Analysis of Primary Graft Dysfunction (PGD) Risk Factors in Lung Transplantation (LuTx) Patients
Abstract
1. Introduction
2. Materials and Methods
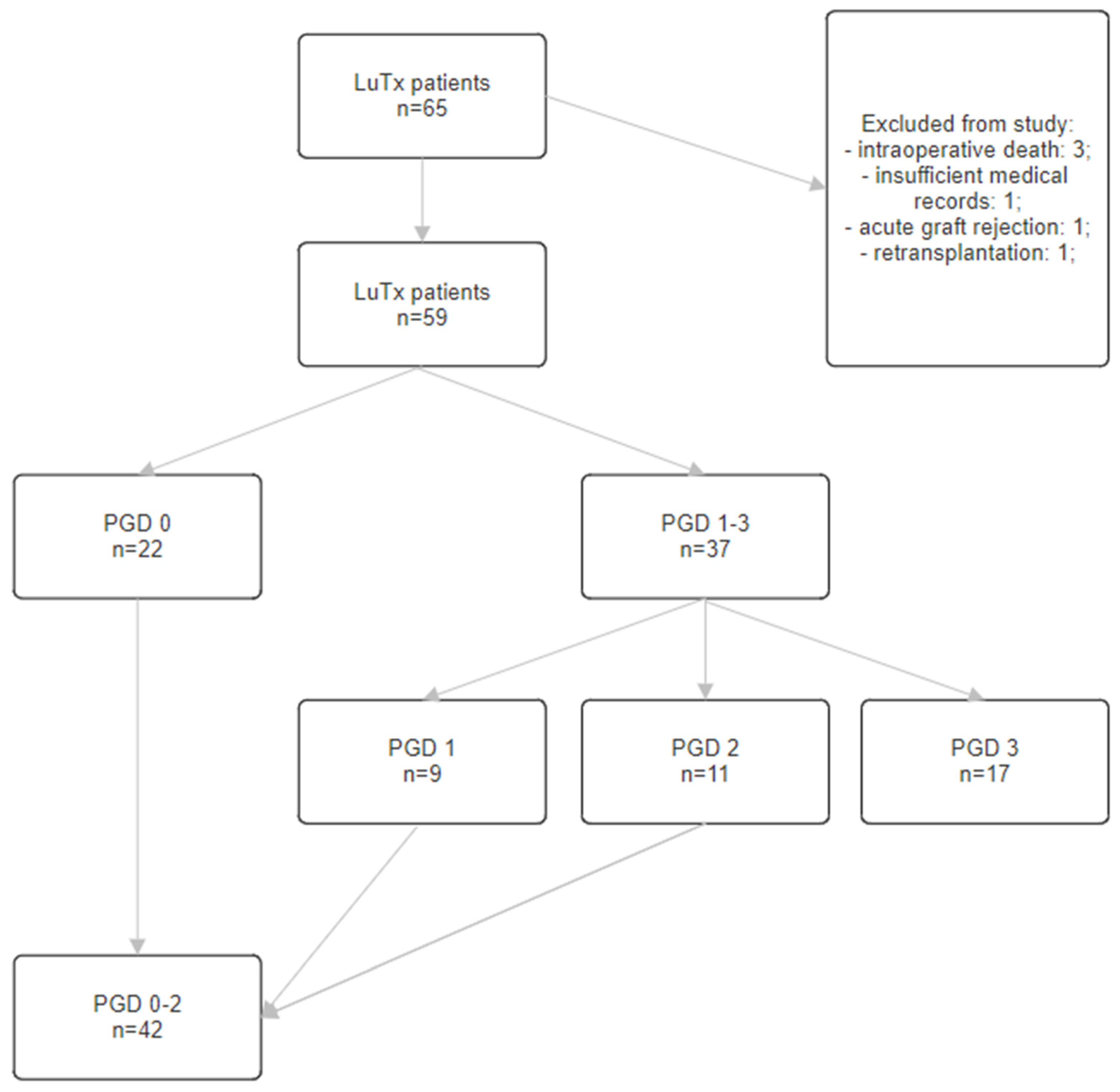
2.1. Population
