Cerebrovascular Accidents After Orthotopic Liver Transplantation in Patients with Hepatopulmonary Syndrome: A Case Series
Abstract
1. Introduction
2. Case Series
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
2.5. Case 5
3. Discussion
4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| HPS | Hepatopulmonary syndrome |
| V/Q | Ventilation–perfusion |
| OLT | Orthotopic liver transplantation |
| PaO2 | Partial pressure of oxygen |
| MELD | Model for end-stage liver disease |
| CVA | Cerebrovascular accident |
| ESLD | End-stage liver disease |
| TTE | Transthoracic echocardiography |
| FiO2 | Fraction of inspired oxygen |
| HFNC | High-flow nasal cannula |
| DM | Diabetes mellitus |
| POD | Post-operative day |
| CT | Computer tomography |
| MASH | Metabolic-dysfunction-associated steatohepatitis |
| GERD | Gastroesophageal reflux disease |
| CKD | Chronic kidney disease |
| 99mTc-MAA | Technetium 99mTc albumin aggregated |
| HCC | Hepatocellular carcinoma |
| ICU | Intensive care unit |
| MRI | Magnetic resonance imaging |
| MR | Magnetic resonance |
| MCA | Middle cerebral artery |
| HITS | High-intensity transient signals |
| PFO | Patent foramen ovale |
| APTT | Activated partial thromboplastin time |
| EEG | Electroencephalogram |
| A-a | Alveolar–arterial |
| AV | Arteriovenous |
| SD | Standard deviations |
| TIA | Transient ischemic attack |
| ICH | Intracranial hemorrhage |
| PAVM | Pulmonary venous malformations |
References
- Schenk, P.; Fuhrmann, V.; Madl, C.; Funk, G.; Lehr, S.; Kandel, O.; Müller, C. Hepatopulmonary syndrome: Prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut 2002, 51, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, B.; Tang, L.; Wang, Y.; Stockard, C.R.; Kadish, I.; Van Groen, T.; Grizzle, W.E.; Ponnazhagan, S.; Fallon, M.B. Pulmonary Angiogenesis in a Rat Model of Hepatopulmonary Syndrome. Gastroenterology 2009, 136, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.A.; Jaffe, C.C.; Hoffer, P.B.; Binder, H.J.; Fallon, M.B. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology 1995, 109, 1283–1288. [Google Scholar] [CrossRef]
