Clinical and Histopathological Correlates of Endometrial Proliferative Lesions in Perimenopausal Women: A Retrospective Study with Internal Validation of a Risk Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Sources and Variables
2.4. Clinical Presentation and Ultrasound Assessment
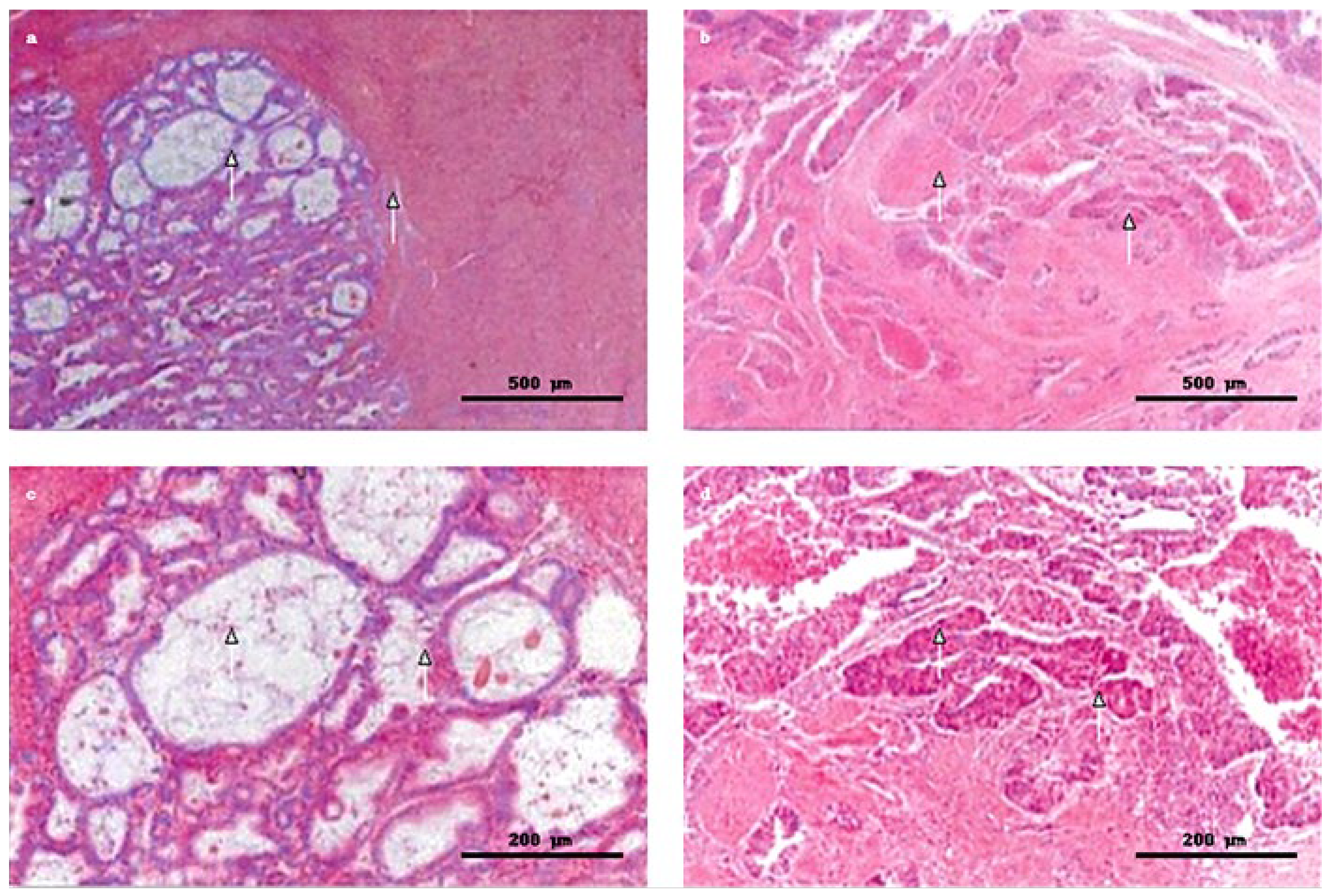
2.5. Endometrial Sampling and Histopathology
2.6. Microphotography and Image Processing
2.7. Advanced Imaging
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. WHO 2014 Classification of Endometrial Lesions
3.3. Distribution of Clinical Risk Factors Across Histopathological Diagnoses
3.4. Clinicopathological Associations Across Histopathological Categories (χ2)
3.5. Predictors of Advanced Endometrial Pathology: Univariable Logistic Regression
3.6. Predictors of Advanced Endometrial Pathology: Multivariable Logistic Regression
3.7. Endometrial Thickness Across Histopathological Diagnoses
3.8. Apparent Performance of the Multivariable Model
3.9. Clinicopathological Correlations with Histopathological Severity
3.10. Internal Validation of the Multivariable Model
4. Discussion
4.1. Principal Findings
4.2. Ultrasound–Pathology Concordance
4.3. Predictive Performance and Internal Validation
4.4. Biological Plausibility and Molecular Context
4.5. Clinical and Diagnostic Implications
4.6. Exploratory Risk Index
5. Limitations
6. Suggestions for Future Research
- -
- Validating the model externally across centers and care settings;
- -
- Assessing calibration and progression endpoints longitudinally;
- -
- Testing augmentation with quantitative ultrasound parameters, refined metabolic markers, and molecular features;
- -
- Performing decision-curve analysis to evaluate clinical utility.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EH | endometrial hyperplasia |
| WHO | World Health Organization |
| EIN | endometrial intraepithelial neoplasia |
| EC | endometrial carcinoma, endometrial cancer |
| PCOS | polycystic ovary syndrome |
| H&E | hematoxylin-and-eosin |
