Key Considerations for Frail Patients Undergoing Hip Fracture Surgery
Abstract
1. Introduction
2. Pain Control in Acute Settings
3. Stratifying the Risk in Frail Patients
3.1. Anemia
3.2. Thrombocytopenia
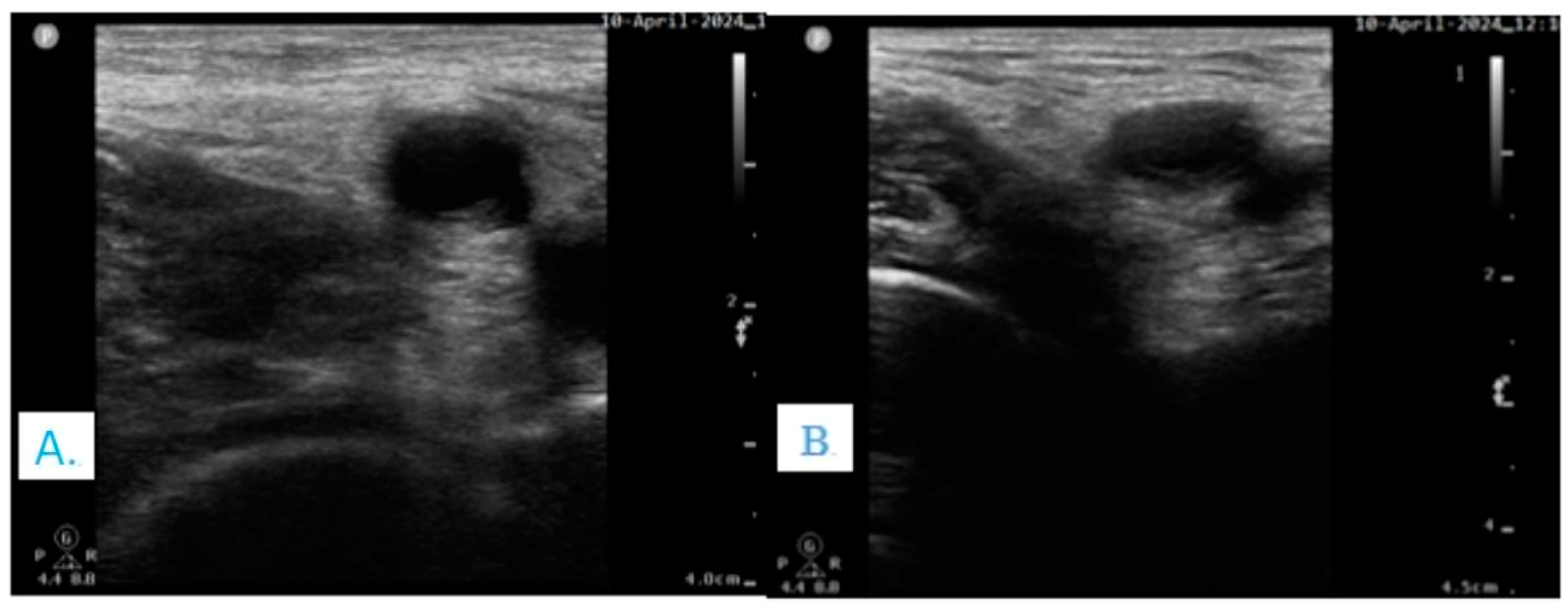
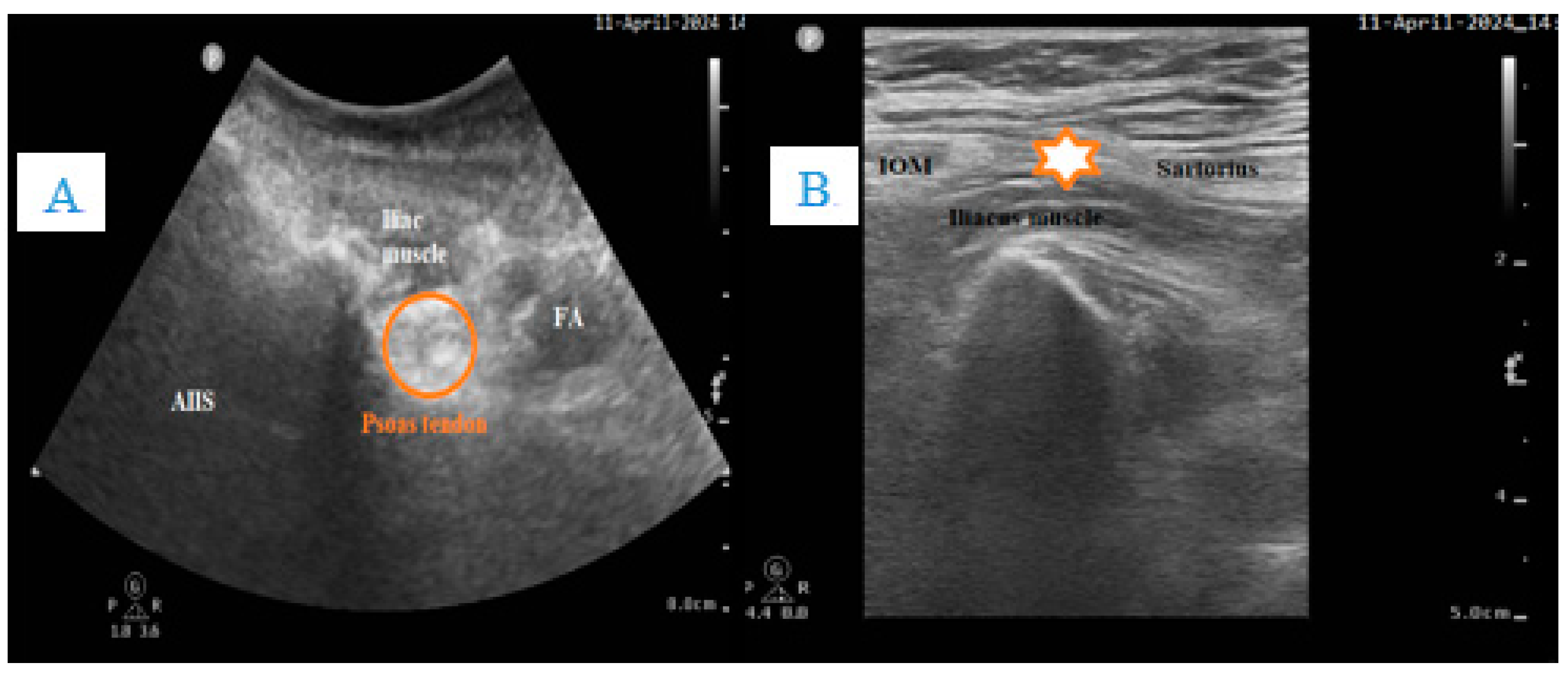
3.3. Volume Depletion
3.4. Cardiovascular Events
3.5. Treatment and Bleeding Risk
3.5.1. Antiplatelet Medication
3.5.2. Anticoagulation Therapy
3.6. Diabetic Patients
3.7. Extensive Pulmonary Assessment
3.8. Chronic Kidney Disease
3.9. Infection
3.10. Chronic Medication
3.11. Cognitive Shifts
4. Other Preoperative Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ilic, I.; Ristic, B.; Stojadinovic, I.; Ilic, M. Epidemiology of Hip Fractures due to Falls. Medicina 2023, 59, 1528. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Groff, H.; Kheir, M.M.; George, J.; Azboy, I.; Higuera, C.A.; Parvizi, J. Causes of in-hospital mortality after hip fractures in the elderly. HIP Int. 2019, 30, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.M.; Crotty, M.; Fairhall, N.; Magaziner, J.; Beaupre, L.A.; Cameron, I.D.; Sherrington, C.; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016, 16, 158. [Google Scholar] [CrossRef]
- Ackermann, L.; Schwenk, E.S.; Lev, Y.; Weitz, H. Update on medical management of acute hip fracture. Clevel. Clin. J. Med. 2021, 88, 237–247. [Google Scholar] [CrossRef]