- Fallon, M.B.; Krowka, M.J.; Brown, R.S.; Trotter, J.F.; Zacks, S.; Roberts, K.E.; Shah, V.H.; Kaplowitz, N.; Forman, L.; Wille, K.; et al. Impact of Hepatopulmonary Syndrome on Quality of Life and Survival in Liver Transplant Candidates. Gastroenterology 2008, 135, 1168–1175. [Google Scholar] [CrossRef]
- Santos, J.; Young, P.; Barjaktarevic, I.; Lazar, C.; Susanto, I.; Wang, T. The Successful Use of Inhaled Nitric Oxide in the Management of Severe Hepatopulmonary Syndrome after Orthotopic Liver Transplantation. Case Reports Hepatol. 2014, 2014, 415109. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarevic, I.; Cortes Lopez, R.; Steadman, R.; Wray, C.; Qadir, N.; Chang, S.Y.; Wang, T. Perioperative Considerations in Liver Transplantation. Semin. Respir. Crit. Care Med. 2018, 39, 609–624. [Google Scholar]
- Molleston, J.P.; Kaufman, B.A.; Cohen, A.; Shackelford, P.G.; Keating, J.P.; Lowell, J.A.; Howard, T.K. Brain Abscess in Hepatopulmonary Syndrome. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 225–226. [Google Scholar] [PubMed]
- Younce, J.R.; Cross, D.T.; Goyal, M.S.; Lee, J.M. Multifocal stroke with proliferation of small cerebral arteries in hepatopulmonary syndrome. Neurol. Clin. Pract. 2018, 8, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Shijo, H.; Sasaki, H.; Nishimaru, K.; Okumura, M. Recurrent Intracranial Hemorrhagic Episodes in Hepatopulmonary Syndrome. Intern. Med. 1992, 31, 786–790. [Google Scholar] [CrossRef][Green Version]
- Sulieman, B.M.; Hunsicker, L.G.; Katz, D.A.; Voigt, M.D. OPTN Policy Regarding Prioritization of Patients with Hepatopulmonary Syndrome: Does It Provide Equitable Organ Allocation? Am. J. Transplant. 2008, 8, 954–964. [Google Scholar] [CrossRef]
- Boudouresques, G.; Hauw, J.; Meininger, V.; Escourolle, R.; Pertuiset, B.; Buge, A.; Lhermitte, F.; Castaigne, P. Hepatic cirrhosis and intracranial hemorrhage: Significance of the association in 53 pathological cases. Ann. Neurol. 1980, 8, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.; Schöniger-Hekele, M.; Fuhrmann, V.; Madl, C.; Silberhumer, G.; Müller, C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology 2003, 125, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Shin, S.; Baek, C.H.; Chang, J.Y.; Kang, D.