Outcomes Following Surgery for Pancreatic Neuro-Endocrine Tumours: A Single-Centre Experience
Abstract
1. Introduction
2. Methods
2.1. Study Design, Ethics Approval and Protocol
2.2. Clinical Data
2.3. Outcome Variables
3. Statistical Analysis
4. Results
4.1. Patients’ Demographics and Pathological Features
4.2. Surgery and Postoperative Morbidity and Mortality
4.3. Histopathological Analysis
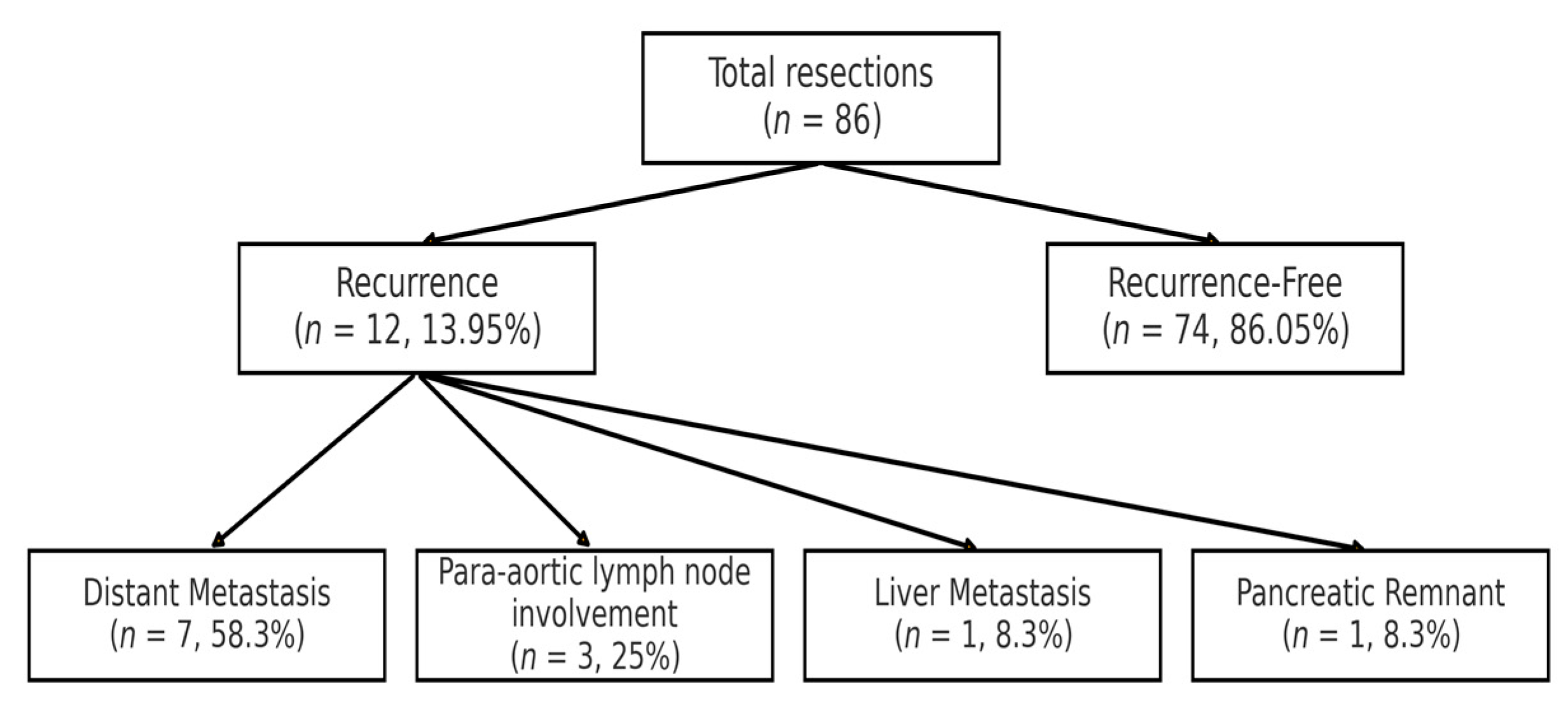
4.4. Recurrence
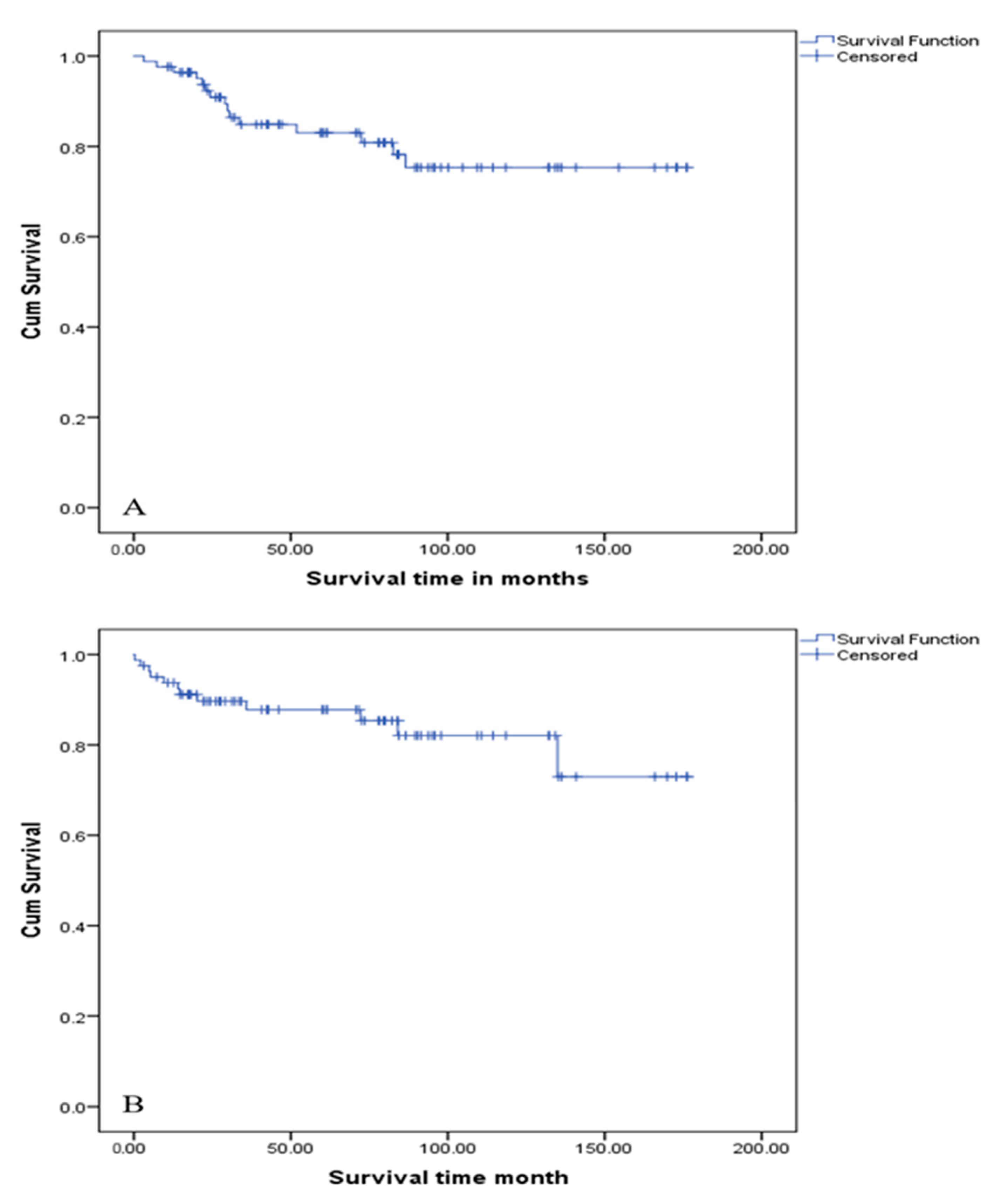
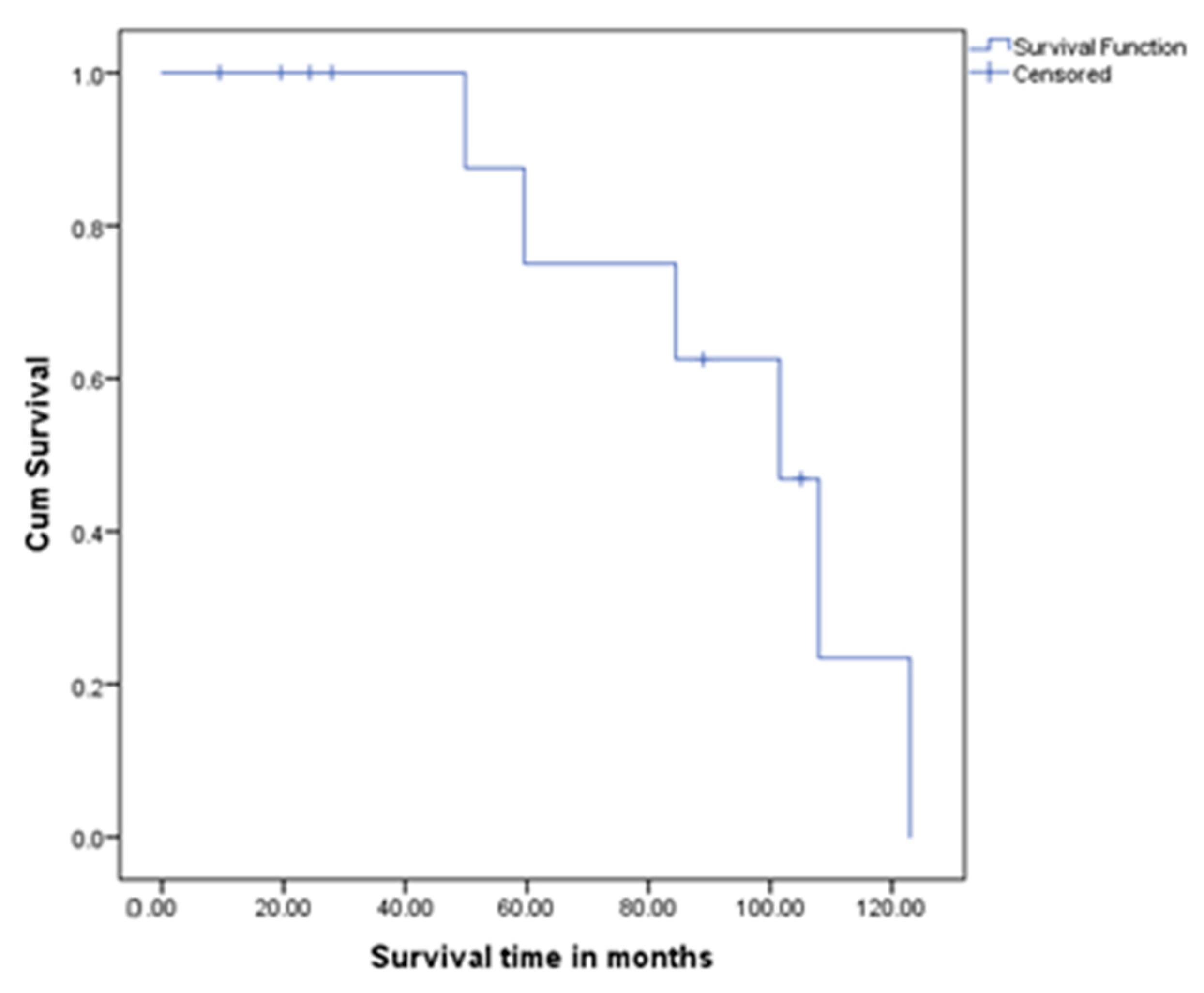
4.5. Survival Outcomes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18, vii. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Öberg, K.; Knigge, U.; Kwekkeboom, D.; Perren, A.; ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii124–vii130. [Google Scholar] [CrossRef]
- Öberg, K. Management of functional neuroendocrine tumors of the pancreas. Gland Surg. 2018, 7, 20–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef]
- Yang, M.; Zeng, L.; Ke, N.-W.; Tan, C.-L.; Tian, B.-L.; Liu, X.-B.; Xiang, B.; Zhang, Y. World Health Organization grading classification for pancreatic neuroendocrine neoplasms: A comprehensive analysis from a large Chinese institution. BMC Cancer 2020, 20, 906. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, L.; Wang, W.; Xu, H.; Jin, K.; Wu, C.; Qi, Z.; Zhang, S.; Liu, C.; Xu, J.; et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018, 412, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Dseagu, V.L.; Devesa, S.S.; Goggins, M.; Stolzenberg-Solomon, R. Pancreatic cancer incidence trends: Evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int. J. Epidemiol. 2018, 47, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, P.; Zakalik, D. Comparison of Demographics, Tumor Characteristics, and Survival Between Pancreatic Adenocarcinomas and Pancreatic Neuroendocrine Tumors: A Population-based Study. Am. J. Clin. Oncol. 