection After Breast Surgery—A Bicentric Retrospective Case–Control Study in Saudi Arabia
Abstract
1. Background
2. Methods
2.1. Study Design and Setting
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Size and Propensity Score Matching
2.5. Data Collection
2.6. Microbiological Assessment
2.7. SSI Definition
2.8. Perioperative Antibiotic Prophylaxis
2.9. Statistical Analysis
3. Results
3.1. Study Population
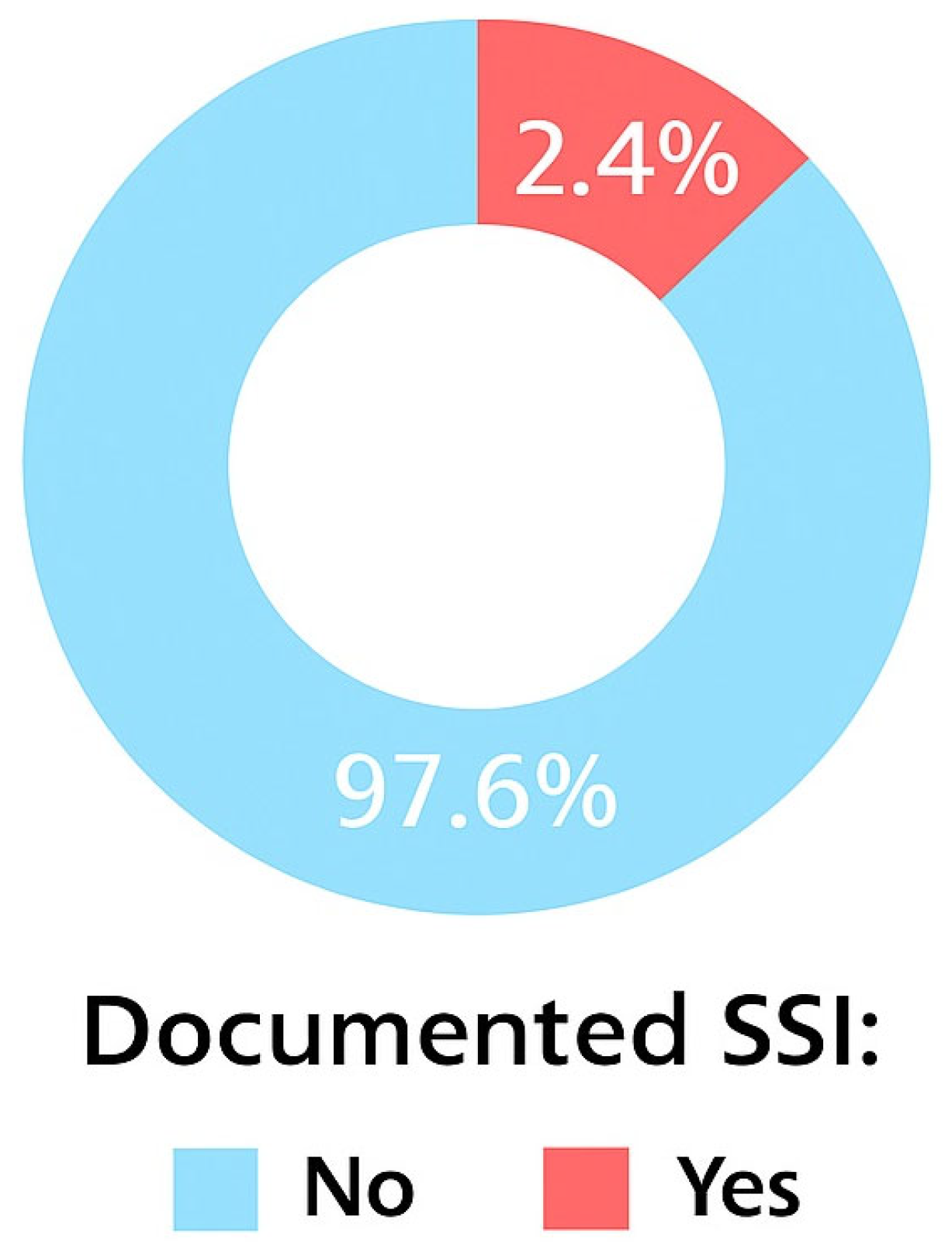
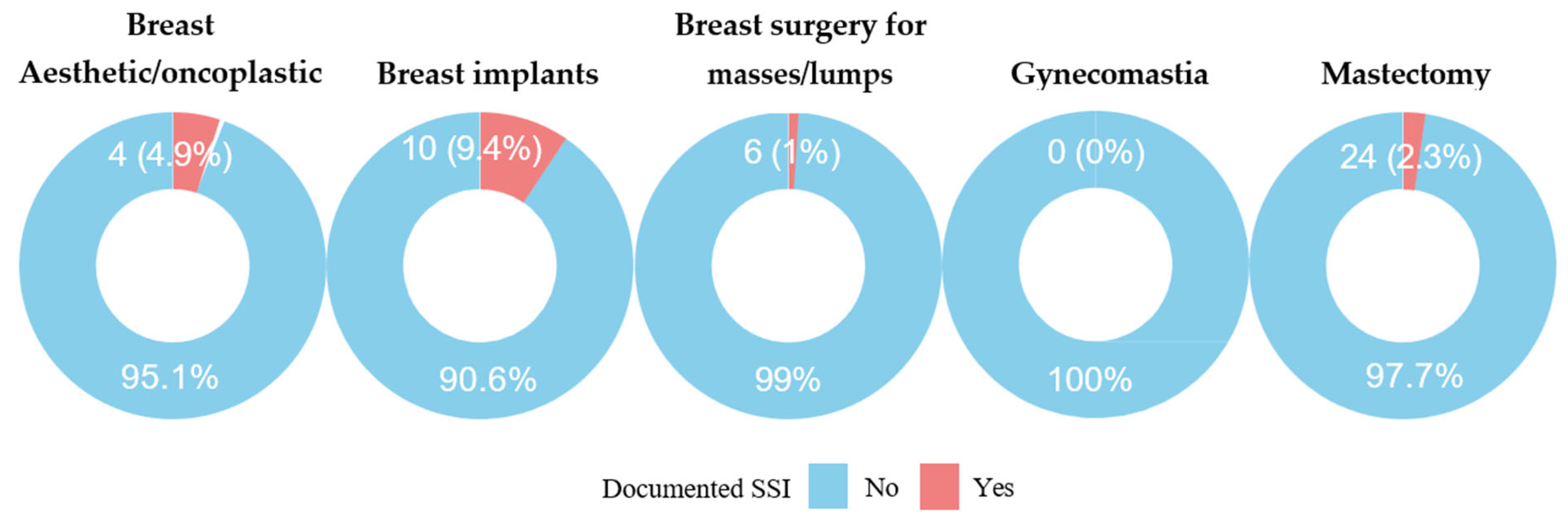
3.2. Incidence of SSI
3.3. Matched Propensity Score Analysis of Factors Associated with SSIs
3.4. Microbiology of SSIs
3.5. Postoperative Onset of SSIs
3.6. Duration of Surgical Drain in Place
3.7. Outcome of SSIs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Boermeester, M.A. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Piednoir, E.; Robert-Yap, J.; Baillet, P.; Lermite, E.; Christou, N. The socioeconomic impact of surgical site infections. Front. Public Health 2021, 9, 712461. [Google Scholar] [CrossRef]
- Nimkar, P.; Kanyal, D. Understanding the financial burden of surgical site infections: A narrative review. Multidiscip. Rev. 2025, 8, 2025084. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Monahan, M.; Jowett, S.; Pinkney, T.; Brocklehurst, P.; Morton, D.G.; Abdali, Z.; Roberts, T.E. Surgical site infection and costs in low- and middle-income countries: A systematic review of the economic burden. PLoS ONE 2020, 15, e0232960. [Google Scholar] [CrossRef]
- Urquhart, J.C.; Gurr, K.R.; Siddiqi, F.; Rasoulinejad, P.; Bailey, C.S. The impact of surgical site infection on patient outcomes after open posterior instrumented thoracolumbar surgery for degenerative disorders. J. Bone Jt. Surg.–Am. Vol. 2021, 103, 2105–2114. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention. NHSN Patient Safety Component Manual: Validation 2023; U.S. Department of Health and Human Services: Washington, DC, USA, 2023. Available online: https://www.cdc.gov/nhsn/pdfs/validation/2023/pcsmanual_2023.pdf (accessed on 7 December 2024).
