Immunosuppression and Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Impact of Age on Survival
2.3. Sex-Based Differences
2.4. Tumor Stage and Survival
2.5. History of Tumor Surgical Treatment
2.6. Immunosuppression as a Key Determinant
3. Results
4. Discussion
Staging Systems and Risk Stratification in cSCC
5. Management of cSCC
5.1. Radiotherapy
5.2. Immunotherapy
5.3. Anti-EGFR (Epidermal Growth Factor Receptor)
5.4. Complications
6. Limitations of This Study and Future Perspectives
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokez, S.; Wakkee, M.; Kan, W.; Venables, Z.C.; Mooyaart, A.L.; Louwman, M.; Nijsten, T.; Hollestein, L.M. Cumulative incidence and disease-specific survival of metastatic cutaneous squamous cell carcinoma: A nationwide cancer registry study. J. Am. Acad. Dermatol. 2022, 86, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Skulsky, S.L.; O’Sullivan, B.; McArdle, O.; Leader, M.; Roche, M.; Conlon, P.J.; O’Neill, J.P. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines in Oncology. Head Neck 2017, 39, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, M.J.; Low, T.-H.; Ebrahimi, A.; Veness, M.; Ashford, B.; Porceddu, S.; Clark, J.R. Evolution of Head and Neck Cutaneous Squamous Cell Carcinoma Nodal Staging—An Australian Perspective. Cancers 2022, 14, 5101. [Google Scholar] [CrossRef]
- Palmer, J.D.; Schneider, C.J.; Hockstein, N.; Hanlon, A.L.; Silberg, J.; Strasser, J.; Mauer, E.A.; Dzeda, M.; Witt, R.; Raben, A. Combination of post-operative radiotherapy and cetuximab for high-risk cutaneous squamous cell cancer of the head and neck: A propensity score analysis. Oral Oncol. 2018, 78, 102–107. [Google Scholar] [CrossRef]
- Luk, P.P.; Ebrahimi, A.; Veness, M.J.; McDowell, L.; Magarey, M.; Gao, K.; Palme, C.E.; Clark, J.R.; Gupta, R. Prognostic value of the 8th edition American Joint Commission Cancer nodal staging system for patients with head and neck cutaneous squamous cell carcinoma: A multi-institutional study. Head Neck 2021, 43, 558–567. [Google Scholar] [CrossRef]
- Farberg, A.S.; Hall, M.A.; Douglas, L.; Covington, K.R.; Kurley, S.J.; Cook, R.W.; Dinehart, S.M. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: Impact on patient management. Curr. Med. Res. Opin. 2020, 36, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, S.; Fanizzi, A.; Quaresmini, D.; Nardone, A.; Armenio, A.; Figliuolo, F.; Filotico, R.; Fucci, L.; Mele, F.; Traversa, M.; et al. Cemiplimab in an elderly frail population of patients with locally advanced or metastatic cutaneous squamous cell carcinoma. Front. Oncol. 2021, 11, 686308. