The Effects of Physical Exercise on Depression and Anxiety in Cancer Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources, Search Strategy and Study Selection
2.2. Data Extraction and Outcome Measures
2.3. Quality Assessment
2.4. Risk of Bias Assessment
3. Results
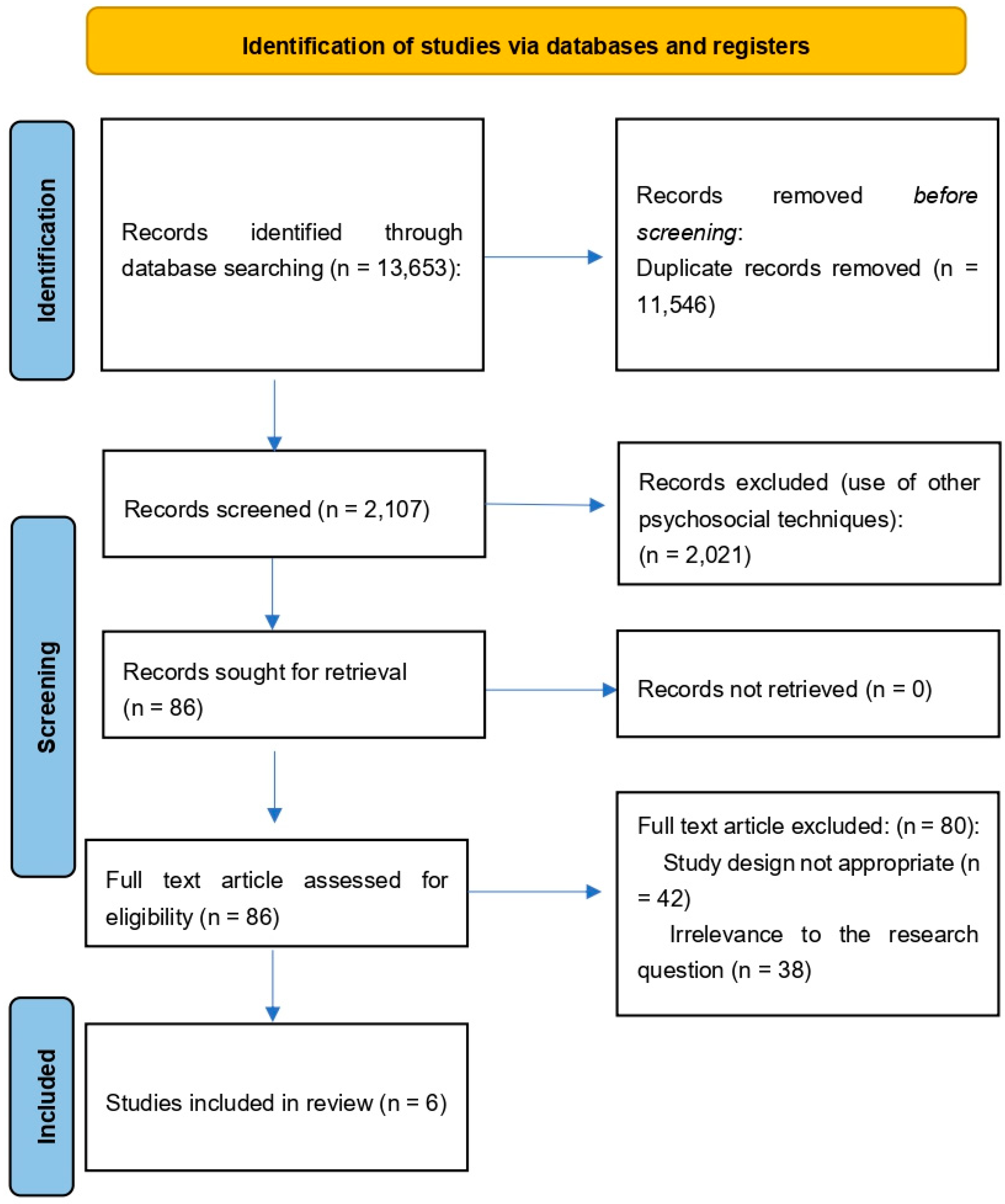
3.1. Identification of Study
3.2. Characteristics of the Included Studies
3.3. Assessment of Methodology and Quality of the Studies
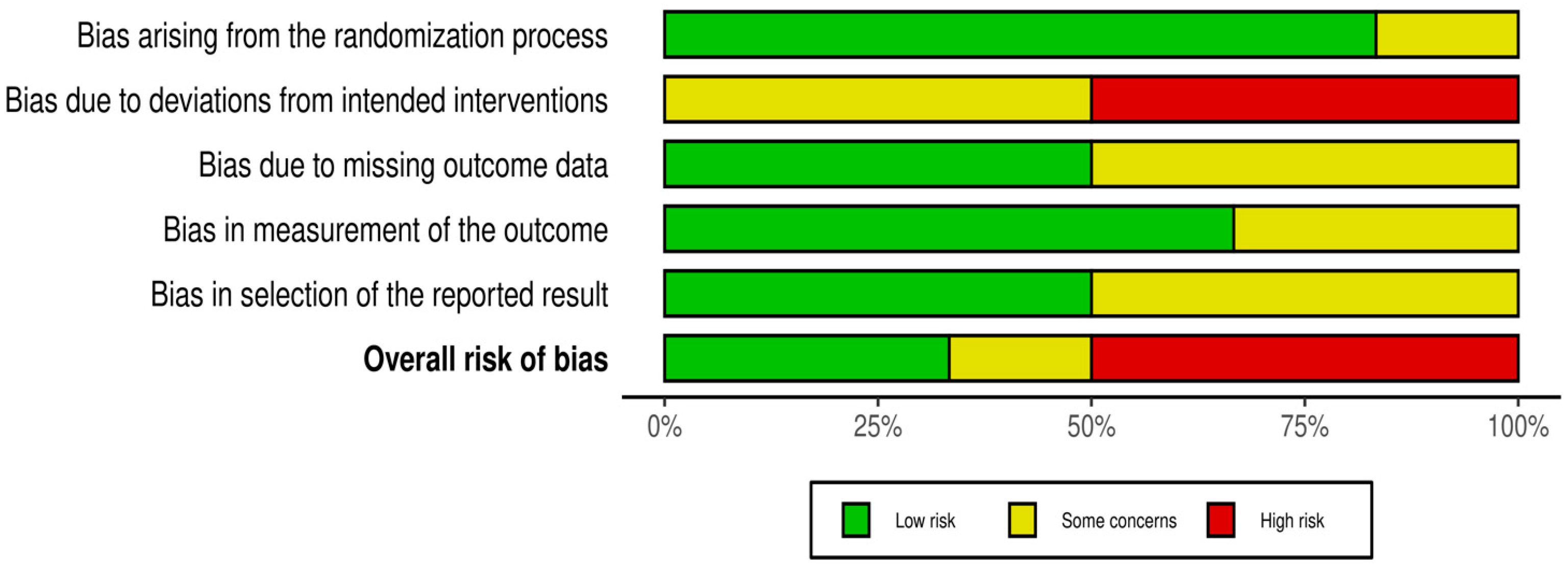
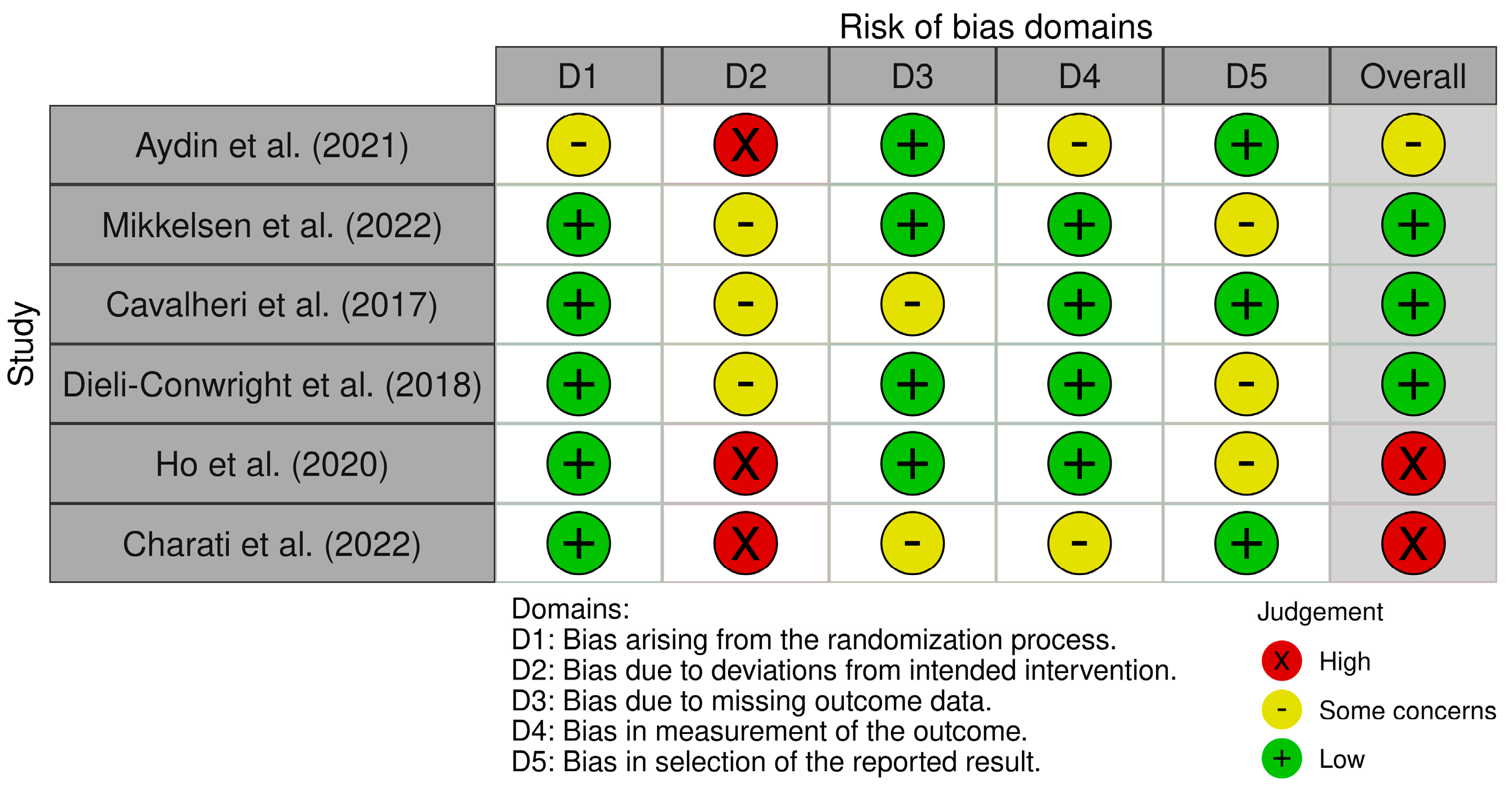
3.4. Evaluation Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCTs | Randomized controlled trials |
| DALYs | Disability-adjusted life years |
| CRCI | cancer-related cognitive impairment |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| MeSH | Medical Subject Headings |
| BDI | Beck Depression Inventory |
| NSCLC | Non-small cell lung cancer |
| 6MWD | Six-minute Walk distance |
| HRQoL | Health-related quality of life |
| FACT-B | Functional Assessment of Cancer Therapy-Breast |
| SF-36 | Short Form-36 Health Survey |
| BFI | Brief Fatigue Inventory |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| HADS | Hospital Anxiety and Depression Scale |
| QoL | Quality of Life |
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in Mental Disorders and Global Disease Burden Implications: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Stubbs, B. The Role of Exercise in Preventing and Treating Depression. Curr. Sports Med. Rep. 2019, 18, 299–304. [Google Scholar] [CrossRef]
- Munir, S.; Takov, V. Generalized Anxiety Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Pitman, A.; Suleman, S.; Hyde, N.; Hodgkiss, A. Depression and Anxiety in Patients with Cancer. BMJ 2018, 361, k1415. [Google Scholar] [CrossRef]
- Massie, M.J. Prevalence of Depression in Patients with Cancer. J. Natl. Cancer Inst. Monogr. 2004, 2004, 57–71. [Google Scholar] [CrossRef]
- Holland, J.C.; Andersen, B.; Breitbart, W.S.; Compas, B.; Dudley, M.M.; Fleishman, S.; Fulcher, C.D.; Greenberg, D.B.; Greiner, C.B.; Handzo, G.F.; et al. Distress Management: Clinical Practice Guidelines in OncologyTM. JNCCN J. Natl. Compr. Cancer Netw. 2010, 8, 448–485. [Google Scholar] [CrossRef]
- Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. Int. J. Mol. Sci. 2021, 22, 11632. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; Notarnicola, A.; DI Paolo, S.; Covelli, I.; Moretti, B. Epidemiology of Injuries in Water Board Sports: Trauma versus Overuse Injury. J. Sports Med. Phys. Fit. 2021, 61, 707–711. [Google Scholar] [CrossRef]
- Xie, S.; Yuan, Y.; Wang, J.; Bai, Y.; Wang, T.; Qiu, B.; Yang, Y.; Lin, S.-C. Optimal Dose and Type of Exercise Improve Walking Velocity in Adults with Parkinson’s Disease: A Systematic Review and Bayesian Network Meta-Analysis. Sci. Rep. 2025, 15, 2239. [Google Scholar] [CrossRef]
- Casciano, F.; Caruso, L.; Zauli, E.; Gonelli, A.; Zauli, G.; Vaccarezza, M. Emerging Mechanisms of Physical Exercise Benefits in Adjuvant and Neoadjuvant Cancer Immunotherapy. Biomedicines 2024, 2, 2528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agostini, F.; Bernetti, A.; Santilli, G.; Damiani, C.; Santilli, V.; Paoloni, M.; Mangone, M. Efficacy of Ultrasound Therapy Combined with Cryotherapy in Pain Management and Rehabilitation in Patients with Achilles Tendinopathy: A Retrospective Observational Study. Clin. Ter. 2023, 174, 148–151. [Google Scholar] [CrossRef]
- Courneya, K.S.; Friedenreich, C.M. Physical exercise and cancer: Biologic mechanisms and outcomes. Compr. Physiol. 2017, 7, 93–118. [Google Scholar]
- Oberste, M.; Schaffrath, N.; Schmidt, K.; Bloch, W.; Jäger, E.; Steindorf, K.; Hartig, P.; Joisten, N.; Zimmer, P. Protocol for the “Chemobrain in Motion—Study” (CIM—Study): A Randomized Placebo-Controlled Trial of the Impact of a High-Intensity Interval Endurance Training on Cancer Related Cognitive Impairments in Women with Breast Cancer Receiving First-Line Chemotherapy. BMC Cancer 2018, 18, 1071. [Google Scholar] [CrossRef]