2018, 41, 485–491. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Pea, A.; Chang, D.K.; Jamieson, N.B. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front. Med. 2020, 7, 385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Titan, A.L.; Norton, J.A.; Fisher, A.T.; Foster, D.S.; Harris, E.J.; Worhunsky, D.J.; Worth, P.J.; Dua, M.M.; Visser, B.C.; Poultsides, G.A.; et al. Evaluation of Outcomes Following Surgery for Locally Advanced Pancreatic Neuroendocrine Tumors. JAMA Netw. Open 2020, 3, e2024318. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, L.; Liu, X.; Tan, C.; Wang, X. Prognosis of small pancreatic neuroendocrine neoplasms: Functionality matters. Am. J. Surg. 2025, 246, 116302. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska, T.; Karczmarski, J.; Paziewska, A.; Kulecka, M.; Kuśnierz, K.; Żeber-Lubecka, N.; Ambrożkiewicz, F.; Mikula, M.; Kos-Kudła, B.; Ostrowski, J. Differences between Well-Differentiated Neuroendocrine Tumors and Ductal Adenocarcinomas of the Pancreas Assessed by Multi-Omics Profiling. Int. J. Mol. Sci. 2020, 21, 4470. [Google Scholar] [CrossRef]
- Kuo, E.J.; Salem, R.R. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann. Surg. Oncol. 2013, 20, 2815–2821. [Google Scholar] [CrossRef]
- Sadot, E.; Reidy-Lagunes, D.L.; Tang, L.H.; Do, R.K.G.; Gonen, M.; D’aNgelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Koerkamp, B.G.; Untch, B.R.; et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann. Surg. Oncol. 2016, 23, 1361–1370. [Google Scholar] [CrossRef]
- Bettini, R.; Partelli, S.; Boninsegna, L.; Capelli, P.; Crippa, S.; Pederzoli, P.; Scarpa, A.; Falconi, M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011, 150, 75–82. [Google Scholar] [CrossRef]