- Bretherton, C.P.; Parker, M.J. Early surgery for patients with a fracture of the hip decreases 30-day mortality. Bone Jt. J. 2015, 97, 104–108. [Google Scholar] [CrossRef]
- Uzoigwe, C.E.; Burnand, H.G.F.; Cheesman, C.L.; Aghedo, D.O.; Faizi, M.; Middleton, R.G. Early and ultra-early surgery in hip fracture patients improves survival. Injury 2013, 44, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Pincus, D.; Ravi, B.; Wasserstein, D.; Huang, A.; Paterson, J.M.; Nathens, A.B.; Kreder, H.J.; Jenkinson, R.J.; Wodchis, W.P. Association Between Wait Time and 30-Day Mortality in Adults Undergoing Hip Fracture Surgery. JAMA 2017, 318, 1994–2003. [Google Scholar] [CrossRef]
- Lizaur-Utrilla, A.; Lopez-Prats, F.A. Hip attack for hip fractures: Is ultra-early surgery necessary? Lancet 2020, 395, 661–662. [Google Scholar] [CrossRef]
- Seong, Y.J.; Shin, W.C.; Moon, N.H.; Suh, K.T. Timing of Hip-fracture Surgery in Elderly Patients: Literature Review and Recommendations. Hip Pelvis 2020, 32, 11–16. [Google Scholar] [CrossRef]
- Dixon, J.; Ashton, F.; Baker, P.; Charlton, K.; Bates, C.; Eardley, W. Assessment and Early Management of Pain in Hip Fractures: The Impact of Paracetamol. Geriatr. Orthop. Surg. Rehabil. 2018, 9, 151459318806443. [Google Scholar] [CrossRef] [PubMed]
- Overview|Hip Fracture: Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/cg124 (accessed on 4 April 2024).