- Battaglini, C.L.; Mills, R.C.; Phillips, B.L.; Lee, J.T.; Story, C.E.; Nascimento, M.G.; Hackney, A.C. Twenty-Five Years of Research on the Effects of Exercise Training in Breast Cancer Survivors: A Systematic Review of the Literature. World J. Clin. Oncol. 2014, 5, 177–190. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, P.; Wang, Y.; Bian, L.; Zhao, K.; Shi, A.; Jiang, Z.; Zhao, L.; Jiang, J.; Zhang, S. The Impact of Exercise during Radiotherapy on Treatment-Related Side Effects in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2025, 163, 104990. [Google Scholar] [CrossRef] [PubMed]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise Therapy for Chronic Fatigue Syndrome. Cochrane Database Syst. Rev. 2024, 12, CD003200. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Mohsina, S.; Gurushankari, B.; Niranjan, R.; Sureshkumar, S.; Sreenath, G.S.; Kate, V. Assessment of the Quality of Randomized Controlled Trials in Surgery Using Jadad Score: Where Do We Stand? J. Postgrad. Med. 2022, 68, 207–212. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Aydin, M.; Kose, E.; Odabas, I.; Bingul, B.M.; Demirci, D.; Aydin, Z. The Effect of Exercise on Life Quality and Depression Levels of Breast Cancer Patients. Asian. Pac. J. Cancer. Prev. 2021, 22, 725–732. [Google Scholar] [CrossRef]
- Mikkelsen, M.K.; Lund, C.M.; Vinther, A.; Tolver, A.; Johansen, J.S.; Chen, I.; Ragle, A.-M.; Zerahn, B.; Engell-Noerregaard, L.; Larsen, F.O.; et al. Effects of a 12-Week Multimodal Exercise Intervention Among Older Patients with Advanced Cancer: Results from a Randomized Controlled Trial. Oncologist 2022, 27, 67–78. [Google Scholar] [CrossRef]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Gain, K.; Phillips, M.J.; Sanders, L.H.; Hill, K. Exercise Training for People Following Curative Intent Treatment for Non-Small Cell Lung Cancer: A Randomized Controlled Trial. Braz. J. Phys. Ther. 2017, 21, 58–68. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and Resistance Exercise Improves Physical Fitness, Bone Health, and Quality of Life in Overweight and Obese Breast Cancer Survivors: A Randomized Controlled Trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Ho, M.; Ho, J.W.C.; Fong, D.Y.T.; Lee, C.F.; Macfarlane, D.J.; Cerin, E.; Lee, A.M.; Leung, S.; Chan, W.Y.Y.; Leung, I.P.F.; et al. Effects of Dietary and Physical Activity Interventions on Generic and Cancer-Specific Health-Related Quality of Life, Anxiety, and Depression in Colorectal Cancer Survivors: A Randomized Controlled Trial. J. Cancer Surviv. 