- Bartolini, I.; Bencini, L.; Risaliti, M.; Ringressi, M.N.; Moraldi, L.; Taddei, A. Current Management of Pancreatic Neuroendocrine Tumors: From Demolitive Surgery to Observation. Gastroenterol. Res. Pract. 2018, 2018, 9647247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halfdanarson, T.R.; Rubin, J.; Farnell, M.B.; Grant, C.S.; Petersen, G.M. Pancreatic endocrine neoplasms: Epidemiology and prognosis of pancreatic endocrine tumors. Endocr. Relat. Cancer 2008, 15, 409–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cloyd, J.M.; Poultsides, G.A. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J. Gastroenterol. 2015, 21, 9512–9525. [Google Scholar] [CrossRef] [PubMed]
- Gratian, L.; Pura, J.; Dinan, M.; Roman, S.; Reed, S.; Sosa, J.A. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann. Surg. Oncol. 2014, 21, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Wu, Z.; Cloyd, J.; Lopez-Aguiar, A.G.; Poultsides, G.; Makris, E.; Rocha, F.; Kanji, Z.; Weber, S.; Fisher, A.; et al. Margin status and long-term prognosis of primary pancreatic neuroendocrine tumor after curative resection: Results from the US Neuroendocrine Tumor Study Group. Surgery 2019, 165, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic Cancer Surgery: The New R-status Counts. Ann. Surg. 2017, 265, 565–573. [Google Scholar] [CrossRef]
- Wu, Z.; Qiu, X.; Zhi, Y.; Shi, X.; Lv, G. The risk and prognostic factors for G1 pancreatic neuroendocrine tumors: A retrospective analysis of the SEER database. Front. Oncol. 2022, 12, 993524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Perren, A.; Couvelard, A.; Scoazec, J.-Y.; Costa, F.; Borbath, I.; Fave, G.D.; Gorbounova, V.; Gross, D.; Grossman, A.; Jensen, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef]