- Ketelaars, R.; Stollman, J.T.; van Eeten, E.; Eikendal, T.; Bruhn, J.; van Geffen, G.-J. Emergency physician-performed ultrasound-guided nerve blocks in proximal femoral fractures provide safe and effective pain relief: A prospective observational study in The Netherlands. Int. J. Emerg. Med. 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Moore, B.; George, R. Improving morbidity and mortality in hip fragility fractures. Curr. Opin. Anaesthesiol. 2024, 37, 316–322. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Liu, S.; Cao, Y.; Zhu, Z. Ultrasound-guided supra-inguinal fascia Iliaca compartment block for older adults admitted to the emergency department with hip fracture: A randomized controlled, double-blind clinical trial. BMC Geriatr. 2021, 21, 669. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Babu, S.; Dixon, P.; Freeman, N.; Hurford, D.; Kelleher, E.; Moppett, I.; Ray, D.; Sahota, O.; Shields, M.; et al. Guideline for the management of hip fractures 2020: Guideline by the Association of Anaesthetists. Anaesthesia 2021, 76, 225–237. [Google Scholar] [CrossRef]
- Pavelescu, D.; Mirea, L.; Păduraru, M.; Beuran, M.; Chiotoroiu, A.; Grinţescu, I. The role of multimodal analgesia in the decrease of postoperative surgical stress response in major neoplastic thoraco-abdominal surgery. Chirurgia 2011, 106, 723–728. [Google Scholar]
- Zhang, X.; Donnan, P.T.; Bell, S.; Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol. 2017, 18, 256. [Google Scholar] [CrossRef]
- Eloy, J.D.; Anthony, C.; Amin, S.; Caparó, M.; Reilly, M.C.; Shulman, S. Gabapentin Does Not Appear to Improve Postoperative Pain and Sleep Patterns in Patients Who Concomitantly Receive Regional Anesthesia for Lower Extremity Orthopedic Surgery: A Randomized Control Trial. Pain Res. Manag. 2017, 2017, 310382. [Google Scholar] [CrossRef]
- Spahn, D.R. Anemia and Patient Blood Management in Hip and Knee Surgery: A Systematic Review of the Literature. Anesthesiology 2010, 113, 482–495. [Google Scholar] [CrossRef]
- Parke, S.; Eaves, C.; Dimond, S.; Bainbridge, C.; Bellwood, J.; Mattinson, D. Perioperative optimization of hip fracture patients. Orthop. Trauma 2020, 34, 80–88. [Google Scholar] [CrossRef]
- Guay, J.; Parker, M.J.; Gajendragadkar, P.R.; Kopp, S. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst. Rev. 2016, 2016, CD000521. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Gu, X.P.; Wu, X.D.; Chen, H.; Kwong, J.S.W.; Zhou, L.Y.; Chen, S.; Ma, Z.-L. Restrictive versus liberal strategy for red blood-cell transfusion: A systematic review and meta-analysis in orthopaedic patients. J. Bone Jt. Surg. 2018, 100, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Ștefan, M.; Tomescu, D.; Predoi, C.; Goicea, R.; Perescu, M.; Popescu, M.; Dorobanțu, D.; Droc, G.; Andrei, Ș.; Știru, O.; et al. Less (Transfusion) Is More—Enhancing Recovery through Implementation of Patient Blood Management in Cardiac Surgery: A Retrospective, Single-Centre Study of 1174 Patients. J. Cardiovasc. Dev. Dis. 2023, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Capdevila, X.; Vielle, B.; Bijok, B.; Lahlou-Casulli, M.; Collange, V.; Grillot, N.; Deserts, M.D.D.; Duchalais, A.; Delannoy, B.; et al. Ferric derisomaltose and tranexamic acid, combined or alone, for reducing blood transfusion in patients with hip fracture (the HiFIT trial): A multicentre, 2 × 2 factorial, randomised, double-blind, controlled trial. Lancet Haematol. 2023, 10, e747–e755. [Google Scholar] [CrossRef]