2020, 14, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Charati, F.G.; Shojaee, L.; Haghighat, S.; Esmaeili, R.; Madani, Z.; Charati, J.Y.; Hosseini, S.H.; Shafipour, V. Motor Exercises Effect on Improving Shoulders Functioning, Functional Ability, Quality of Life, Depression and Anxiety For Women With Breast Cancer. Clin. Breast Cancer 2022, 22, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; Fiore, P.; Ricci, V.; Zonno, A.; Joksimovic, M.; Petruzzella, D.; Gioia, G.; Giarrizzo, D.; Mastrorillo, S.; Coretti, B.; et al. The Impact of the COVID-19 Pandemic on Outdoor Physical Activities for People with Disabilities, Including the Risks for Psychophysical Well-Being. Sustainability 2023, 15, 1436. [Google Scholar] [CrossRef]
- Farì, G.; Di Paolo, S.; Ungaro, D.; Luperto, G.; Farì, E.; Latino, F. The Impact of COVID-19 on Sport and Daily Activities in an Italian Cohort of Football School Children. Int. J. Athl. Ther. Train. 2021, 26, 274–278. [Google Scholar] [CrossRef]
- Kalpana, M.; Katta, R.; Madhusudhan, U.; Gaur, A.; Ganji, V.; Taranikanti, M.; Nitin, J.; Kasturi, V.K. COVID-19 among Physically Active and Physically Inactive Individuals. Maedica 2024, 19, 594–599. [Google Scholar] [CrossRef]
- Szabo-Reed, A.N.; Watts, A.; Vidoni, E.D.; Mahnken, J.; Van Sciver, A.; Finley, K.; Clutton, J.; Holden, R.; Key, M.N.; Burns, J.M. Lifestyle Empowerment for Alzheimer’s Prevention Prescribed by Physicians: Methods and Adaptations to COVID-19. Contemp. Clin. Trials 2024, 147, 107729. [Google Scholar] [CrossRef] [PubMed]
- Schaepe, K.S.; Basford, J.R.; Cheville, A.L. Integrating Fitness Training in Oncologic Care: Lessons Learned from a Large Telemedicine Trial. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100367. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Battaglia, M.; Lin, P.-J.; Sanapala, C.; Watson, E.E.; Mendler, J.H.; Liesveld, J.; Wang, Y.; Hayward, E.; LoCastro, M.; Mortaz, S.; et al. Changes in Muscle Performance among Older Adults with Myeloid Malignancies Engaging in a Mobile Health (mHealth) Exercise Intervention: A Single Arm Pilot Study. BMC Geriatr. 2025, 25, 22. [Google Scholar] [CrossRef] [PubMed]