- Spolverato, G.; Bagante, F.; Aldrighetti, L.; Poultsides, G.; Bauer, T.W.; Field, R.C.; Marques, H.P.; Weiss, M.; Maithel, S.K.; Pawlik, T.M. Neuroendocrine Liver Metastasis: Prognostic Implications of Primary Tumor Site on Patients Undergoing Curative Intent Liver Surgery. J. Gastrointest. Surg. 2017, 21, 2039–2047. [Google Scholar] [CrossRef]
- Vouros, D.; Bramis, K.; Alexakis, N.; Kotsarinis, V.; Antonakis, P.; Memos, N.; Konstadoulakis, M.; Toutouzas, K. Completion Pancreatectomy. Indications and Outcomes: A Systematic Review. Am. Surg. 2023, 89, 6134–6146. [Google Scholar] [CrossRef]
- Gaujoux, S.; Gonen, M.; Tang, L.; Klimstra, D.; Brennan, M.F.; D’angelica, M.; DeMatteo, R.; Allen, P.J.; Jarnagin, W.; Fong, Y. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann. Surg. Oncol. 2012, 19, 4270–4277. [Google Scholar] [CrossRef]
- Zaidi, M.Y.; Lopez-Aguiar, A.G.; Switchenko, J.M.; Lipscomb, J.; Andreasi, V.; Partelli, S.; Gamboa, A.C.; Lee, R.M.; Poultsides, G.A.; Dillhoff, M.; et al. A Novel Validated Recurrence Risk Score to Guide a Pragmatic Surveillance Strategy After Resection of Pancreatic Neuroendocrine Tumors: An International Study of 1006 Patients. Ann. Surg. 2019, 270, 422–433. [Google Scholar] [CrossRef]
- Heidsma, C.M.; van Roessel, S.; van Dieren, S.; Engelsman, A.F.; Strobel, O.; Buechler, M.W.; Schimmack, S.; Perinel, J.; Adham, M.; Deshpande, V.; et al. International Validation of a Nomogram to Predict Recurrence after Resection of Grade 1 and 2 Nonfunctioning Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 571–579. [Google Scholar] [CrossRef]

| Clinical and Pathological Factors | Number of Patients | Percentage |
|---|---|---|
| Mean Age (years) | 61.5 | |
| Male/Female | 1: 1 | |
| Site of Tumour | ||
| Head of Pancreas | 15 | 17.40 |
| Neck of Pancreas | 1 | 1.20 |
| Body of Pancreas | 11 | 12.80 |
| Body and Tail | 9 | 10.50 |
| Tail of Pancreas | 49 | 57.00 |
| Uncinate Process | 1 | 1.20 |
| Functionality | ||
| Not Functioning | 62 | 72.09 |