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Boldrini, L.; Menna, S.; Venuti, V.; Pernice, G.; Fanzese, C.; Angileri, T.; Deidone, A. Hypofractionated radiotherapy in head and neck cancer elderly patients: A feasibility and safety systematic review for the clinician. Front. Oncol. 2021, 11, 761393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Kim, L.; Thariat, J.; Baumert, B.G.; Mazibuko, T.; Gorobets, O.; Vinh-Hung, V.; Giap, H.; Mehmood, T.; Vincent, F.; et al. Immunotherapy and modern radiotherapy technique for older patients with locally advanced head and neck cancer: A proposed paradigm by the International Geriatric Radiotherapy Group. Cancers 2022, 14, 5285. [Google Scholar] [CrossRef]
- Muto, P.; Pastore, F. Radiotherapy in the adjuvant and advanced setting of CSCC. Dermatol. Pract. Concept. 2021, 11 (Suppl. S2), e2021168S. [Google Scholar] [CrossRef]
- Massey, P.R.; Schmults, C.D.; Li, S.J.; Arron, S.T.; Asgari, M.M.; Bavinck, J.N.B.; Billingsley, E.; Blalock, T.W.; Blasdale, K.; Carroll, B.T.; et al. Consensus-Based Recommendations on the Prevention of Squamous Cell Carcinoma in Solid Organ Transplant Recipients: A Delphi Consensus Statement. JAMA Dermatol. 2021, 157, 1219–1226. [Google Scholar] [CrossRef]
- Sharma, A.; Birnie, A.J.; Bordea, C.; Cheung, S.T.; Mann, J.; A Morton, C.; Salim, A.; Hasan, Z.-U.; Hashme, M.; Kiaee, Z.M.; et al. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma in situ (Bowen disease) 2022. Br. J. Dermatol. 2023, 188, 186–194. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Chudek, D.A.; Flynn, C.D.; Gaskell, P.; Loh, C.; Tandon, S.; Roland, N.J.; Jones, T.M.; Lancaster, J. Outcomes and prognosticators in regionally recurrent cutaneous squamous cell carcinoma of the head and neck. Eur. J. Surg. Oncol. 2020, 46, 2035–2041. [Google Scholar] [CrossRef]
- Daloiso, A.; Franz, L.; Mondello, T.; Tisato, M.; Negrisolo, M.; Zanatta, P.; de Filippis, C.; Astolfi, L.; Marioni, G. Head and Neck Squamous Cell Carcinoma with Distant Metastasis: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3887. [Google Scholar] [CrossRef] [PubMed]
- Manyam, B.V.; Garsa, A.A.; Chin, R.-I.; Reddy, C.A.; Gastman, B.; Thorstad, W.; Yom, S.S.; Nussenbaum, B.; Wang, S.J.; Vidimos, A.T.; et al. A multi-institutional comparison of outcomes of immunosuppressed and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Cancer 2017, 123, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Lukowiak, T.M.; Aizman, L.; Perz, A.; Miller, C.J.; Sobanko, J.F.; Shin, T.M.; Giordano, C.N.; Higgins, H.W.; Etzkorn, J.R. Association of Age, Sex, Race, and Geographic Region with Variation of the Ratio of Basal Cell to Cutaneous Squamous Cell Carcinomas in the United States. JAMA Dermatol. 