| Title | Authors and Year of Publication | Sample | Research Design | Collected Data and Outcomes | Results |
|---|---|---|---|---|---|
| The Effect of Exercise on Life Quality and Depression Levels of Breast Cancer Patients | Aydin et al. (2021) [23] | 48 women diagnosed with breast cancer who completed treatment. | This RCT divided the study population into two groups:
| Depression levels were measured using the WHOQOL-BREF, EORTC-QLQ-C30 quality of life assessments and BDI, before and after the intervention. | Following the 12-week program, the intervention group demonstrated a significant reduction in depression levels. In contrast, no notable changes were observed in the control group. These findings suggest that a structured exercise program can effectively alleviate symptoms of depression in breast cancer survivors. |
| Exercise Intervention Among Older Patients with Advanced Cancer: Results from a Randomized Controlled Trial | Mikkelsen et al. (2022) [24] | The study involved 84 older adults (aged ≥65 years) diagnosed with advanced-stage (III/IV) pancreatic, biliary tract, or non-small cell lung cancer, all of whom were undergoing systemic oncological treatment. | RCT; a 12-week multimodal exercise intervention comprising the following components:
| Symptoms of depression and anxiety plus physical function. | A 12-week multimodal exercise program with targeted support proved effective in enhancing physical function, endurance, muscle strength, overall physical activity level and mental well-being in older adults with advanced cancer undergoing oncological treatment. Participants in the intervention group also experienced reductions in symptoms of depression and anxiety. |