| AH/EIN | atypical hyperplasia/endometrial intraepithelial neoplasia |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| ORs | odds ratios |
| CIs | confidence intervals |
| aORs | adjusted confidence intervals |
| AMH | anti-Müllerian hormone |
| LH | luteinizing hormone |
| MSI | microsatellite instability |
References
- Dias Da Silva, I.; Wuidar, V.; Zielonka, M.; Pequeux, C. Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview. Cells 2024, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of endometrial cancer risk with postmenopausal bleeding in women: A systematic review and meta-analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef]
- Daan, N.M.; Fauser, B.C. Menopause prediction and potential implications. Maturitas 2015, 82, 257–265. [Google Scholar] [CrossRef]
- Doherty, M.T.; Sanni, O.B.; Coleman, H.G.; Cardwell, C.R.; McCluggage, W.G.; Quinn, D.; Wylie, J.; McMenamin, Ú.C. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232231. [Google Scholar] [CrossRef]
- Ellenson, L.H.; Ronnett, B.M.; Kurman, R.J. Precursor lesions of endometrial carcinoma. In Blaustein’s Pathology of the Female Genital Tract, 6th ed.; Kurman, R.J., Ellenson, L.H., Ronnett, B.M., Eds.; Springer: New York, NY, USA, 2011; p. 360. [Google Scholar]
- Emons, G.; Beckmann, M.W.; Schmidt, D.; Mallmann, P. Uterus Commission of the Gynecological Oncology Working Group (AGO). New WHO classification of endometrial hyperplasias. Geburtshilfe Frauenheilkd. 2015, 75, 135. [Google Scholar]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia: A long-term study of untreated hyperplasia in 170 patients. Cancer 1985, 56, 403. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.G.; Kurman, R.J.; Nogales, F. Tumors of the uterine corpus (epithelial tumors and related lesions). In World Health Organization Classification of Tumours; Tavassoli, F.A., Devilee, P., Eds.; IARC Press: Lyon, France, 2003; p. 221. [Google Scholar]
- World Health Organization (WHO). GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed on 8 February 2025).