-W.; Kwon, S.U.; Kim, J.S.; Kim, B.J. Characteristics of stroke after liver and kidney transplantation. Front. Neurol. 2023, 14, 1123518. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.J.; Moon, J.I.; Kato, T.; Nishida, S.; Selvaggi, G.; Levi, D.M.; Island, E.R.; Pyrsopoulos, N.; Weppler, D.; Ganz, S.; et al. A Cause-Specific Hazard Rate Analysis of Prognostic Factors Among 877 Adults Who Received Primary Orthotopic Liver Transplantation. Transplantation 2007, 84, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, J.; Nguyen-Buckley, C.; Nguyen-Lee, J.; Wray, C.; Agopian, V.; Busuttil, R.W.; Steadman, R.H.; Xia, V.W. Intraoperative Hypertension and Thrombocytopenia Associated With Intracranial Hemorrhage After Liver Transplantation. Transplantation 2020, 104, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M.; De Groen, P.C.; Wiesner, R.H.; Krom, R.A.F. Intracerebral Hemorrhage in Liver Transplant Recipients. Mayo Clin. Proc. 1995, 70, 443–446. [Google Scholar] [CrossRef]
- Rodríguez-Roisin, R.; Krowka, M.J. Hepatopulmonary syndrome—A liver-induced lung vascular disorder. N. Engl. J. Med. 2008, 358, 2378–2387. [Google Scholar] [CrossRef]
- Berthelot, P.; Walker, J.G.; Sherlock, S.; Reid, L. Arterial Changes in the Lungs in Cirrhosis of the Liver—Lung Spider Nevi. N. Engl. J. Med. 1966, 274, 291–298. [Google Scholar] [CrossRef]
- Das, A.; Greisman, J.D.; Vazquez, S.; Feldstein, E.; Spirollari, E.; Lui, A.; Yang, K.; Dominguez, J.F.; Epelbaum, O.; Harris, K.; et al. Acute Ischemic Stroke in Patients With Pulmonary Arteriovenous Malformations: Paradoxical Embolism or Epiphenomenon? Stroke: Vasc. Interv. Neurol. 2023, 3, e000571. [Google Scholar] [CrossRef]
- Gossage, J.R.; Kanj, G. Pulmonary arteriovenous malformations: A state of the art review. Am. J. Respir. Crit. Care Med. 1998, 158, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Aljeradat, B.; Koneru, M.; Oliveira, R.; Shaikh, H. Bilateral Arteriovenous Shunting Through Pial and Perforating Vessels With Multiple Strokes in a Patient With Hepatopulmonary Syndrome. Cureus 2023, 15, e42756. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V. Cerebral amyloid angiopathy. A critical review. Stroke 1987, 18, 311–324. [Google Scholar] [CrossRef]