- Griffiths, R.; Alper, J.; Beckingsale, A.; Goldhill, D.; Heyburn, G.; Holloway, J.; Leaper, E.; Parker, M.; Ridgway, S.; White, S.; et al. Management of proximal femoral fractures 2011: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2012, 67, 85–98. [Google Scholar]
- Kumar, D.; Mbako, A.N.; Riddick, A.; Patil, S.; Williams, P. On admission haemoglobin in patients with hip fracture. Injury 2011, 42, 167–170. [Google Scholar] [CrossRef]
- Porter, C.J.; Moppett, I.K.; Juurlink, I.; Nightingale, J.; Moran, C.G.; Devonald, M.A.J. Acute and chronic kidney disease in elderly patients with hip fracture: Prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol. 2017, 18, 20. [Google Scholar] [CrossRef]
- Parker, C.W.; Kolimas, A.M.; Kotini-Shah, P. Velocity-Time Integral: A Bedside Echocardiography Technique Finding a Place in the Emergency Department. J. Emerg. Med. 2022, 63, 382–388. [Google Scholar] [CrossRef]
- Di Nicolò, P.; Tavazzi, G.; Nannoni, L.; Corradi, F. Inferior Vena Cava Ultrasonography for Volume Status Evaluation: An Intriguing Promise Never Fulfilled. J. Clin. Med. 2023, 12, 2217. [Google Scholar] [CrossRef]
- Klinck, J.; McNeill, L.; Di Angelantonio, E.; Menon, D.K. Predictors and outcome impact of perioperative serum sodium changes in a high-risk population. Br. J. Anaesth. 2015, 114, 615–622. [Google Scholar] [CrossRef][Green Version]
- Norring-Agerskov, D.; Madsen, C.M.; Abrahamsen, B.; Riis, T.; Pedersen, O.B.; Jørgensen, N.R.; Bathum, L.; Lauritzen, J.B.; Jørgensen, H.L. Hyperkalemia is Associated with Increased 30-Day Mortality in Hip Fracture Patients. Calcif. Tissue Int. 2017, 101, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.N.; Awad, S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury?: Con. Kidney Int. 2014, 86, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Cumming, K.; Hoyle, G.E.; Hutchison, J.D.; Soiza, R.L. Prevalence, Incidence and Etiology of Hyponatremia in Elderly Patients with Fragility Fractures. PLoS ONE 2014, 9, e88272. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, H.; Zhao, S.; Ouyang, T.; Passos, C.; Sarang, A.; Cheung, P.W.; Waikar, S.S.; Steele, D.J.; Kalim, S.; Allegretti, A.S.; et al. Severe Hyponatremia Correction, Mortality, and Central Pontine Myelinolysis. NEJM Evid. 2023, 2, EVIDoa2300107. [Google Scholar] [CrossRef]
- Braun, M.M.; Barstow, C.H.; Pyzocha, N.J. Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia. Am. Fam. Physician 2015, 91, 299–307. [Google Scholar]
- Rostagno, C.; Polidori, G.; Ceccofiglio, A.; Cartei, A.; Boccaccini, A.; Peris, A.; Rubbieri, G.; Civinini, R.; Innocenti, M. Takotsubo Syndrome: Is This a Common Occurrence in Elderly Females after Hip Fracture? J. Crit. Care Med. 2020, 6, 146–151. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Duceppe, E.; Patel, A.; Chan, M.T.V.; Berwanger, O.; Ackland, G.; Kavsak, P.A.; Rodseth, R.; Biccard, B.; Chow, C.K.; Borges, F.K.; et al. Preoperative N-Terminal Pro-B-Type Natriuretic Peptide and Cardiovascular Events after Noncardiac Surgery: A Cohort Study. Ann. Intern. Med. 2020, 172, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Glance, L.G.; Faden, E.; Dutton, R.P.; Lustik, S.J.; Li, Y.; Eaton, M.P.; Dick, A.W. Impact of the Choice of Risk Model for Identifying Low-risk Patients Using the 2014 American College of Cardiology/American Heart Association Perioperative Guidelines. Anesthesiology 2018, 129, 889–900. [Google Scholar] [CrossRef]