2.2. LuTx Recipient Data
2.3. LuTx Donor Data
2.4. Statistical Analysis
3. Results
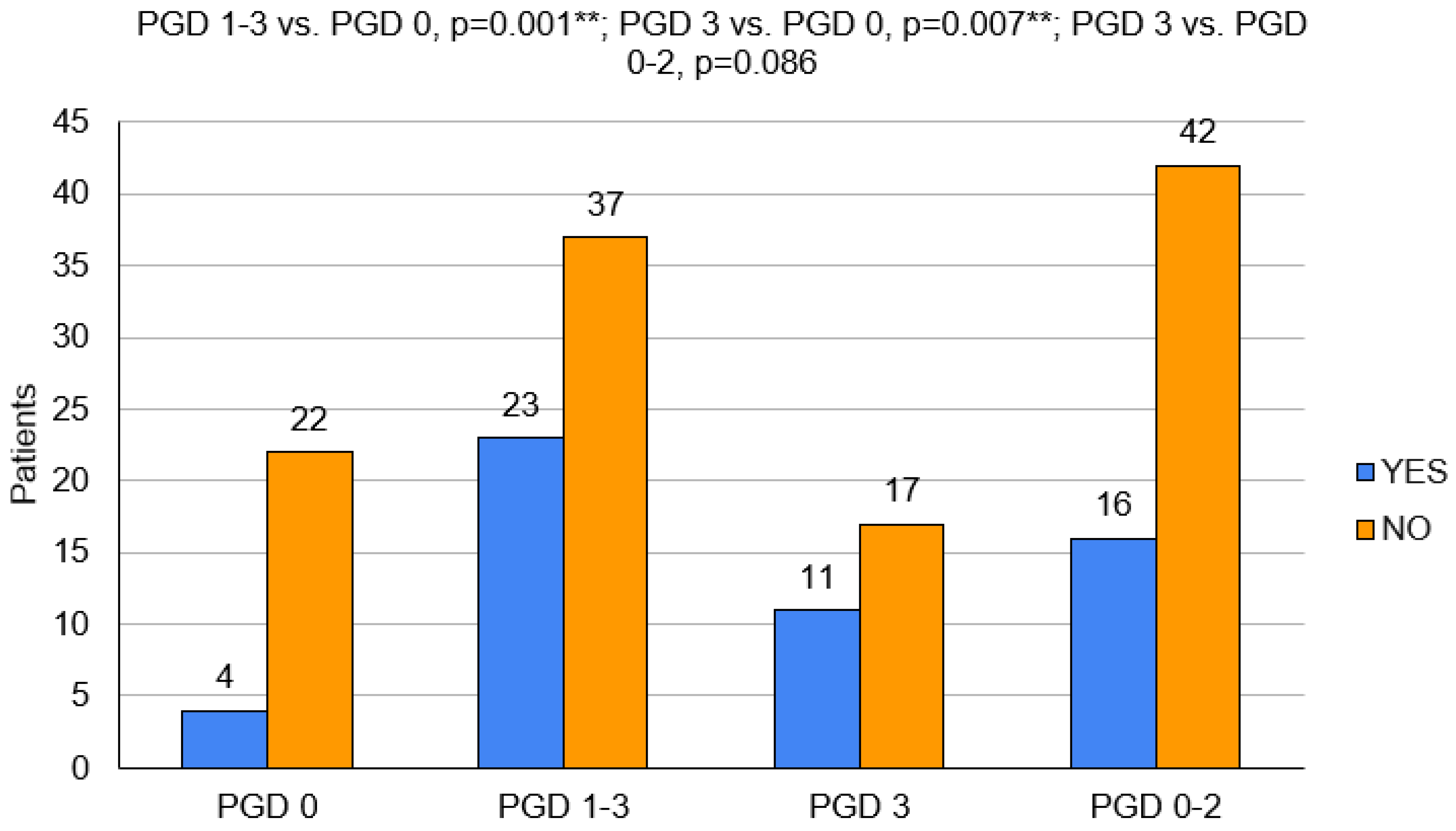
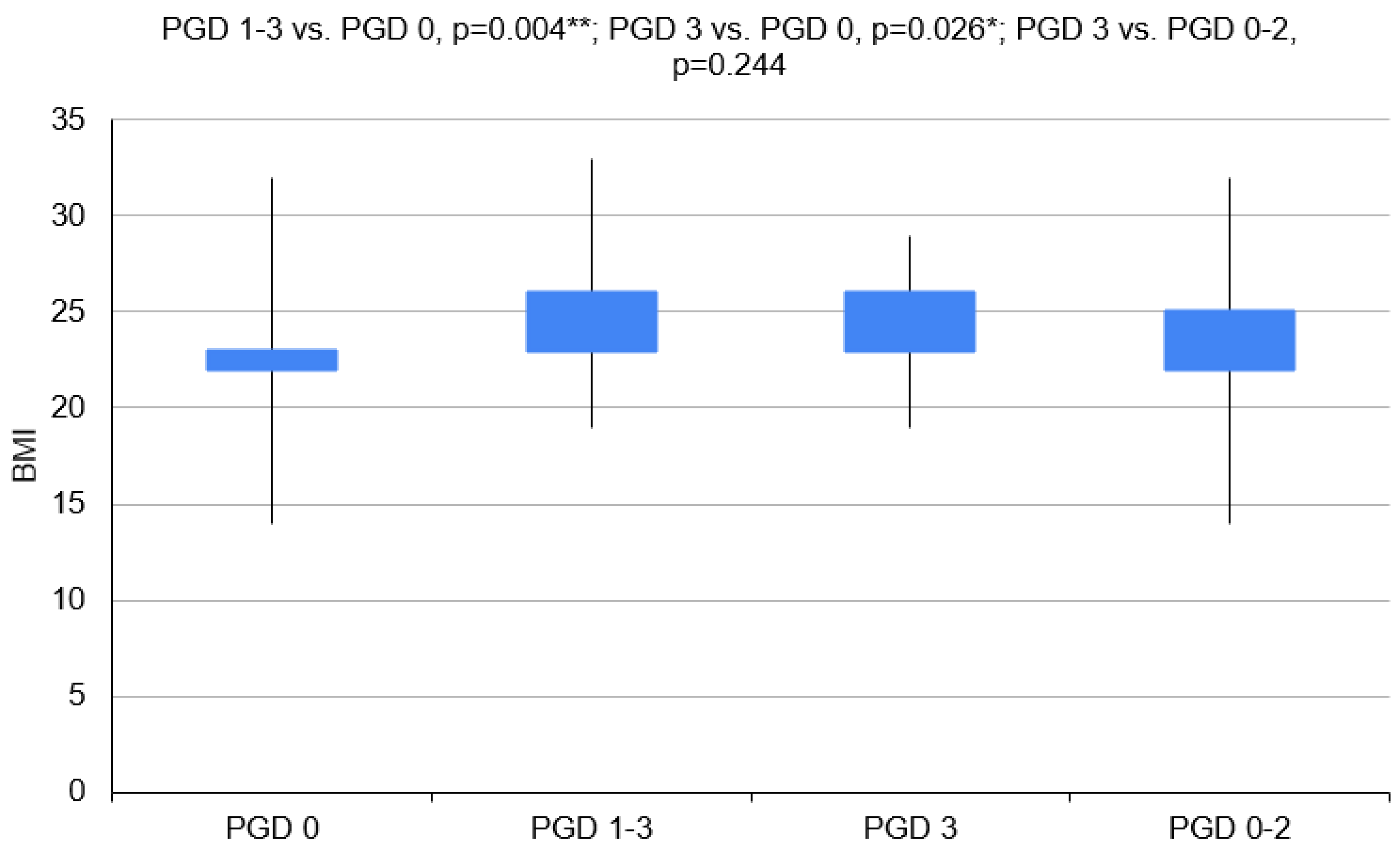
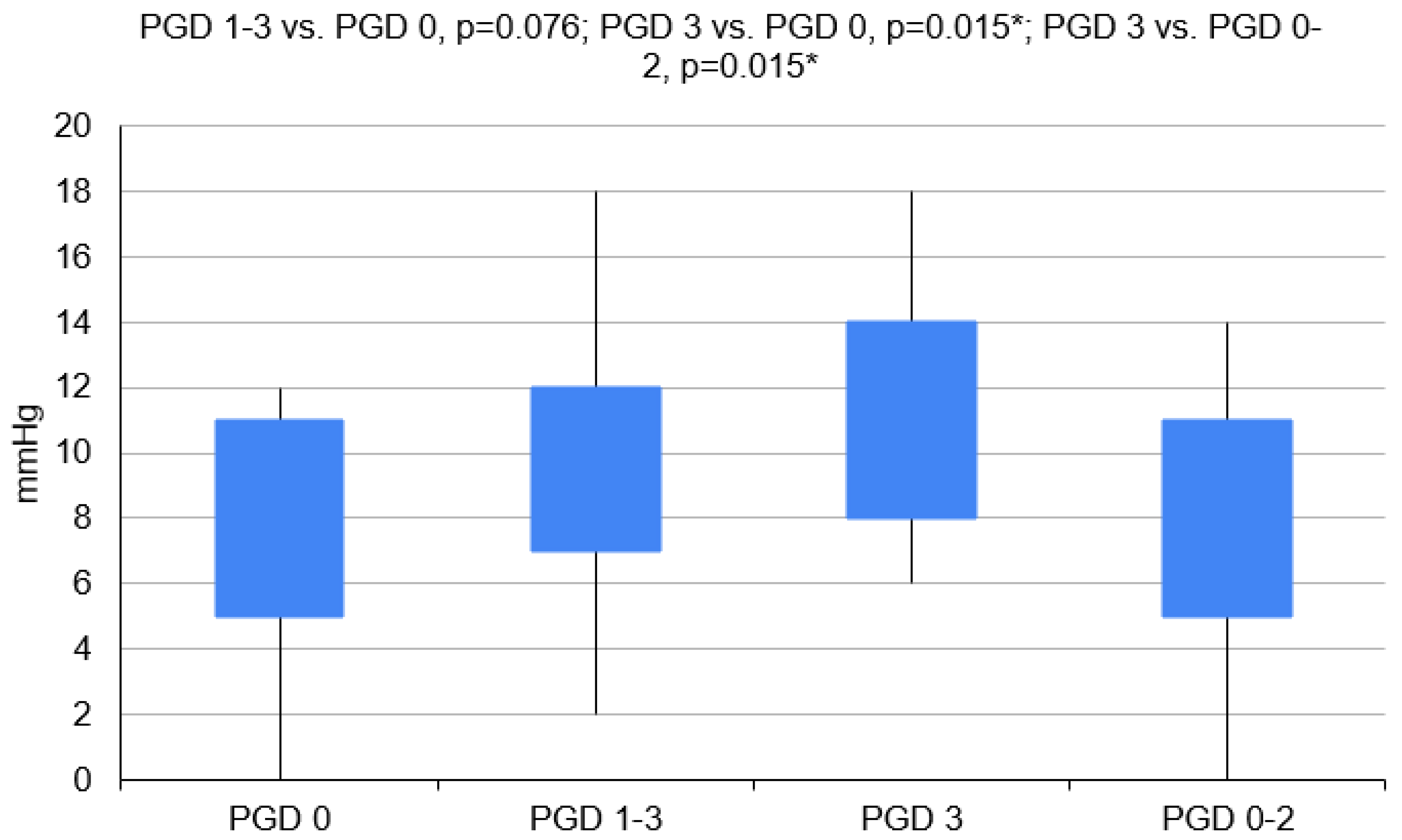