- Carvalho, A.; Rodrigues, M.; Rocha, M.; Nunes, J.; Cunha, A.; Costa, H.; Barros, P. Multiple recurrent intracerebral hemorrhages and hepatopulmonary syndrome. Neurol. Clin. Pract. 2019, 9, e27–e29. [Google Scholar] [CrossRef] [PubMed]


| Case no. | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Biodata | Age (years), Gender at Birth | 45 Female | 33 Female | 31 Female | 60 Male | 49 Male |
| Etiology of End- Stage Liver Disease (ESLD) | Hepatitis C | Metabolic Dysfunction-associated Steatohepatitis (MASH) | Biliary Atresia | MASH complicated by Hepatocellular Carcinoma | Alcohol-associated Cirrhosis | |
| Medical History Risk factors (1) | none | Chronic Kidney Disease | none | Previous post- traumatic small subarachnoid hemorrhage 6 weeks prior to OLT | Previous transient ischemic attack | |
| Hepatopulmonary Syndrome (HPS) History | Oxygen Requirement at index admission | FiO2 0.45 via High Flow Nasal Cannula, SpO2 82–94% | PaO2 76 mmHg on room air, SpO2 93% | PaO2 66 mmHg on room air | PaO2 88 mmHg on room air | PaO2 66 mmHg on room air |
| Diagnostic Findings | Transthoracic Echocardiography (TTE): Patent Foramen Ovale (PFO) + HPS Shunt study: 23.2% shunt fraction | TTE bubble study: Mild right-to-left shunting at rest, consistent with transpulmonary shunt | TTE: PFO + HPS, large shunt 99mTc-MAA scintigraphy: right-to-left shunt, 15.6% | T TE bubble study: positive with late transition, consistent with transpulmonary shunt | TTE bubble study: large degree of right-to-left shunting, likely due to both interatrial and transpulmonary shunt | |
| Management | Bubble filters | Bubble filters | PO garlic 1000 mg two times a day | none | PO garlic 1000 mg two times a day | |
| Orthotopic Liver Transplant (OLT) History | Physiologic MELD score at transplant | 12 | 30 | 33 | 31 | 26 |
| Complications of ESLD | Hepatorenal Syndrome | Ascites, Esophageal Varices | Ascites, esophageal varices, hyponatremia, thrombocytopenia, recurrent cholangitis | Hepatic encephalopathy, esophageal and gastric varices, thrombocytopenia | Ascites, esophageal varices, pancytopenia | |