| Exercise training for people following curative intent treatment for non-small cell lung cancer: a randomized controlled trial | Cavalheri et al. (2017) [25] | 17 individuals, mean age of 67 years (Standard Deviation: 9 years). Participants 6–10 weeks post-lobectomy for NSCLC or 4–8 weeks post-adjuvant chemotherapy | RCT to investigate the effects of supervised exercise training on multiple health outcomes in individuals who had completed curative intent treatment for NSCLC.
| Outcomes measured included anxiety and depression levels, exercise capacity (assessed via peak oxygen consumption [VO2peak] and the 6MWD), physical activity and sedentary behavior, peripheral muscle strength (quadriceps and handgrip), HRQoL, fatigue, and lung function. | An 8-week supervised exercise training program led to improvements in exercise capacity. However, it did not lead to significant changes in physical activity levels, fatigue, anxiety, depression, or lung function. These results indicate that, while supervised exercise may enhance physical performance, its impact on broader health outcomes in this population appears to be limited. |
| Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial | Dieli-Conwright et al. (2018) [26] | 160 overweight and obese breast cancer survivors (female, ages 25–70 years) | RCT A 16-week supervised aerobic and resistance exercise program, three times a week. | FACT-B, SF-36, BFI, CES-D, Physical fitness tests (e.g., aerobic capacity, strength), bone health assessments (e.g., bone mineral density). | Participants in both exercise groups reported improvements in physical and emotional well-being. Notably, the exercise group showed a significant reduction in depression levels compared to the control group, with a between-group difference of 14.7 points (95% CI: 18.2, 9.7; p < 0.001). |
| Effects of dietary and physical activity interventions on generic and cancer-specific health-related quality of life, anxiety, and depression in colorectal cancer survivors: a randomized controlled trial | Ho et al. (2020) [27] | 118 colorectal cancer survivors aged 18–80 years, with a mean age of 62 years. The inclusion criteria included having completed cancer treatment and having no significant comorbidities. | RCT with a two-group design.