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Adekanmbi, V.; Hsu, C.D.; Hoang, T.N.; Soliman, P.T.; Baillargeon, J.G.; Berenson, A.B. Trends in Endometrial Cancer Incidence Among Premenopausal and Postmenopausal Women in the United States Between 2001 and 2021. Cancers 2025, 17, 1035. [Google Scholar] [CrossRef]
- Sherman, M.E.; Carreon, J.D.; Lacey, J.V., Jr.; Devesa, S.S. Impact of hysterectomy on endometrial carcinoma rates in the United States. J. Natl. Cancer Inst. 2005, 97, 1700. [Google Scholar] [CrossRef]
- Turcan, N.; Baros, A.; Zugravu, C.; Mergeanu, M.; Sajin, M.; Andreescu, C.V.; Frincu, F.; Carp-Veliscu, A.; Edu, A.; Mehedintu, C.; et al. Trend of incidence in the last five years of breast, cervical, ovarian and uterine cancer in the main hospital in Romania. Ro. J. Med. Pract. 2021, 16 (Suppl. S6), 62–68. [Google Scholar] [CrossRef]
- Bansal, N.; Yendluri, V.; Wenham, R.M. The molecular biology of endometrial cancers and the implications for pathogenesis, classification and targeted therapies. Cancer Control 2009, 16, 8. [Google Scholar] [CrossRef]
- Hecht, J.L.; Mutter, G.L. Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol. 2006, 24, 4783. [Google Scholar] [CrossRef]
- Anca-Stanciu, M.-B.; Manu, A.; Olinca, M.V.; Coroleucă, C.; Comandașu, D.-E.; Coroleuca, C.A.; Maier, C.; Bratila, E. Comprehensive Review of Endometrial Cancer: New Molecular and FIGO Classification and Recent Treatment Changes. J. Clin. Med. 2025, 14, 1385. [Google Scholar] [CrossRef] [PubMed]
- Samarnthai, N.; Hall, K.; Yeh, I.T. Molecular profiling of endometrial malignancies. Obstet. Gynecol. Int. 2010, 2010, 162363. [Google Scholar] [CrossRef]
- Kitson, S.J.; Evans, D.G.; Crosbie, E.J. Identifying high-risk women for endometrial cancer prevention strategies: Proposal of an endometrial cancer risk prediction model. Cancer Prev. Res. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Santoro, N.; Randolph, J.F., Jr. Reproductive hormones and the menopause transition. Obstet. Gynecol. Clin. N. Am. 2011, 38, 455–466. [Google Scholar] [CrossRef]
- Jain, A.; Santoro, N. Endocrine mechanism and management for abnormal bleeding due to perimenopausal changes. Clin. Obstet. Gynecol. 2005, 48, 285. [Google Scholar] [CrossRef]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. North Am. 2015, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Lee, C.H.; Baerwald, A. Interactions between serum FSH, inhibin B and antral follicle count in the decline of serum AMH during the menstrual cycle in late reproductive age. Endocrinol. Diabetes Metab. 2020, 4, e00172. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Lasley, B.; McConnell, D. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study. J. Clin. Endocrinol. Metab. 2004, 89, 2622. [Google Scholar] [CrossRef]
- Kwee, J.; Schats, R.; McDonnell, J. Evaluation of antimüllerian hormone as a test for the prediction of ovarian reserve. Fertil. Steril. 2008, 90, 737. [Google Scholar] [CrossRef]
- La Marca, A.; Sighinolfi, G.; Radi, D. Antimüllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 2010, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.-J.; Bing, R.-S.; Ding, D.-C. Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer. Diagnostics 2024, 14, 2471. [Google Scholar] [CrossRef]