- Lilani, S.P.; Jangale, N.; Chowdhary, A.; Daver, G.B. Surgical site infection in clean and clean contaminated cases. Indian J. Med. Microbiol. 2005, 23, 249–252. [Google Scholar] [CrossRef]
- National Nosocomial Infections Surveillance System. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2004. Am. J. Infect. Control 2004, 32, 470–485. [Google Scholar] [CrossRef]
- Gaynes, R.P.; Culver, D.H.; Horan, T.C.; Edwards, J.R.; Richards, C.; Tolson, J.S. Surgical site infections (SSI) rates in the United States, 1992–1998: The National Nosocomial Infections Surveillance System basic SSI risk index. Clin. Infect. Dis. 2001, 33 (Suppl. 2), S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C.; Hall, J.L. Antibiotic prophylaxis for patients undergoing breast surgery. J. Hosp. Infect. 2000, 46, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Canavese, G.; Catturich, A.; Vecchio, C.; Bruzzi, P.; Tomei, D.; Giannotti, A. Surgical complications related to peri-operative adjuvant chemotherapy in breast cancer: Results of a prospective, controlled, randomized clinical trial. Eur. J. Surg. Oncol. 1997, 23, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, J.; Sabbagh, M.D.; Roh, S.-G.; Nguyen, M.-D.T.; Lemaine, V.; Tran, N.V.; Jacobson, S.R.; Boughey, J.C.; Jakub, J.W.; Hieken, T.J.; et al. Infections following immediate implant-based breast reconstruction: A case-control study over 11 years. Plast. Reconstr. Surg. 2019, 144, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.Q.; Qian, C.; Yang, L.; Wang, X.F. Risk factors for surgical site infections after breast surgery: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2012, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ding, S.; Li, J.; Liao, X.; Ru, K.; Liu, L.; Shang, W. Diagnostic value of inflammatory indicators for surgical site infection in patients with breast cancer. Front. Cell. Infect. Microbiol. 2023, 13, 1286313. [Google Scholar] [CrossRef]
- Vilar-Compte, D.; Jacquemin, B.; Robles-Vidal, C.; Volkow, P. Surgical site infections in breast surgery: Case control study. World J. Surg. 2004, 28, 242–246. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Pastoriza, J.; McNelis, J.; Parsikia, A.; Lewis, E.; Ward, M.; Marini, C.P.; Castaldi, M.T. Predictive factors for surgical site infections in patients undergoing surgery for breast carcinoma. Am. Surg. 2021, 87, 68–76. [Google Scholar] [CrossRef]
- Palubicka, A.; Jaworski, R.; Wekwejt, M.; Swieczko-Zurek, B.; Pikula, M.; Jaskiewicz, J.; Zielinski, J. Surgical site infection after breast surgery: A retrospective analysis of 5-year postoperative data from a single center in Poland. Medicina 2019, 55, 512. [Google Scholar] [CrossRef]
- Olsen, M.A.; Nickel, K.B.; Fox, I.K.; Margenthaler, J.A.; Ball, K.E.; Mines, D.; Wallace, A.E.; Fraser, V.J. Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect. Control Hosp. Epidemiol. 2015, 36, 907–914. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2025. Available online: https://www.R-project.org (accessed on 10 May 2025).
- Di Napoli, A.; Pepe, G.; Giarnieri, E.; Cippitelli, C.; Bonifacino, A.; Mattei, M.; Martelli, M.; Falasca, C.; Cox, M.C.; Santino, I.; et al. Cytological diagnostic features of late breast implant seromas: From reactive to anaplastic large cell lymphoma. PLoS ONE 2017, 12, e0181097. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Throckmorton, A.D.; Alvarado, M.D.; Ewing, C.A.; Lehman, C.D.; Esserman, L.J. Bacteriologic features of surgical site infections following breast surgery. Am. J. Surg. 2009, 198, 529–531. [Google Scholar] [CrossRef]