| Mechanical Ventilation days | 23 | N/A | 1 | 5 | 7 | |
| ICU stay Post-OLT (days) | 27 | 4 | 12 | 17 | 35 | |
| Cerebrovascular Accident (CVA) History | Date of CVA (POD) | 39 | 3 | 5 | 10 | 9 |
| Patient Location | Subacute rehabilitation unit | Inpatient, ICU | Inpatient, ICU | Inpatient, medical floor | Inpatient, ICU | |
| Coagulation Profile | INR: 1.1 Plt: 189 × 109/L | INR: 1.2 Plt: 44 | INR: 1.3 Plt: 156 | INR: 1.1 Plt: 95 | INR: 1.1 APTT: 40.7 s Plt: 34 | |
| Liver Function Test | AST: 40 U/L ALT: 115 U/L ALP: 301 U/L Bilirubin (total): 0.9 mg/dL | AST: 93 ALT: 134 ALP: 71 Bilirubin (total): 9.0 | AST: 13 ALT: 93 ALP: 69 Bilirubin (total): 3.3 | AST: 46 ALT: 59 ALP: 210 Bilirubin (total): 2.1 | AST: 14 ALT: 60 ALP: 79 Bilirubin (total): 2.0 | |
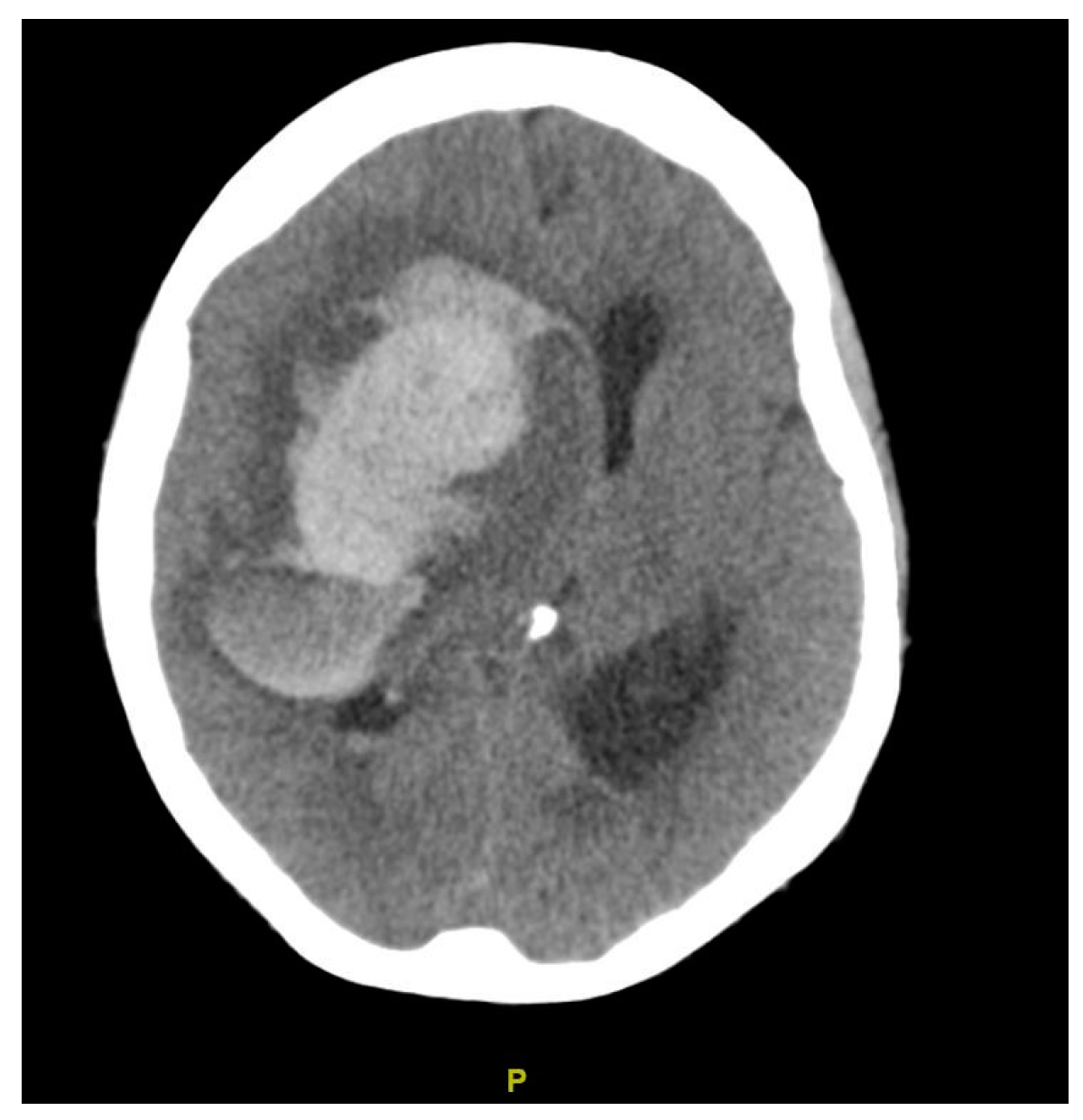
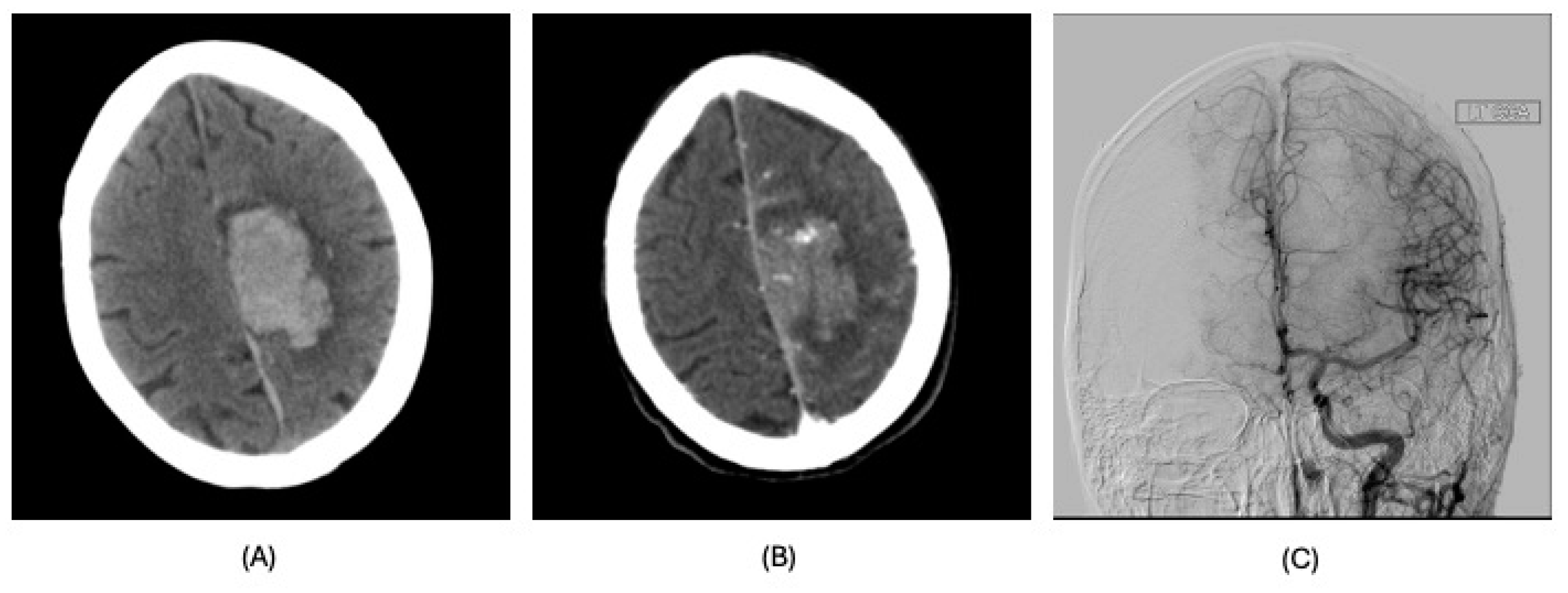
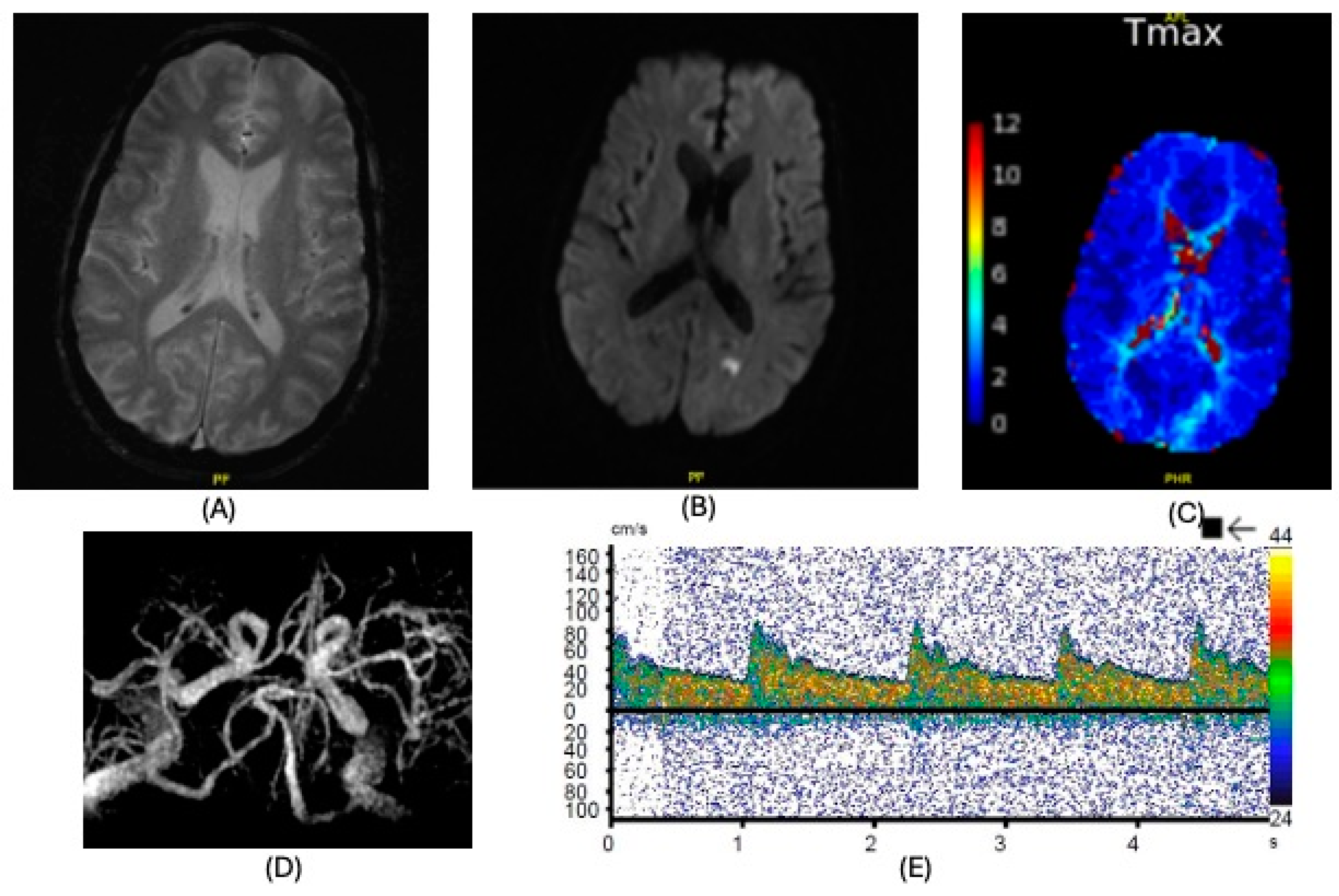
| Diagnosis | Lobar intracranial hemorrhage (ICH) | Basal Ganglia ICH | Lobar ICH | Embolic ischemic stroke (IS) | Embolic IS | |
| Clinical Syndrome | Left headache, right hemianopia | Persistent drowsiness post-OLT, GCS E1VTM2, dilated right pupil | Headache, right hemiparesis, right lower limb sensory deficits | Aphasia | Altered mental status, right gaze preference, left flaccid paralysis | |
| Imaging | CT scan | CT scan | CT scan MRI CT angiography | CT scan MRI MR angiography | CT scan CT angiography MRI | |
| Findings | CT: Left occipital intraparenchymal hematoma Volume: 15 mL | CT: Large right frontal temporal parenchymal hematoma, severe surrounding mass effect with leftward midline shift, right uncal herniation Volume: 93.5 mL | CT: Right frontoparietal parenchymal hematoma with locoregional mass effect, rightward midline shift Volume: 25 mL | CT: Left occipital infarct Transcranial doppler: 2 spontaneous emboli, more than 100 high intensity transient signals (HITS) in bilateral middle cerebral arteries (MCAs) after agitated saline | CT: Right parietal lobe hypodensity Transcranial doppler: more than 300 HITS in bilateral MCAs after agitated saline | |