- Pakish, J.B.; Lu, K.H.; Sun, C.C. Endometrial cancer–associated symptoms: A case-control study. J. Womens Health 2016, 25, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Saccone, G.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. Endometrial hyperplasia and progression to cancer: Which classification system stratifies the risk better? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 1233. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Mollo, A.; De Placido, G.; Insabato, L.; Zullo, F. Endometrial hyperplasia and the risk of coexistent cancer: WHO versus EIN criteria. Histopathology 2019, 74, 676. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International patterns and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Yen, T.T.; Wang, T.L.; Fader, A.N.; Shih, I.M.; Gaillard, S. Molecular classification and emerging targeted therapy in endometrial cancer. Int. J. Gynecol. Pathol. 2020, 39, 26–35. [Google Scholar] [CrossRef]
- Nero, C.; Ciccarone, F.; Pietragalla, A.; Scambia, G. PTEN and gynecological cancers. Cancers 2019, 11, 1458. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin, Clinical Management Guidelines for Obstetrician-Gynecologists, Number 65, August 2005: Management of endometrial cancer. Obstet. Gynecol. 2005, 106, 413–425. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Chauvin, F.; Leblanc, E.; Caux, C.; Hoarau, H.; Bonnetain, F.; Christophe, V.; Sastre-Garau, X.; Lazennec, G.; Poulain, L.; et al. Le PAIR-gynécologie: Recherche multi/interdisciplinaire en cancérologie gynécologique. Les problèmes à résoudre en 2012 [PAIR-gynaecology: Multi/interdisciplinary for gynecologic cancer research. Problems needed to be resolved]. Bull. Cancer 2012, 99, 479–498. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple functions in human malignant tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef]
- Bozkurt, K.K.; Yalçın, Y.; Erdemoğlu, E.; Tatar, B.; Erdemoğlu, E.; Çerçi, S.S.; Çiriş, İ.M.; Başpınar, Ş.; Uğuz, A.; Kapucuoğlu, N. The role of immunohistochemical adrenomedullin and Bcl-2 expression in development of type-1 endometrial adenocarcinoma: Adrenomedullin expression in endometrium. Pathol. Res. Pract. 2016, 212, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.P.; Meeker, A.; Guido, R.; Gunter, M.J.; Huang, G.S.; Luhn, P.; d’Ambrosio, L.; Wentzensen, N.; Sherman, M.E. PTEN Expression in Benign Human Endometrial Tissue and Cancer in Relation to Endometrial Cancer Risk Factors. Cancer Causes Control 2015, 26, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Saccone, G.; Campanino, M.R.; Mollo, A.; De Placido, G.; Insabato, L.; Zullo, F. Loss of PTEN expression as diagnostic marker of endometrial precancer: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2019, 98, 275–286. [Google Scholar] [CrossRef]
- Okuda, T.; Sekizawa, A.; Purwosunu, Y.; Nagatsuka, M.; Morioka, M.; Hayashi, M.; Okai, T. Genetics of endometrial cancers. Obstet. Gynecol. Int. 2010, 2010, 984013. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The association and significance of p53 in gynecologic cancers: The potential of targeted therapy. Int. J. Mol. Sci. 2019, 20, 5482. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Yan, X.J.; Guo, Y.J.; Wang, J.; Wen, X.D.; Wang, N.; Yang, Y. Transvaginal real-time shear wave elastography in the diagnosis of endometrial lesions. Int. J. Gen. Med. 2021, 14, 2849–2856. [Google Scholar] [CrossRef]