| Functioning | 24 | 27.91 |
| Insulinoma | 10 | 41.66 |
| Carcinoid | 5 | 20.83 |
| Gastrinoma | 1 | 4.17 |
| Glucagonoma | 7 | 29.17 |
| VIPOMA | 1 | 4.17 |
| Associated Genetic Syndrome | ||
| None | 78 | 90.70 |
| MEN 1 | 5 | 5.81 |
| VHL | 2 | 2.33 |
| NF1 | 1 | 1.16 |
| Type of Operation | ||
| Distal Pancreatectomy | 65 | 75.60 |
| Pyloric Preserving Pancreaticoduodenectomy | 5 | 5.80 |
| Standard Whipple | 13 | 15.10 |
| Total Pancreatectomy | 3 | 3.50 |
| Number Of Tumours | ||
| One | 76 | 88.40 |
| Two | 4 | 4.70 |
| Three | 1 | 1.20 |
| Multifocal | 5 | 5.80 |
| UICC (8th edition) TNM Staging System | ||
| T (Tumour) | ||
| T1 | 35 | 40.69 |
| T2 | 30 | 34.88 |
| T3 | 18 | 20.93 |
| T4 | 3 | 3.48 |
| N (Nodes) | ||
| N0 | 66 | 76.74 |
| N1 | 19 | 22.09 |
| N2 | 1 | 1.16 |
| M (Metastasis) | ||
| M0 | 85 | 98.83 |
| M1 | 1 | 1.16 |
| Tumour Grade | ||
| I | 56 | 65.12 |
| II | 23 | 26.74 |
| III | 7 | 8.14 |
| KI 67 Expression | ||
| <3 | 56 | 65.12 |
| 3–20 | 23 | 26.74 |
| >20 | 7 | 8.14 |
| Perineural Invasion | ||
| No | 74 | 86.05 |
| Yes | 12 | 13.95 |
| Vascular Invasion | ||
| No | 61 | 70.93 |
| Yes | 25 | 29.07 |
| R Status (Resection Margin Status) | ||
| 0 | 67 | 77.90 |
| 1 | 19 | 22.10 |
| Complications | 32 | 37.2 |
| Postoperative collection | 7 | 8.1 |
| Small bowel Ileus | 6 | 6.9 |
| Chest infection | 4 | 4.7 |
| Minor leak/pancreatic fistula | 2 | 2.3 |
| Pancreatico-jejunostomy (PJ) leak | 2 | 2.3 |
| Pancreatitis | 2 | 2.3 |
| Intraoperative bleeding | 2 | 2.3 |
| Chyle leak | 1 | 1.2 |
| Major 2ry postoperative bleeding | 1 | 1.2 |
| SMV thrombosis | 1 | 1.2 |
| Pleural effusion | 1 | 1.2 |
| Clavien–Dindo Classification | ||
| 2 | 26 | 81.3 |
| 3 A | 5 | 15.6% |
| 5 | 1 | 3.1 |
| Mortality | 15 | 17.44 |
| Reason for Mortality | ||
| Metastatic PNET | 7 | 46.70 |
| Metastatic breast cancer | 2 | 13.30 |