2020, 156, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Brown, M.; Golda, N.; Goldberg, D.; Miller, C.; Pugliano-Mauro, M.; Schmults, C.; Shin, T.; Stasko, T.; Xu, Y.G.; et al. Nodal staging of high-risk cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2019, 81, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Post-Operative Radiation with Cetuximab for Locally Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck. 2022. Available online: https://clinicaltrials.gov/study/NCT01979211 (accessed on 29 April 2023).
- A Study of Tremelimumab and IV Durvalumab Plus Poly-ICLC in Subjects with Biopsy-Accessible Cancers. 2022. Available online: https://clinicaltrials.gov/study/NCT02643303 (accessed on 15 May 2023).
- Trigriluzole with Nivolumab and Pembrolizumab in Treating Patients with Metastatic or Unresectable Solid Malignancies or Lymphoma. 2022. Available online: https://clinicaltrials.gov/study/NCT03229278 (accessed on 15 May 2023).
- Ph1 Study of SL-172154 Administered Intratumorally in Subjects with Squamous Cell Carcinoma of the Head and Neck or Skin. 2022. Available online: https://clinicaltrials.gov/study/NCT04502888 (accessed on 15 May 2023).
- Post-Operative Concurrent Chemo-Radiotherapy Versus Post-Operative Radiotherapy for Cancer of the Head and Neck. 2016. Available online: https://clinicaltrials.gov/study/NCT00193895 (accessed on 25 February 2024).
- Palbociclib and Cetuximab Versus Cetuximab Monotherapy for Patients with CDKN2A-Altered, HPV-Unrelated Head and Neck Squamous Cell Carcinoma Who Experienced Disease Progression on a PD-1/L1 Inhibitor. 2024. Available online: https://clinicaltrials.gov/study/NCT04966481 (accessed on 3 March 2024).
- Jia, W.; Gao, Q.; Han, A.; Zhu, H.; Yu, J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol. Med. 2019, 16, 655–670. [Google Scholar] [CrossRef]
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Egloff, A.M.; Afeyan, A.B.; Wolff, J.O.; Zeng, Z.; Chernock, R.D.; Zhou, L.; Messier, C.; Lizotte, P.; Pfaff, K.L.; et al. Preexisting tumor-resident T cells with cytotoxic potential associate with response to neoadjuvant anti-PD-1 in head and neck cancer. Sci. Immunol. 2023, 8, eadf4968. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hirakawa, H.; Suzuki, M.; Higa, T.; Agena, S.; Hasegawa, N.; Kawakami, J.; Toyama, M.; Higa, T.; Kinjyo, H.; et al. Biomarkers for Predicting Anti-Programmed Cell Death-1 Antibody Treatment Effects in Head and Neck Cancer. Curr. Oncol. 2023, 30, 5409–5424. [Google Scholar] [CrossRef] [PubMed]
- Furgiuele, S.; Descamps, G.; Lechien, J.R.; Dequanter, D.; Journe, F.; Saussez, S. Immunoscore Combining CD8, FoxP3, and CD68-Positive Cells Density and Distribution Predicts the Prognosis of Head and Neck Cancer Patients. Cells 2022, 11, 2050. [Google Scholar] [CrossRef]