- Canty, D.J.; Heiberg, J.; Yang, Y.; Royse, A.G.; Margale, S.; Nanjappa, N.; Scott, D.A.; Maier, A.B.; Sessler, D.I.; Chuan, A.; et al. One-year results of the pilot multicentre randomised trial of preoperative focused cardiac ultrasound in hip fracture surgery. Anaesth. Intensive Care 2019, 47, 207–208. [Google Scholar] [CrossRef]
- Borges, F.K.; Bhandari, M.; Guerra-Farfan, E.; Patel, A.; Sigamani, A.; Umer, M.; Tiboni, M.E.; Villar-Casares, M.d.M.; Tandon, V.; Tomas-Hernandez, J.; et al. Accelerated surgery versus standard care in hip fracture (HIP ATTACK): An international, randomised, controlled trial. Lancet 2020, 395, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, C.; Trikha, V.; Janani, S.; Bajwa, S.S.; Sharma, V.; Khanna, M. Impact of preoperative echocardiography on perioperative management in geriatric hip trauma: A retrospective observational study. Int. J. Appl. Basic Med. Res. 2017, 7, 104. [Google Scholar] [PubMed]
- Smeets, S.J.M.; Poeze, M.; Verbruggen, J.P.A.M. Preoperative cardiac evaluation of geriatric patients with hip fracture. Injury 2012, 43, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Bellas, N.; Stohler, S.; Staff, I.; Majk, K.; Lewis, C.; Davis, S.; Kumar, M. Impact of Preoperative Specialty Consults on Hospitalist Comanagement of Hip Fracture Patients. J. Hosp. Med. 2020, 15, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Bilgili, F.; Mutlu, S.; Karaman, O.; Cakal, B.; Ozkaya, U. The effects of preoperative non-invasive cardiac tests on delay to surgery and subsequent mortality in elderly patients with hip fracture. J. Back Musculoskelet. Rehabil. 2016, 29, 49–54. [Google Scholar] [CrossRef]
- Patti, G.; Nusca, A.; Mangiacapra, F.; Gatto, L.; D’Ambrosio, A.; Di Sciascio, G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J. Am. Coll. Cardiol. 2008, 52, 1128–1133. [Google Scholar]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar]
- Tarrant, S.M.; Kim, R.G.; McGregor, K.L.; Palazzi, K.; Attia, J.; Balogh, Z.J. Dual Antiplatelet Therapy and Surgical Timing in Geriatric Hip Fracture. J. Orthop. Trauma 2020, 34, 559–565. [Google Scholar] [CrossRef]
- Lizaur-Utrilla, A.; Gonzalez-Navarro, B.; Vizcaya-Moreno, M.F.; Miralles Muñoz, F.A.; Gonzalez-Parreño, S.; Lopez-Prats, F.A. Reasons for delaying surgery following hip fractures and its impact on one year mortality. Int. Orthop. 2019, 43, 441–448. [Google Scholar] [CrossRef]
- Doherty, J.U.; Ty Gluckman, C.J.; William Hucker, F.J.; Januzzi, J.L.; Thomas Ortel, F.L.; Saxonhouse, S.J.; Spinler, S.A. 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients with Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J. Am. Coll. Cardiol. 2017, 69, 871–898. [Google Scholar] [CrossRef]
- Mullins, B.; Akehurst, H.; Slattery, D.; Chesser, T. Should surgery be delayed in patients taking direct oral anticoagulants who suffer a hip fracture? A retrospective, case-controlled observational study at a UK major trauma centre. BMJ Open 2018, 8, e20625. [Google Scholar] [CrossRef]
- Vio, R.; Proietti, R.; Rigato, M.; Calò, L.A. Clinical Evidence for the Choice of the Direct Oral Anticoagulant in Patients with Atrial Fibrillation According to Creatinine Clearance. Pharmaceuticals 2021, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Vanderwerf, J.D.; Kumar, M.A. Management of neurologic complications of coagulopathies. Handb. Clin. Neurol. 2017, 141, 743–764. [Google Scholar] [PubMed]