| Cardiovascular/medical disease | 6 | 40.00 |
| Variables | Number of Patients | Percentage |
|---|---|---|
| Type of surgery | ||
| Distal pancreatectomy | 6 | 50 |
| Standard pancreatoduodenectomy | 3 | 25 |
| Pylorus-preserving pancreatoduodenectomy | 2 | 16.7 |
| Total pancreatectomy | 1 | 8.3 |
| Tumour focality | ||
| Unifocal | 11 | 91.7 |
| Multifocal | 1 | 8.3 |
| UICC (8th edition) TNM staging system | ||
| T (Tumour) | ||
| T1 | 1 | 8.3 |
| T2 | 5 | 41.6 |
| T3 | 4 | 33.3 |
| T4 | 2 | 16.7 |
| N (Nodes) | ||
| N0 | 7 | 58.3 |
| N1 | 5 | 41.6 |
| N2 | 0 | 0 |
| M (Metastasis) | ||
| M0 | 5 | 41.6 |
| M1 | 7 | 58.3 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p Value | HR (95th C.I.) | p Value | HR (95th C.I.) | |
| Age | 0.818 | 0.995 (0.958–1.035) | ||
| Sex (M/F) | 0.039 | 4.026 (1.07–15.147) | 0.531 | 1.783 (0.293–10.864) |
| Size of tumour | 0.009 | 1.013 (1.003–1.023) | 0.007 | 1.017 (1.005–1.029) |
| Vascular invasion | 0.003 | 6.353 (1.885–21.409) | 0.27 | 2.715 (0.46–16.029) |
| Perineural invasion | 0.042 | 3.526 (1.046–11.883) | 0.114 | 3.688 (0.73–18.631) |
| R status | 0.498 | 1.576 (0.423–5.872) | ||
| CCI (Charlson Comorbidity Index) | 0.001 | 1.63 (1.235–2.152) | 0.031 | 1.618 (1.044–2.508) |
| KI 67 expression % | 0.006 | 1.142 (1.039–1.256) | 0.002 | 1.261 (1.087–1.463) |
| Function | 0.113 | 0.185 (0.023–1.495) | ||
| T (1–2/3–4) | 0.158 | 2.298 (0.724–7.289) | ||
| N (0/1 and 2) | 0.015 | 4.527 (1.342–15.272) | 0.778 | 0.725 (0.077–6.83) |
| Tumour grade 1 versus 2 and 3 | 0.006 | 5.653 (1.651–19.35) | 0.826 | 1.275 (0.147–11.027) |
| Number (single/multiple) | 0.561 | 0.544 (0.07–4.237) | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p Value | HR (95th C.I.) | p Value | HR (95th C.I.) | |
| Age | 0.055 | 1.041 (0.999–1.085) | ||
| Sex (F//M) | 0.624 | 1.289 (0.467–3.561) | ||