- Becker, A.-S.; Kluge, C.; Schofeld, C.; Zimpfer, A.H.; Schneider, B.; Strüder, D.; Redwanz, C.; Ribbat-Idel, J.; Idel, C.; Maletzki, C. Correction: Becker et al. Identifying Predictive Biomarkers for Head and Neck Squamous Cell Carcinoma Response. Cancers 2023, 15, 5597, Erratum in Cancers 2024, 16, 3554. https://doi.org/10.3390/cancers16203554. [Google Scholar] [CrossRef]
- Amaral, T.; Osewold, M.; Presser, D.; Meiwes, A.; Garbe, C.; Leiter, U. Advanced cutaneous squamous cell carcinoma: Real world data of patient profiles and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fulga, A.; Cristea Ene, D.; Bujoreanu Bezman, L.; Dragostin, O.M.; Fulga, I.; Stamate, E.; Piraianu, A.I.; Bujoreanu, F.; Tatu, A.L. Pharyngeal-Esophageal Malignancies with Dermatologic Paraneoplastic Syndrome. Life 2022, 12, 1705. [Google Scholar] [CrossRef]
- Voruganti, I.S.; Poon, I.; Husain, Z.A.; Bayley, A.; Barnes, E.A.; Zhang, L.; Chin, L.; Erler, D.; Higgins, K.; Enepekides, D.; et al. Stereotactic body radiotherapy for head and neck skin cancer. Radiother. Oncol. 2021, 165, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Lahmi, L.; Meillan, N.; Pietta, G.; Albert, S.; Maingon, P. Treatment Strategy for Distant Synchronous Metastatic Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2019, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Bobeica, C.; Onisor, C.; Gurau, G.; Nechita, A.; Radaschin, D.S.; Tutunaru, D.; Bujoreanu-Bezman, L.; Tatu, A.L. Basal cell carcinoma perineural invasion and suggestive signs of perineural invasion-findings and perspectives. Life 2023, 13, 1406. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Kondo, T.; Okamoto, I.; Sato, H.; Koyama, N.; Fushimi, C.; Okada, T.; Masubuchi, T.; Miura, K.; Matsuki, T.; Yamashita, T.; et al. Age-based efficacy and safety of nivolumab for recurrent or metastatic head and neck squamous cell carcinoma: A multicenter retrospective study. Asia Pac. J. Clin. Oncol. 2020, 16, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Botticelli, A.; Mezi, S.; Pomati, G.; Sciattella, P.; Cerbelli, B.; Roberto, M.; Mammone, G.; Cirillo, A.; Cassano, A.; Di Dio, C.; et al. The impact of locoregional treatment on response to nivolumab in advanced platinum refractory head and neck cancer: The need trial. Vaccines 2020, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Bellia, S.R.; Feliciani, G.; del Ducca, M.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, I.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing allpha emitters radiation therapy: A case report. J. Contemp. Brachytherapy 2019, 5, 449–457. [Google Scholar] [CrossRef]