- Yassa, R.; Khalfaoui, M.Y.; Hujazi, I.; Sevenoaks, H.; Dunkow, P. Management of anticoagulation in hip fractures: A pragmatic approach. EFORT Open Rev. 2017, 2, 394–402. [Google Scholar] [CrossRef]
- Kietaibl, S.; Ferrandis, R.; Godier, A.; Llau, J.; Lobo, C.; Macfarlane, A.J.; Schlimp, C.J.; Vandermeulen, E.; Volk, T.; Von Heymann, C.; et al. Regional anaesthesia in patients on antithrombotic drugs Joint ESAIC/ESRA guidelines. Eur. J. Anaesthesiol. 2022, 39, 100–132. [Google Scholar] [CrossRef] [PubMed]
- Gulcelik, N.E.; Bayraktar, M.; Caglar, O.; Alpaslan, M.; Karakaya, J. Mortality after hip fracture in diabetic patients. Exp. Clin. Endocrinol. Diabetes 2011, 119, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Meng, K.; Zeng, Z.; Ma, B.; Liu, X.; Qi, B.; Cui, S.; Cao, P.; Yang, Y. Stress-Induced Hyperglycemia After Hip Fracture and the Increased Risk of Acute Myocardial Infarction in Nondiabetic Patients. Diabetes Care 2013, 36, 3328–3332. [Google Scholar] [CrossRef]
- Grintescu, I.M.; Luca Vasiliu, I.; Cucereanu Badica, I.; Mirea, L.; Pavelescu, D.; Balanescu, A.; Grintescu, I.C. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients—A randomized-controlled clinical study. Clin. Nutr. 2015, 34, 377–382. [Google Scholar] [CrossRef]
- Allan, B. Management of Adults with Diabetes Undergoing Surgery and Elective Procedures: Improving Standards Summary Supporting Organisations JBDS IP Group with Special Thanks to Christine Jones (Norwich) for Her Administrative Work and Help with These Guidelines and with JBDS-IP n.d. Available online: https://abcd.care/sites/default/files/site_uploads/JBDS_Guidelines_Archive/JBDS_03_Surgical_guidelines_2015_full_FINAL_amended_Mar_2016_Archive.pdf (accessed on 18 September 2024).
- Lo, I.L.; Siu, C.W.; Tse, H.F.; Lau, T.W.; Leung, F.; Wong, M. Pre-operative pulmonary assessment for patients with hip fracture. Osteoporos. Int. 2010, 21, 579–586. [Google Scholar] [CrossRef]
- Loggers, S.A.I.; Giannakopoulos, G.F.; Vandewalle, E.; Erwteman, M.; Berger, F.; Zuidema, W.P. Preoperative chest radiographs in hip fracture patients: Is there any additional value? Eur. J. Orthop. Surg. Traumatol. 2017, 27, 953–959. [Google Scholar] [CrossRef]
- Patterson, J.T.; Bohl, D.D.; Basques, B.A.; Arzeno, A.H.; Grauer, J.N. Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery? Am. J. Orthop. 2017, 46, E177–E185. [Google Scholar]
- Jamali, S.; Dagher, M.; Bilani, N.; Mailhac, A.; Habbal, M.; Zeineldine, S.; Tamim, H. The Effect of Preoperative Pneumonia on Postsurgical Mortality and Morbidity: A NSQIP Analysis. World J. Surg. 2018, 42, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Badrick, T.; Turner, P. The Uncertainty of the eGFR. Indian J. Clin. Biochem. 2013, 28, 242. [Google Scholar] [CrossRef][Green Version]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Marakala, V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury—A systematic review. Clin. Chim. Acta 2022, 536, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.E.; Kim, T.Y.; Yoo, J.H.; Kim, J.K.; Kim, S.G.; Kim, H.J.; Song, Y.R. Acute kidney injury can predict in-hospital and long-term mortality in elderly patients undergoing hip fracture surgery. PLoS ONE 2017, 12, e0176259. [Google Scholar] [CrossRef] [PubMed]