|
| The combined dietary and physical activity intervention effectively reduced anxiety and depression while increasing physical activity in colorectal cancer survivors. These results indicate that lifestyle interventions can significantly improve both physical and mental health outcomes in this population. Participants in the intervention group showed significantly lower levels of anxiety and depression compared to those in the control group. |
| Motor Exercises Effect on Improving Shoulder Functioning, Functional Ability, Quality of Life, Depression and Anxiety For Women With Breast Cancer | Charati et al. (2022) [28] | 70 women with breast cancer. | RCT. The participants were divided into intervention group that performed motor exercises for five weeks (n = 35) and control (n = 35) groups. | Depression and Anxiety: Measured using the HADS at baseline and five weeks post-surgery. | Significant reductions in depression and anxiety levels were observed in the intervention group compared to control (p < 0.05). In conclusion motor exercises effectively alleviated symptoms of anxiety and depression, thereby improving overall quality of life. |
| Authors | Was the Treatment Randomly Allocated? | Was the Randomization Procedure Described and Was Appropriate? | Was There a Description of Withdrawals and Dropout? | Was There a Clear Description of the Inclusion/Exclusion Criteria? | Were the Methods of Statistical Analysis Described? | Jadad score (0–5) |
|---|---|---|---|---|---|---|
| Aydin et al. (2021) [23] | Yes | No | Yes | Yes | Yes | 4 |
| Mikkelsen et al. (2022) [24] | Yes | Yes | Yes | Yes | Yes | 5 |
| Cavalheri et al. (2017) [25] | Yes | Yes | Yes | Yes | No | 4 |
| Dieli-Conwright et al. (2018) [26] | Yes | Yes | Ye | Yes | Yes | 5 |
| Ho et al. (2020) [27] | Yes | No | Yes | Yes | No | 3 |
| Charati et al. (2022) [28] | Yes | No | Yes | Yes | No | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farì, G.; Fai, A.; Quarta, F.; Pitruzzella, M.; Sconza, C.; Leoni, M.L.G.; Varrassi, G.; Filipponi, M.; Rollo, G.; Baricich, A.; et al. The Effects of Physical Exercise on Depression and Anxiety in Cancer Patients: A Systematic Review. Clin. Pract. 2025, 15, 180. https://doi.org/10.3390/clinpract15100180
Farì G, Fai A, Quarta F, Pitruzzella M, Sconza C, Leoni MLG, Varrassi G, Filipponi M, Rollo G, Baricich A, et al. The Effects of Physical Exercise on Depression and Anxiety in Cancer Patients: A Systematic Review. Clinics and Practice. 2025; 15(10):180. https://doi.org/10.3390/clinpract15100180
Chicago/Turabian StyleFarì, Giacomo, Annatonia Fai, Francesco Quarta, Morena Pitruzzella, Cristiano Sconza, Matteo Luigi Giuseppe Leoni, Giustino Varrassi, Marco Filipponi, Giuseppe Rollo, Alessio Baricich, and et al. 2025. "The Effects of Physical Exercise on Depression and Anxiety in Cancer Patients: A Systematic Review" Clinics and Practice 15, no. 10: 180. https://doi.org/10.3390/clinpract15100180
APA StyleFarì, G., Fai, A., Quarta, F., Pitruzzella, M., Sconza, C., Leoni, M. L. G., Varrassi, G., Filipponi, M., Rollo, G., Baricich, A., & Bernetti, A. (2025). The Effects of Physical Exercise on Depression and Anxiety in Cancer Patients: A Systematic Review. Clinics and Practice, 15(10), 180. https://doi.org/10.3390/clinpract15100180