- Funk-Debleds, P.; Ducroux, E.; Guillaud, O.; Ursic-Bedoya, J.; Decullier, E.; Vallin, M.; Euvrard, S.; Pageaux, G.-P.; Boillot, O.; Dumortier, J. Subsequent nonmelanoma skin cancers and impact of immunosuppression in liver transplant recipients. J. Am. Acad. Dermatol. 2018, 79, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Prabhakar, N.K.; Davuluri, R.; Morrison, C.M.; Yi, S.K. Role of Concurrent Systemic Therapy with Adjuvant Radiation Therapy for Locally Advanced Cutaneous Head and Neck Squamous Cell Carcinoma. Cureus 2017, 9, e1784. [Google Scholar] [CrossRef] [PubMed]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, G.; Zitelli, J.A.; Brodland, D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J. Am. Acad. Dermatol. 2019, 80, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Macedo, S.; Fernandes, M.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. TERT Promoter Mutations Are Associated with Poor Prognosis in Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 80, 660–669.e6. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Ridgway, R.A.; Cammareri, P.; Treanor-Taylor, M.; Bailey, U.-M.; Schoenherr, C.; Bone, M.; Schreyer, D.; Purdie, K.; Thomson, J.; et al. Driver Gene Combinations Dictate Cutaneous Squamous Cell Carcinoma Disease Continuum Progression. Nat. Commun. 2023, 14, 5211. [Google Scholar] [CrossRef] [PubMed]
- Danishevich, A.; Bilyalov, A.; Nikolaev, S.; Khalikov, N.; Isaeva, D.; Levina, Y.; Makarova, M.; Nemtsova, M.; Chernevskiy, D.; Sagaydak, O.; et al. CDKN2A Gene Mutations: Implications for Hereditary Cancer Syndromes. Biomedicines 2023, 11, 3343. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kemp, V.; Lamfers, M.L.M.; van der Pluijm, G.; van den Hoogen, B.G.; Hoeben, R.C. Developing oncolytic viruses for clinical use: A consortium approach. Cytokine Growth Factor Rev. 2020, 56, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yom, S.S.; Torres-Saavedra, P.A.; Kuperwasser, C.; Kumar, S.; Gupta, P.B.; Ha, P.; Geiger, J.L.; Banerjee, R.; Thorstad, B.; Blakaj, D.; et al. Association of plasma tumor tissue modified viral HPV DNA with tumor burden, treatment type, and outcome: A translational analysis from NRG-HN002. J. Clin. Oncol. 2022, 40, 6006. [Google Scholar] [CrossRef]
- Szatmari, T.; Mocan, S.; Neagos, C.M.; Pap, Z. Biomarker Profiles and Clinicopathological Features in Head and Neck Squamous Cell Carcinoma Patients. Medicina 2024, 60, 1681. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Resteghini, C.; Paielli, N.; Licitra, L.; Pilotti, S.; Perrone, F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 74362–74379. [Google Scholar] [CrossRef] [PubMed]