- Graver, A.; Merwin, S.; Collins, L.; Kohn, N.; Goldman, A. Comorbid Profile Rather than Age Determines Hip Fracture Mortality in a Nonagenarian Population. HSS J.® 2015, 11, 223–235. [Google Scholar] [CrossRef]
- Juthani-Mehta, M. Asymptomatic Bacteriuria and Urinary Tract Infection in Older Adults. Clin. Geriatr. Med. 2007, 23, 585–594. [Google Scholar] [CrossRef]
- Bliemel, C.; Buecking, B.; Hack, J.; Aigner, R.; Eschbach, D.A.; Ruchholtz, S.; Oberkircher, L. Urinary tract infection in patients with hip fracture: An underestimated event? Geriatr. Gerontol. Int. 2017, 17, 2369–2375. [Google Scholar] [CrossRef]
- Gillespie, W.J.; Walenkamp, G.H. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst. Rev. 2010, 2010, CD000244. [Google Scholar] [CrossRef]
- Bonaccorsi, H.A.; Burns, B. Perioperative Cardiac Management; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Whinney, C. Perioperative medication management: General principles and practical applications. Clevel. Clin. J. Med. 2009, 76 (Suppl. S4), S126–S132. [Google Scholar] [CrossRef]
- Freter, S.; Dunbar, M.; Koller, K.; MacKnight, C.; Rockwood, K. Prevalence and Characteristics of Pre-Operative Delirium in Hip Fracture Patients. Gerontology 2016, 62, 396–400. [Google Scholar] [CrossRef]
- Cotae, A.M.; Mirea, L.; Cobilinschi, C.; Ungureanu, R.; Grintescu, I.M. Early postoperative cognitive decline—Are there any preventive strategies for surgical patients in the emergency setting? Signa Vitae 2024, 20. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jo, H.Y.; Na, H.S.; Han, S.H.; Do, S.H.; Shin, H.J. The Effect of Peripheral Nerve Block on Postoperative Delirium in Older Adults Undergoing Hip Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 2459. [Google Scholar] [CrossRef] [PubMed]
- Al-Husinat, L.; Jouryyeh, B.; Al Sharie, S.; Al Modanat, Z.; Jurieh, A.; Al Hseinat, L.; Varrassi, G. Bone Cement and Its Anesthetic Complications: A Narrative Review. J. Clin. Med. 2023, 12, 2105. [Google Scholar] [CrossRef]
- Moldovan, F. Bone Cement Implantation Syndrome: A Rare Disaster Following Cemented Hip Arthroplasties—Clinical Considerations Supported by Case Studies. J. Pers. Med. 2023, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; Ene, R.; Popescu, A.; Cîrstoiu, M.; Sinescu, R.; Cîrstoiu, C. ToTal hip joinT replacemenT in young male paTienT wiTh osTeoporosis, secondary To hypogonadoTropic hypogonadism. Acta Endocrinol. 2015, 11, 109–113. [Google Scholar] [CrossRef]
- El-Othmani, M.M.; Zalikha, A.K.; Cooper, H.J.; Shah, R.P. Femoral Stem Cementation in Primary Total Hip Arthroplasty. JBJS Rev. 2022, 10, e22.00111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitriu, A.-M.; Ene, R.; Mirea, L. Key Considerations for Frail Patients Undergoing Hip Fracture Surgery. Clin. Pract. 2024, 14, 2256-2266. https://doi.org/10.3390/clinpract14060177
Dumitriu A-M, Ene R, Mirea L. Key Considerations for Frail Patients Undergoing Hip Fracture Surgery. Clinics and Practice. 2024; 14(6):2256-2266. https://doi.org/10.3390/clinpract14060177
Chicago/Turabian StyleDumitriu, Ana-Maria, Rǎzvan Ene, and Liliana Mirea. 2024. "Key Considerations for Frail Patients Undergoing Hip Fracture Surgery" Clinics and Practice 14, no. 6: 2256-2266. https://doi.org/10.3390/clinpract14060177
APA StyleDumitriu, A.-M., Ene, R., & Mirea, L. (2024). Key Considerations for Frail Patients Undergoing Hip Fracture Surgery. Clinics and Practice, 14(6), 2256-2266. https://doi.org/10.3390/clinpract14060177