- Cohen, J.B.; Carroll, C.; Tenenbaum, M.M.; Myckatyn, T.M. Breast implant–associated infections: The role of the National Surgical Quality Improvement Program and the local microbiome. Plast. Reconstr. Surg. 2015, 136, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Washer, L.L.; Gutowski, K. Breast implant infections. Infect. Dis. Clin. N. Am. 2012, 26, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Schlager, J.G.; Hartmann, D.; Wallmichrath, J.; Ruiz San Jose, V.; Patzer, K.; French, L.E.; Kendziora, B. Patient--dependent risk factors for wound infection after skin surgery: A systematic review and meta-analysis. Int. Wound J. 2022, 19, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Xu, L.; Ye, J.M.; Wang, D.M.; Zhao, J.X.; Zhang, L.B.; Duan, X.N.; Liu, Y.H. Analysis of risk factors of surgical site infections in breast cancer. Chin. Med. J. 2010, 123, 559–562. [Google Scholar] [CrossRef]
- Vilar-Compte, D.; Rosales, S.; Hernandez-Mello, N.; Maafs, E.; Volkow, P. Surveillance, control, and prevention of surgical site infections in breast cancer surgery: A 5-year experience. Am. J. Infect. Control 2009, 37, 674–679. [Google Scholar] [CrossRef]
- Ruvalcaba-Limón, E.; Robles-Vidal, C.; Poitevin-Chacón, A.; Chávez-MacGregor, M.; Gamboa-Vignolle, C.; Vilar-Compte, D. Complications after breast cancer surgery in patients treated with concomitant preoperative chemoradiation: A case–control analysis. Breast Cancer Res. Treat. 2006, 95, 147–152. [Google Scholar] [CrossRef]
- Di Pompeo, F.S.; Firmani, G.; Paolini, G.; Amorosi, V.; Briganti, F.; Sorotos, M. Immediate prepectoral breast reconstruction using an ADM with smooth round implants: A prospective observational cohort study. J. Plast. Reconstr. Aesthetic Surg. 2023, 80, 56–65. [Google Scholar] [CrossRef]
- Gupta, R.; Pate, K.; Varshney, S.; Goddard, J.; Royle, G.T. A comparison of 5-day and 8-day drainage following mastectomy and axillary clearance. Eur. J. Surg. Oncol. 2001, 27, 26–30. [Google Scholar] [CrossRef]
- Purushotham, A.D.; McLatchie, E.; Young, D.; George, W.D.; Stallard, S.; Doughty, J.; Brown, D.C.; Farish, C.; Walker, A.; Millar, K.; et al. Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer. Br. J. Surg. 2002, 89, 286–292. [Google Scholar] [CrossRef]
- Talbot, M.L.; Magarey, C.J. Reduced use of drains following axillary lymphadenectomy for breast cancer. ANZ J. Surg. 2002, 72, 488–490. [Google Scholar] [CrossRef]
- Francis, S.H.; Ruberg, R.L.; Stevenson, K.B.; Beck, C.E.; Ruppert, A.S.; Harper, J.T.; Boehmler, J.H.; Miller, M.J. Independent risk factors for infection in tissue expander breast reconstruction. Plast. Reconstr. Surg. 2009, 124, 1790–1796. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Smoking in the Kingdom of Saudi Arabia: Findings from the Saudi Health Interview Survey. 2014. Available online: https://www.healthdata.org/sites/default/files/files/Projects/KSA/Smoking-KSA-Findings-from-the-Saudi-Health-Interview-Survey.pdf (accessed on 10 May 2025).
- Papa, G.; Frasca, A.; Renzi, N.; Stocco, C.; Pizzolato, G.; Ramella, V.; Arnež, Z.M. Protocol for Prevention and Monitoring of Surgical Site Infections in Implant-Based Breast Reconstruction: Preliminary Results. Medicina 2021, 57, 151. [Google Scholar] [CrossRef]
| Case, n = 36 1 | Control, n = 136 1 | Difference 2 | |
|---|---|---|---|
| Patient age at surgery, years | 53 (45, 63) | 55 (45, 63) | −0.04 |
| BMI, kg/m2 | 32.3 (24.8, 36.0) | 29.9 (26.7, 34.6) | 0.19 |
| Hospital | 0.01 | ||
| MADINAH | 6 (17%) | 22 (16%) | |
| RIYADH | 30 (83%) | 114 (84%) | |