- Dorobisz, K.; Dorobisz, T.; Pazdro-Zastawny, K. Analysis of Risk Factors with Assessment of the Impact of the Microbiome on the Risk of Squamous Cell Carcinoma of the Larynx. J. Clin. Med. 2024, 13, 6101. [Google Scholar] [CrossRef]
- Miranda-Galvis, M.; Loveless, R.; Kowalski, L.P.; Teng, Y. Impacts of Environmental Factors on Head and Neck Cancer Pathogenesis and Progression. Cells 2021, 10, 389. [Google Scholar] [CrossRef]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef]
- Wu, I.-C.; Chen, Y.-C.; Karmakar, R.; Mukundan, A.; Gabriel, G.; Wang, C.-C.; Wang, H.-C. Advancements in Hyperspectral Imaging and Computer-Aided Diagnostic Methods for the Enhanced Detection and Diagnosis of Head and Neck Cancer. Biomedicines 2024, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.K.; Zhou, X.; Palsgrove, D.; Sumer, B.D.; Chen, A.Y.; Fei, B. An ensemble learning method for detection of head and neck squamous cell carcinoma using polarized hyperspectral microscopic imaging. In Proceedings of the Medical Imaging 2024: Digital and Computational Pathology, Edinburgh, UK, 16–19 September 2024; pp. 169–178. [Google Scholar]
- Crossman, B.E.; Harmon, R.L.; Kostecki, K.L.; McDaniel, N.K.; Iida, M.; Corday, L.W.; Glitchev, C.E.; Crow, M.T.; Harris, M.A.; Lin, C.Y.; et al. From Bench to Bedside: A Team’s Approach to Multidisciplinary Strategies to Combat Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2024, 13, 6036. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, N.K.; Iida, M.; Nickel, K.P.; Longhurst, C.A.; Fischbach, S.R.; Rodems, T.S.; Kranjac, C.A.; Bo, A.Y.; Luo, Q.; Gallagher, M.M.; et al. AXL Mediates Cetuximab and Radiation Resistance Through Tyrosine 821 and the c-ABL Kinase Pathway in Head and Neck Cancer. Clin. Cancer Res. 2020, 26, 4349–4359. [Google Scholar] [CrossRef]
- Miniuk, M.; Reszeć-Giełażyn, J.; Bortnik, P.; Borsukiewicz, A.; Mroczek, A. Novel Predictive Biomarkers in the Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Med. 2024, 13, 5876. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Hoffmann, F.; Franzen, A.; de Vos, L.; Wuest, L.; Kulcsár, Z.; Fietz, S.; Maas, A.P.; Hollick, S.; Diop, M.Y.; Gabrielpillai, J.; et al. CTLA4 DNA methylation is associated with CTLA-4 expression and predicts response to immunotherapy in head and neck squamous cell carcinoma. Clin. Epigenet. 2023, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Nwabudike, L.C.; Tatu, A.L. Reply to Gambichler T et al.: Altered epigenetic pathways and cell cycle dysregulation in healthy appearing skin of patients with koebnerized squamous cell carcinomas following skin surgery. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e3–e4. [Google Scholar] [CrossRef]
- Ehst, B.D.; Minzer-Conzetti, K.; Swerdlin, A.; Devere, T.S. Cutaneous Manifestations of Internal Malignancy. Curr. Probl. Surg. 2010, 47, 384–445. [Google Scholar] [CrossRef] [PubMed]