| Management | Conservative | Non-operative in view of poor prognosis | Endoscopic left craniotomy for evacuation of hematoma | Conservative | Conservative management; tracheostomized and decannulated 1 month after CVA | |
| Post-CVA Status | Length-of-Stay (LOS) | Hospital LOS 28 days | Post-OLT LOS 4 days | Post-OLT LOS 22 days | Post-OLT LOS 45 days | Post-OLT LOS 84 days |
| Sequelae, Discharge | Discharged with persistent right hemianopia | Death discharge | Discharged with residual right-sided weakness | Discharged with mild dysphoria, no other deficits | Discharged, able to ambulate with a walker and moderate assistance | |
| Status at 360 Days | Alive | Deceased | Alive | Alive | Alive at 4 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.K.; Buitrago Blanco, M.M.; Feduska, N.J.; Agopian, V.G.; Ebaid, S.S.; Wang, T.; Tamhaney, A.; Barjaktarevic, I. Cerebrovascular Accidents After Orthotopic Liver Transplantation in Patients with Hepatopulmonary Syndrome: A Case Series. Clin. Pract. 2025, 15, 5. https://doi.org/10.3390/clinpract15010005
Chan SK, Buitrago Blanco MM, Feduska NJ, Agopian VG, Ebaid SS, Wang T, Tamhaney A, Barjaktarevic I. Cerebrovascular Accidents After Orthotopic Liver Transplantation in Patients with Hepatopulmonary Syndrome: A Case Series. Clinics and Practice. 2025; 15(1):5. https://doi.org/10.3390/clinpract15010005
Chicago/Turabian StyleChan, Steffi K., Manuel M. Buitrago Blanco, Nicholas J. Feduska, Vatche G. Agopian, Samer S. Ebaid, Tisha Wang, Ami Tamhaney, and Igor Barjaktarevic. 2025. "Cerebrovascular Accidents After Orthotopic Liver Transplantation in Patients with Hepatopulmonary Syndrome: A Case Series" Clinics and Practice 15, no. 1: 5. https://doi.org/10.3390/clinpract15010005
APA StyleChan, S. K., Buitrago Blanco, M. M., Feduska, N. J., Agopian, V. G., Ebaid, S. S., Wang, T., Tamhaney, A., & Barjaktarevic, I. (2025). Cerebrovascular Accidents After Orthotopic Liver Transplantation in Patients with Hepatopulmonary Syndrome: A Case Series. Clinics and Practice, 15(1), 5. https://doi.org/10.3390/clinpract15010005