| Diabetic | 12 (33.3%) | 33 (24.4%) | 0.09 |
| ASA score | 0.04 | ||
| 1 | 0 (0%) | 4 (2.9%) | |
| 2 | 17 (47%) | 59 (43%) | |
| 3 | 19 (53%) | 73 (54%) | |
| NHSN Index | 0.25 | ||
| Clean | 14 (39%) | 58 (43%) | |
| Clean-Contaminated | 22 (61%) | 78 (57%) | |
| Type of breast surgery | 1.6 | ||
| Breast aesthetic or oncoplastic surgeries | 4 (11%) | 3 (2.2%) | |
| Breast implants | 7 (19%) | 36 (26%) | |
| Breast surgery for masses/lumps | 4 (11%) | 85 (63%) | |
| Mastectomy | 21 (58%) | 12 (8.8%) | |
| Use of surgical drain | 28 (77.8%) | 56 (41.2%) | 0.37 |
| Surgery duration, minutes | 126 (96, 178) | 97 (70, 148) | 0.50 |
| Characteristic | n | OR | 95% CI | p Value |
|---|---|---|---|---|
| Body mass index | 172 | 1.03 | 0.98, 1.08 | 0.30 |
| ASA score | 172 | 1.07 | 0.55, 2.15 | 0.84 |
| Diabetic | 171 | — | — | — |
| No | — | — | — | |
| Yes | 1.55 | 0.68, 3.39 | 0.28 | |
| Malignant tumor | 172 | — | — | — |
| No | — | — | — | |
| Yes | 2.14 | 1.00, 4.86 | 0.057 | |
| Immunocompromised | 172 | — | — | — |
| No | — | — | — | |
| Yes | 3.95 | 1.81, 9.21 | <0.001 | |
| MRSA infection or colonization before surgery | 172 | — | — | — |
| No | — | — | — | |
| Unknown | 1.19 | 0.51, 3.03 | 0.70 | |
| Yes | 9.50 | 1.58, 77.8 | 0.018 | |
| Surgery duration (minutes) | 172 | 1.01 | 1.00, 1.01 | 0.007 |
| Use of surgical drain | 172 | — | — | — |
| No | — | — | — | |
| Yes | 5.00 | 2.21, 12.5 | <0.001 |
| Factor | OR | 95% CI | p Value |
|---|---|---|---|
| Immunocompromised | |||
| No | - | - | |
| Yes | 3.32 | 1.35, 8.14 | 0.009 |
| Use of surgical drain | |||
| No | - | - | |
| Yes | 4.07 | 1.68, 9.87 | 0.002 |
| Microbiological Results | Number | Percentage |
|---|---|---|
| Corynebacterium spp. | 1 | 2.8% |
| Gram-positive cocci | 1 | 2.8% |
| Streptococcus agalactiae (Group B) | 1 | 2.8% |
| Mixed Gram-positive and mixed Gram-negative organisms | 2 | 5.6% |
| Serratia marcescens | 2 | 5.6% |
| Enterobacter cloacae | 3 | 8.3% |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 4 | 11% |
| Pseudomonas aeruginosa | 4 | 11% |
| Klebsiella pneumoniae | 5 | 14% |
| Staphylococcus aureus | 5 | 14% |
| No organism | 8 | 22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSaadi, M.; Alghamdi, S.; Mazari, F.; Alshuhri, S.; Bashtawi, R.; Aljehani, R.; Alwuqaisi, B.; Almohammadi, R.; Alfirikh, M.; Desai, S.; et al. Surgical Site Infection After Breast Surgery—A Bicentric Retrospective Case–Control Study in Saudi Arabia. Clin. Pract. 2025, 15, 231. https://doi.org/10.3390/clinpract15120231
AlSaadi M, Alghamdi S, Mazari F, Alshuhri S, Bashtawi R, Aljehani R, Alwuqaisi B, Almohammadi R, Alfirikh M, Desai S, et al. Surgical Site Infection After Breast Surgery—A Bicentric Retrospective Case–Control Study in Saudi Arabia. Clinics and Practice. 2025; 15(12):231. https://doi.org/10.3390/clinpract15120231
Chicago/Turabian StyleAlSaadi, Moteb, Salem Alghamdi, Fayyaz Mazari, Sabah Alshuhri, Rustom Bashtawi, Raghad Aljehani, Basmah Alwuqaisi, Rawan Almohammadi, Mahmoud Alfirikh, Sameer Desai, and et al. 2025. "Surgical Site Infection After Breast Surgery—A Bicentric Retrospective Case–Control Study in Saudi Arabia" Clinics and Practice 15, no. 12: 231. https://doi.org/10.3390/clinpract15120231
APA StyleAlSaadi, M., Alghamdi, S., Mazari, F., Alshuhri, S., Bashtawi, R., Aljehani, R., Alwuqaisi, B., Almohammadi, R., Alfirikh, M., Desai, S., & Mahmoud, E. (2025). Surgical Site Infection After Breast Surgery—A Bicentric Retrospective Case–Control Study in Saudi Arabia. Clinics and Practice, 15(12), 231. https://doi.org/10.3390/clinpract15120231