- Baușic, A.; Coroleucă, C.; Coroleucă, C.; Comandașu, D.; Matasariu, R.; Manu, A.; Frîncu, F.; Mehedințu, C.; Brătilă, E. Transvaginal Ultrasound vs. Magnetic Resonance Imaging (MRI) Value in Endometriosis Diagnosis. Diagnostics 2022, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Noventa, M.; Scioscia, M.; Schincariol, M.; Cavallin, F.; Pontrelli, G.; Virgilio, B.; Vitale, S.G.; Laganà, A.S.; Dessole, F.; Cosmi, E.; et al. Imaging modalities for diagnosis of deep pelvic endometriosis: Comparison between transvaginal sonography, rectal endoscopy sonography and magnetic resonance imaging. A head-to-head meta-analysis. Diagnostics 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Loiacono, R.M.; Trojano, G.; Del Gaudio, N.; Kardhashi, A.; Deliso, M.A.; Falco, G.; Sforza, R.; Laera, A.F.; Galise, I.; Trojano, V. Hysteroscopy as a valid tool for endometrial pathology in patients with postmenopausal bleeding or asymptomatic patients with a thickened endometrium: Hysteroscopic and histological results. Gynecol. Obstet. Investig. 2015, 79, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Girschik, J.; Miller, L.J.; Addiscott, T.; Daube, M.; Katirs, P.; Ransom, D.; Slevin, T.; Threfall, T.; Weeramanthri, T.S. Precision in setting cancer prevention priorities: Synthesis of data, literature, and expert opinion. Front. Public Health 2017, 5, 125. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | n (%) or Summary |
|---|---|
| Age group, years | |
| 45–50 | 87 (27.6) |
| 51–55 | 228 (72.4) |
| Systemic comorbidities | |
| Cardiovascular disease (primarily hypertension) | 84 (26.7) |
| Obesity | 73 (23.2) |
| Diabetes mellitus | 28 (8.9) |
| Gynecologic/breast comorbidities | |
| Uterine fibroids/polyfibromatosis | 52 (16.5) |
| Polycystic ovary syndrome (PCOS) | 18 (5.7) |
| Infertility | 36 (11.4) |
| Fibrocystic breast disease | 18 (5.7) |
| Estrogen-secreting ovarian tumors | 6 (1.9) |
| Ultrasound findings | |
| Endometrial thickness, mm | range 5–17 |
| Cystic/polypoid structures (suggestive of polyps) | 32 (10.2) |
| Heterogeneous endometrial architecture (suspicious for carcinoma) | 12 (3.8) |
| Category | n/N | % (95% CI) |
|---|---|---|
| Hyperplasia without atypia | 235/315 | 74.6 (69.5–79.1) |
| Atypical hyperplasia/EIN (AH/EIN) | 63/315 | 20.0 (16.0–24.8) |
| Endometrial adenocarcinoma | 17/315 | 5.4 (3.4–8.5) |
| Risk Factor | Simple Hyperplasia | Complex Hyperplasia (No Atypia) | Complex Atypical Hyperplasia | EIN | Adenocarcinoma | Total (%) |
|---|---|---|---|---|---|---|
| Obesity | 24 (14.7%) | 23 (31.9%) | 16 (41.0%) | 6 (25.0%) | 4 (23.5%) | 73 (23.2%) |
| Hypertension | 34 (20.9%) | 22 (30.6%) | 10 (25.6%) | 9 (37.5%) | 9 (52.9%) | 84 (26.7%) |
| Diabetes | 11 (6.7%) | 4 (5.6%) | 4 (10.3%) | 6 (25.0%) | 3 (17.6%) | 28 (8.9%) |
| Infertility | 18 (11.0%) | 8 (11.1%) | 6 (15.4%) | 2 (8.3%) | 2 (11.8%) | 36 (11.4%) |
| Fibroids/Polyps | 29 (17.8%) | 13 (18.1%) | 6 (15.4%) | 3 (12.5%) | 1 (5.9%) | 52 (16.5%) |
| Fibrocystic breast disease | 7 (4.3%) | 7 (9.7%) | 2 (5.1%) | 1 (4.2%) | 1 (5.9%) | 18 (5.7%) |
| PCOS | 9 (5.5%) | 5 (6.9%) | 2 (5.1%) | 1 (4.2%) | 1 (5.9%) | 18 (5.7%) |
| Estrogen-producing tumors | 3 (1.8%) | 2 (2.8%) | 1 (2.6%) | 0 (0.0%) | 0 (0.0%) | 6 (1.9%) |
| Factor | Chi-Square | df | p-Value | Cramér’s V |
|---|---|---|---|---|
| Obesity | 16.67 | 4 | 0.0022 | 0.23 |
| Hypertension | 10.83 | 4 | 0.0285 | 0.19 |
| Diabetes | 11.30 | 4 | 0.0234 | 0.19 |
| History of non-atypical hyperplasia | 11.26 | 4 | 0.0238 | 0.19 |
| Oral contraceptives (long-term) | 5.76 | 4 | 0.2180 | 0.14 |
| Fibrocystic breast disease | 2.89 | 4 | 0.5766 | 0.10 |
| Fibroids/Polyps | 2.03 | 4 | 0.7306 | 0.08 |
| Estrogen-producing tumors | 1.18 | 4 | 0.8807 | 0.06 |
| Estrogen replacement therapy | 1.23 | 4 | 0.8731 | 0.06 |
| Infertility | 0.86 | 4 | 0.9298 | 0.05 |
| PCOS | 0.35 | 4 | 0.9867 | 0.03 |