- Trignano, E.; Tettamanzi, M.; Rampazzo, S.; Trignano, C.; Boccaletti, R.; Fadda, G.M.; Sanna, F.; Bussu, F.; Cossu, A.; Rubino, C. Squamous cell carcinoma of the scalp: A combination of different therapeutic strategies. Case Rep. Plast. Surg. Hand Surg. 2023, 10, 2210670. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Fionda, B.; Bussu, F.; Parrilla, C.; Lancellotta, V.; Deodato, F.; Cammelli, S.; Boldrini, L.; Gambacorta, M.A.; Morganti, A.G.; et al. Interventional radiotherapy (brachytherapy) for squamous cell carcinoma of the nasal vestibule: A multidisciplinary systematic review. Eur. J. Dermatol. 2019, 29, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.N.; Watson, C.; Barlev, A.; Stevenson, M.; Dharnidharka, V.R. Mean lifetime survival estimates following solid organ transplantation in the US and UK. J. Med. Econ. 2022, 25, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.-J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Olatunji, I.; Cui, F. Multimodal AI for prediction of distant metastasis in carcinoma patients. Front. Bioinform. 2023, 3, 1131021. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Fargnoli, M.C.; Fantini, F.; Gattoni, M.; Gualdi, G.; Pastore, F.; Pellacani, G.; Quaglino, P.; Queirolo, P.; Troiani, T. Identifying candidates for immunotherapy with cemiplimab to treat advanced cutaneous squamous cell carcinoma: An expert opinion. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066272. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Harden, P.N.; Wood, K.J. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. J. Am. Soc. Nephrol. 2016, 27, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Machado, C.; Martín-Vallejo, J.; Becerril-Andrés, S.; Revilla-Nebreda, D.; Moralejo, L.; Pérez-Losada, J.; Cañueto, J. Immunosuppression is associated with an increased risk of distant metastases in high-risk cutaneous squamous cell carcinoma: A retrospective cohort study. JAAD Int. 2023, 15, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J., III; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral Oncol. 2021, 112, 105038. [Google Scholar] [CrossRef] [PubMed]
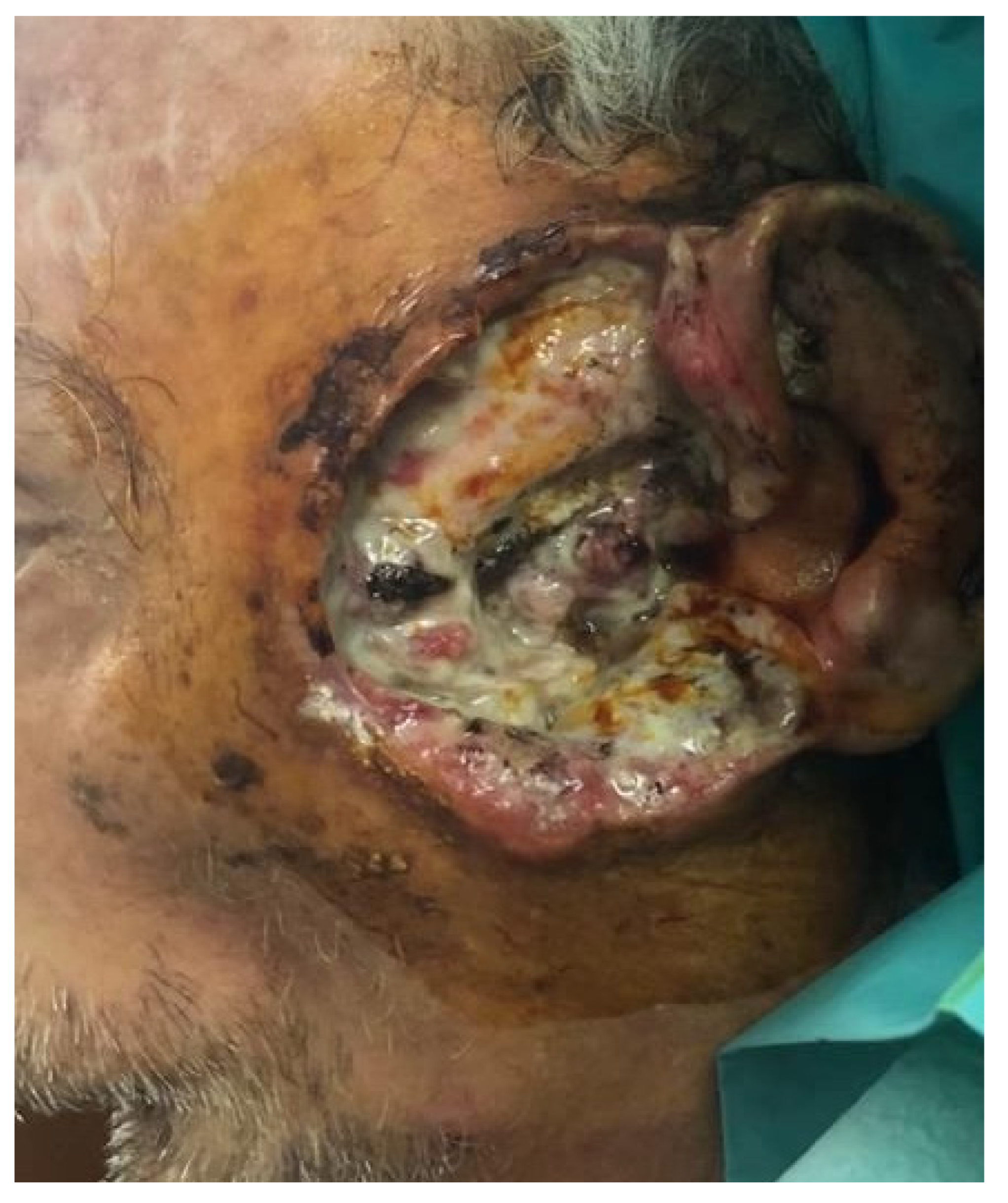
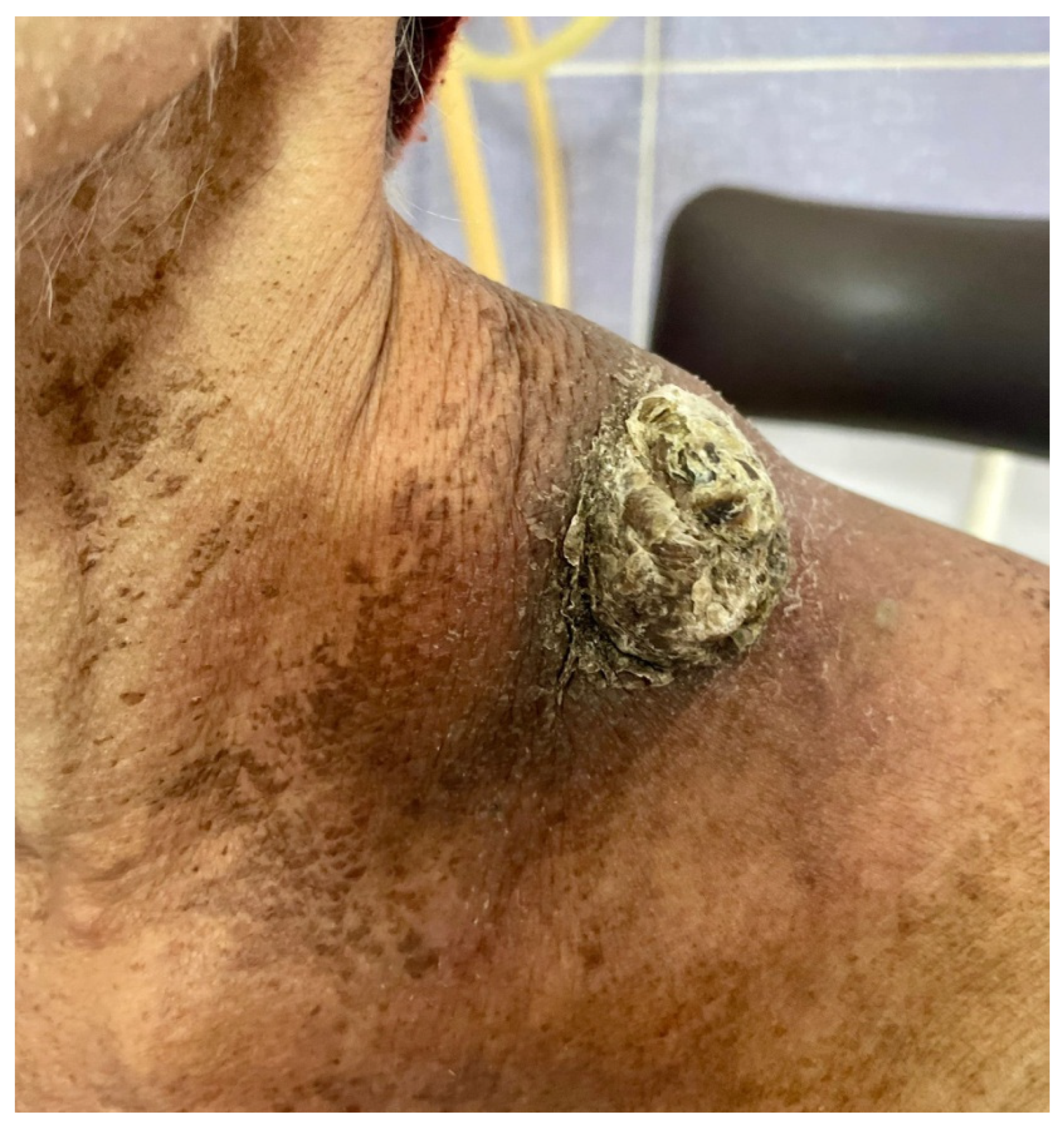
| Trial Registration Number | Clinical Condition | Number of Participants | Time Period | Results |
|---|---|---|---|---|
| NCT01979211 [18] | Locally advanced HNcSCC | 24 | 2013–2022 | 67.5% 5-year median overall survival |
| NCT02643303 [19] | Advanced, measurable, biopsy-accessible head and neck cancer | 58 | 2015–2022 | 338 days median overall survival |