| Size of Tumour | 0.039 | 1.009 (1–1.018) | 0.007 | 1.015 (1.004–1.026) |
| Vascular Invasion (no/yes) | 0.097 | 2.363 (0.856–6.525) | ||
| Perineural Invasion (no/yes) | 0.677 | 1.374 (0.308–6.122) | ||
| CCI (Charlson Comorbidity Index) | 0.002 | 1.396 (1.128–1.728) | <0.001 | 2.116 (1.395–3.21) |
| KI 67 expression % | 0.134 | 1.077 (0.977–1.187) | 0.025 | 1.193 (1.022–1.394) |
| N (0/1 and 2) | 0.198 | 0.464 (0.144–1.493) | ||
| T (1–2/3–4) | 0.425 | 1.549 (0.528–4.538) | ||
| Tumour grade 1 versus 2 and 3 | 0.13 | 2.205 (0.793–6.128) | 0.046 | 0.139 (0.02–0.968) |
| Number (single/multiple) | 0.373 | 0.397 (0.052–3.024) | ||
| R Status | 0.254 | 1.953 (0.618–6.179) | ||
| Function | 0.216 | 0.39 (0.088–1.731) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouad, M.; Almahari, S.A.; Zaitoun, A.M.; Sonoo, P.; Malek, S.; Sourial, K.; Gomez, D. Outcomes Following Surgery for Pancreatic Neuro-Endocrine Tumours: A Single-Centre Experience. Clin. Pract. 2025, 15, 202. https://doi.org/10.3390/clinpract15110202
Fouad M, Almahari SA, Zaitoun AM, Sonoo P, Malek S, Sourial K, Gomez D. Outcomes Following Surgery for Pancreatic Neuro-Endocrine Tumours: A Single-Centre Experience. Clinics and Practice. 2025; 15(11):202. https://doi.org/10.3390/clinpract15110202
Chicago/Turabian StyleFouad, Mina, Sayed Ali Almahari, Abed Moeti Zaitoun, Prithvirao Sonoo, Sepand Malek, Karim Sourial, and Dhanny Gomez. 2025. "Outcomes Following Surgery for Pancreatic Neuro-Endocrine Tumours: A Single-Centre Experience" Clinics and Practice 15, no. 11: 202. https://doi.org/10.3390/clinpract15110202
APA StyleFouad, M., Almahari, S. A., Zaitoun, A. M., Sonoo, P., Malek, S., Sourial, K., & Gomez, D. (2025). Outcomes Following Surgery for Pancreatic Neuro-Endocrine Tumours: A Single-Centre Experience. Clinics and Practice, 15(11), 202. https://doi.org/10.3390/clinpract15110202