| Factor | OR | 95% CI (Low–High) | p-Value |
|---|---|---|---|
| Diabetes | 2.85 | 1.29–6.28 | 0.0096 |
| Obesity | 1.93 | 1.09–3.39 | 0.0230 |
| History of non-atypical hyperplasia | 0.29 | 0.13–0.67 | 0.0037 |
| Oral contraceptives (long-term) | 0.38 | 0.17–0.89 | 0.0251 |
| Hypertension | 1.72 | 0.99–2.98 | 0.0525 |
| Fibroids/Polyps | 0.66 | 0.31–1.38 | 0.2662 |
| Estrogen replacement therapy | 1.18 | 0.56–2.49 | 0.6671 |
| PCOS | 0.83 | 0.27–2.60 | 0.7503 |
| Fibrocystic breast disease | 0.83 | 0.27–2.60 | 0.7503 |
| Estrogen-producing tumors | 0.58 | 0.07–5.06 | 0.6240 |
| Infertility | 1.15 | 0.53–2.50 | 0.7275 |
| Factor | aOR | 95% CI (Low–High) | p-Value |
|---|---|---|---|
| Diabetes | 2.75 | 1.14–6.61 | 0.0237 |
| History of non-atypical hyperplasia | 0.31 | 0.13–0.72 | 0.0068 |
| Obesity | 1.79 | 0.98–3.26 | 0.0582 |
| Oral contraceptives (long-term) | 0.42 | 0.18–1.00 | 0.0512 |
| Hypertension | 1.68 | 0.93–3.03 | 0.0838 |
| Fibroids/Polyps | 0.72 | 0.33–1.58 | 0.4096 |
| Estrogen-producing tumors | 0.43 | 0.05–4.12 | 0.4673 |
| Fibrocystic breast disease | 0.63 | 0.18–2.21 | 0.4749 |
| Estrogen replacement therapy | 1.25 | 0.55–2.83 | 0.5987 |
| PCOS | 0.73 | 0.22–2.41 | 0.6072 |
| Infertility | 1.08 | 0.47–2.48 | 0.8488 |
| Age 51–55 vs. 45–50 | 0.97 | 0.52–1.81 | 0.9204 |
| Source | Sum of Squares | df | F | p-Value |
|---|---|---|---|---|
| Diagnosis | 1357.77 | 4 | 61.33 | <0.0001 |
| Residual | 1715.64 | 310 |
| Metric | Value | Interpretation |
|---|---|---|
| AUC (ROC) | 0.68 | Moderate discrimination |
| Hosmer–Lemeshow χ2 (df = 8), p | 15.4, p = 0.052 | Acceptable calibration (no lack-of-fit) |
| Nagelkerke R2 | 0.14 | Modest explained variance |
| Metric | Apparent | Optimism-corrected |
|---|---|---|
| AUC (ROC) | 0.68 | 0.66 |
| Calibration slope | — | 0.92 |
| Calibration intercept | — | ≈0.01 |
| Brier score | — | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brăila, A.D.; Tudor, V.; Poalelungi, C.-V.; Damian, C.M.; Bogdan-Andreescu, C.F.; Burcea, A.; Bănățeanu, A.-M.; Cadar, E.; Albu, C.-C. Clinical and Histopathological Correlates of Endometrial Proliferative Lesions in Perimenopausal Women: A Retrospective Study with Internal Validation of a Risk Model. Clin. Pract. 2025, 15, 177. https://doi.org/10.3390/clinpract15100177
Brăila AD, Tudor V, Poalelungi C-V, Damian CM, Bogdan-Andreescu CF, Burcea A, Bănățeanu A-M, Cadar E, Albu C-C. Clinical and Histopathological Correlates of Endometrial Proliferative Lesions in Perimenopausal Women: A Retrospective Study with Internal Validation of a Risk Model. Clinics and Practice. 2025; 15(10):177. https://doi.org/10.3390/clinpract15100177
Chicago/Turabian StyleBrăila, Anca Daniela, Viorica Tudor, Cristian-Viorel Poalelungi, Constantin Marian Damian, Claudia Florina Bogdan-Andreescu, Alexandru Burcea, Andreea-Mariana Bănățeanu, Emin Cadar, and Cristina-Crenguţa Albu. 2025. "Clinical and Histopathological Correlates of Endometrial Proliferative Lesions in Perimenopausal Women: A Retrospective Study with Internal Validation of a Risk Model" Clinics and Practice 15, no. 10: 177. https://doi.org/10.3390/clinpract15100177
APA StyleBrăila, A. D., Tudor, V., Poalelungi, C.-V., Damian, C. M., Bogdan-Andreescu, C. F., Burcea, A., Bănățeanu, A.-M., Cadar, E., & Albu, C.-C. (2025). Clinical and Histopathological Correlates of Endometrial Proliferative Lesions in Perimenopausal Women: A Retrospective Study with Internal Validation of a Risk Model. Clinics and Practice, 15(10), 177. https://doi.org/10.3390/clinpract15100177