| NCT03229278 [20] | Solid malignancy or lymphoma that is metastatic or unresectable | 14 | 2017–2022 | 21% 6-month progression-free survival rate |
| NCT04502888 [21] | HNcSCC | 18 | 2020–2022 | Not submitted |
| NCT00193895 [22] | HNcSCC | 321 | 2005–2016 | 83% Freedom from locoregional relapse |
| NCT04966481 [23] | HPV-unrelated recurrent or metastatic head and neck squamouscell carcinoma | 81 | 2022–2027 | 9.7 months median overall survival |
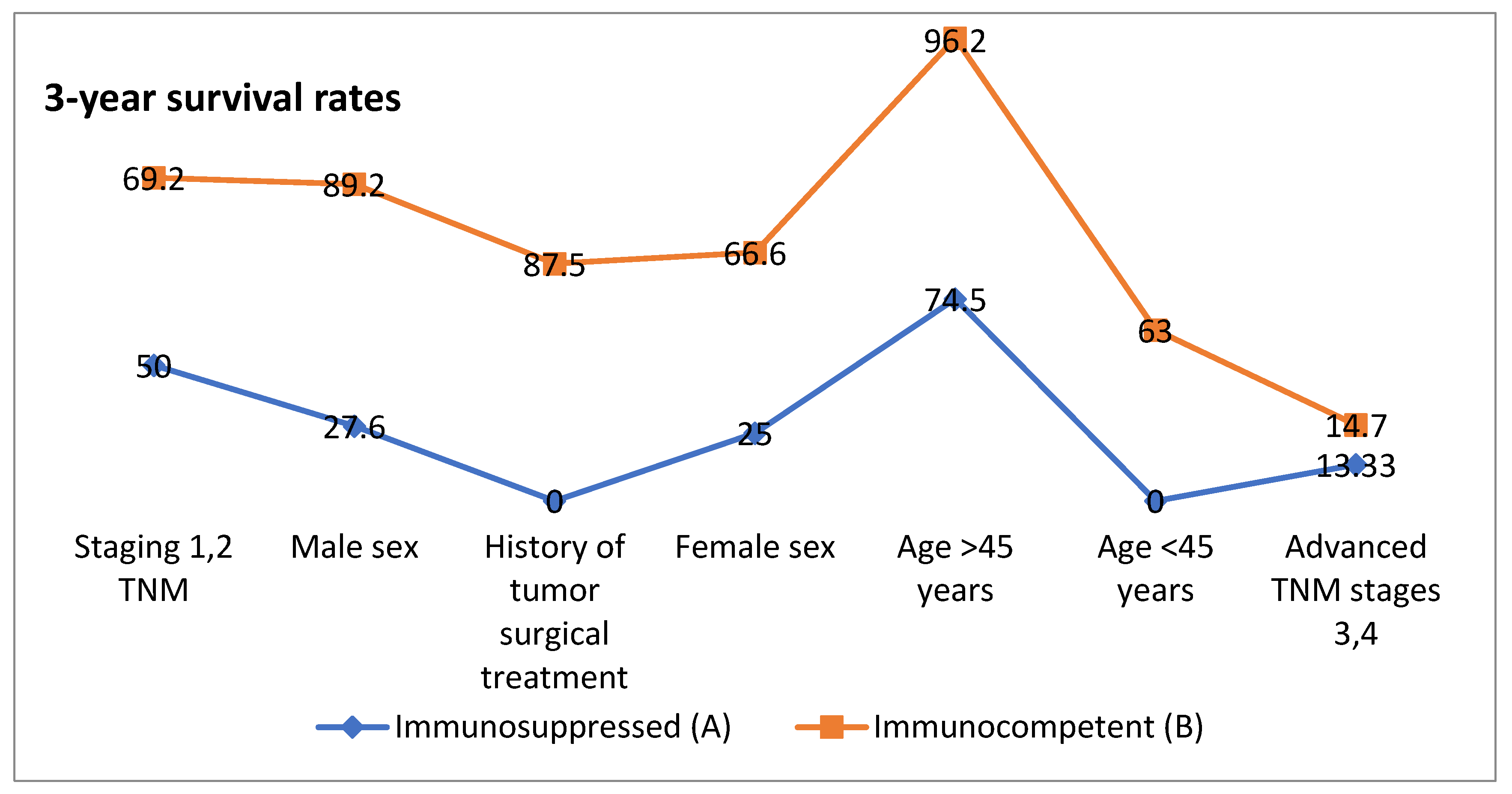
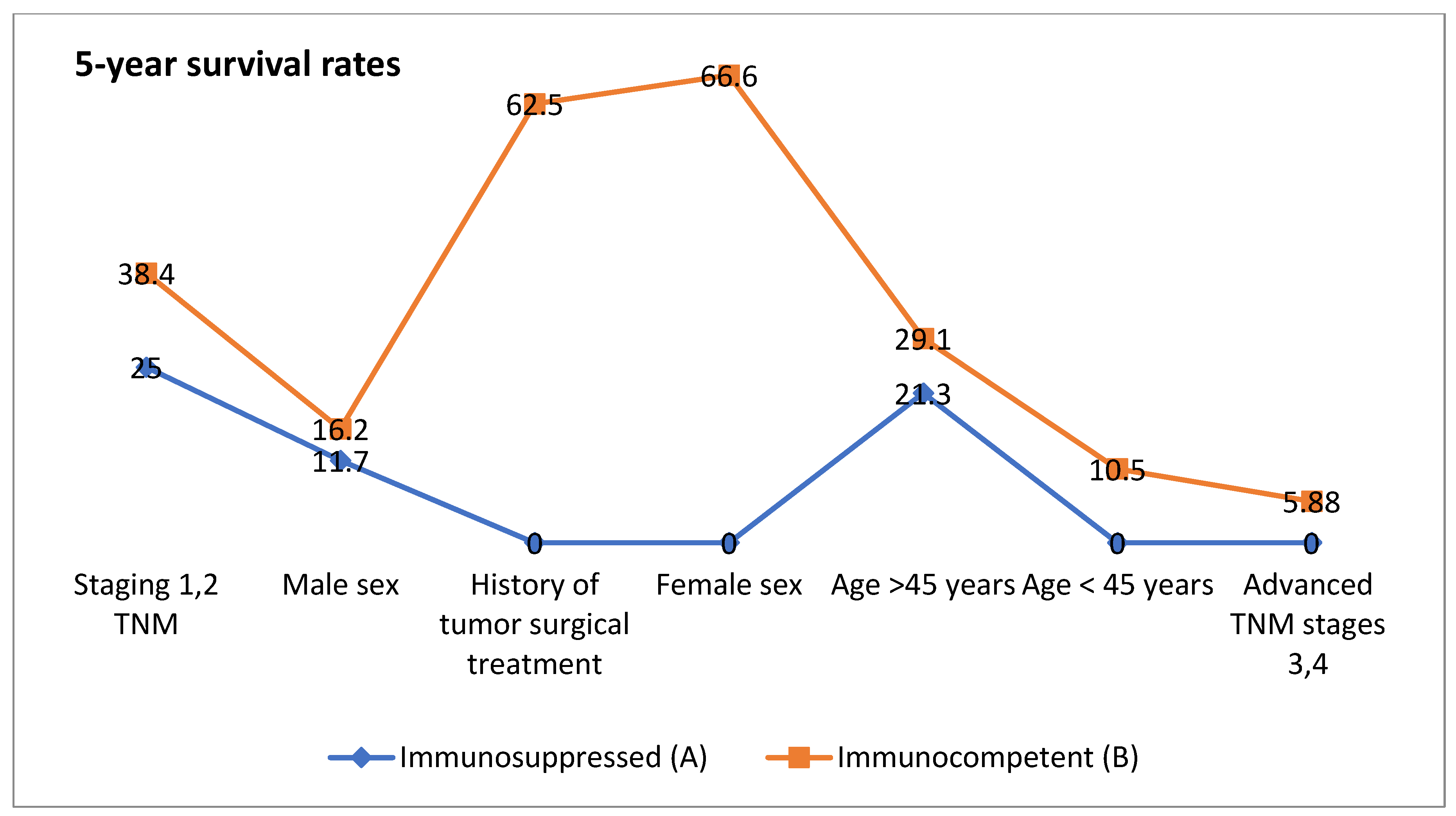
| CLINICAL Characteristics | Group A | Group B | Median Overall Survival (Months) | 3-Year Survival Rates% (Months) | 5-Year Survival Rates % (Months) | |||
|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | |||
| Age < 45 years | 2 | 10 | 19 | 37 | 0 | 63 | 0 | 10.5 |
| Age > 45 years | 19 | 37 | 15 | 31 | 74.5 | 96.2 | 21.3 | 29.1 |
| Male sex | 17 | 41 | 14 | 22 | 27.6 | 89.2 | 11.7 | 16.2 |
| Female sex | 4 | 6 | 17 | 41 | 25 | 66.6 | 0 | 66.6 |
| Staging 1,2 TNM | 6 | 13 | 21 | 43 | 50 | 69.2 | 25 | 38.4 |
| Advanced TNM stages 3,4 | 15 | 34 | 11 | 18 | 13.33 | 14.7 | 0 | 5.88 |
| History of tumor surgical treatment | 1 | 8 | 16 | 51 | 0 | 87.5 | 0 | 62.5 |
| p Value | p = 0.005 | p = 0.013 | p = 0.040 | |||||
| Immunosuppression Diseases | Number of Cases |
|---|---|
| Other malignancies | 7 |
| Systemic lupus erythematosus | 1 |
| Type 1 or type 2 diabetes | 6 |
| HIV | 1 |
| Scleroderma | 2 |
| Psoriasis | 2 |
| Lymphoma | 3 |
| Leukemia | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, D.; Fulga, A.; Vesa, D.; Fulga, I.; Tutunaru, D.; Zenovia, A.; Piraianu, A.I.; Stamate, E.; Sterian, C.; Dimofte, F.; et al. Immunosuppression and Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clin. Pract. 2025, 15, 21. https://doi.org/10.3390/clinpract15010021
Iancu D, Fulga A, Vesa D, Fulga I, Tutunaru D, Zenovia A, Piraianu AI, Stamate E, Sterian C, Dimofte F, et al. Immunosuppression and Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clinics and Practice. 2025; 15(1):21. https://doi.org/10.3390/clinpract15010021
Chicago/Turabian StyleIancu, Doriana, Ana Fulga, Doina Vesa, Iuliu Fulga, Dana Tutunaru, Andrei Zenovia, Alin Ionut Piraianu, Elena Stamate, Corina Sterian, Florentin Dimofte, and et al. 2025. "Immunosuppression and Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck" Clinics and Practice 15, no. 1: 21. https://doi.org/10.3390/clinpract15010021
APA StyleIancu, D., Fulga, A., Vesa, D., Fulga, I., Tutunaru, D., Zenovia, A., Piraianu, A. I., Stamate, E., Sterian, C., Dimofte, F., Badea, M. A., & Tatu, A. L. (2025). Immunosuppression and Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck. Clinics and Practice, 15(1), 21. https://doi.org/10.3390/clinpract15010021