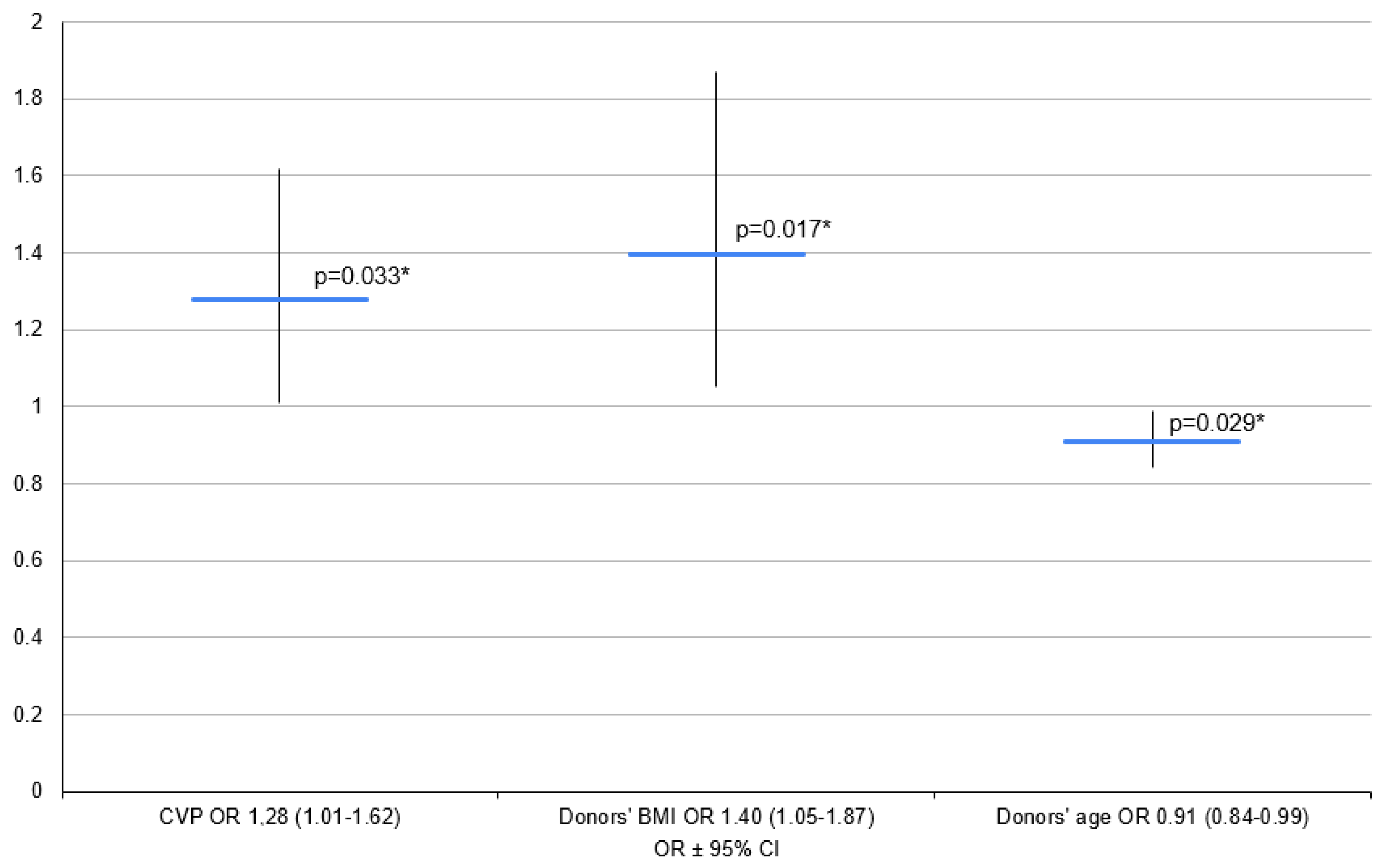
3.1. Variant 1—PGD 1–3 Group vs. PGD 0 Group
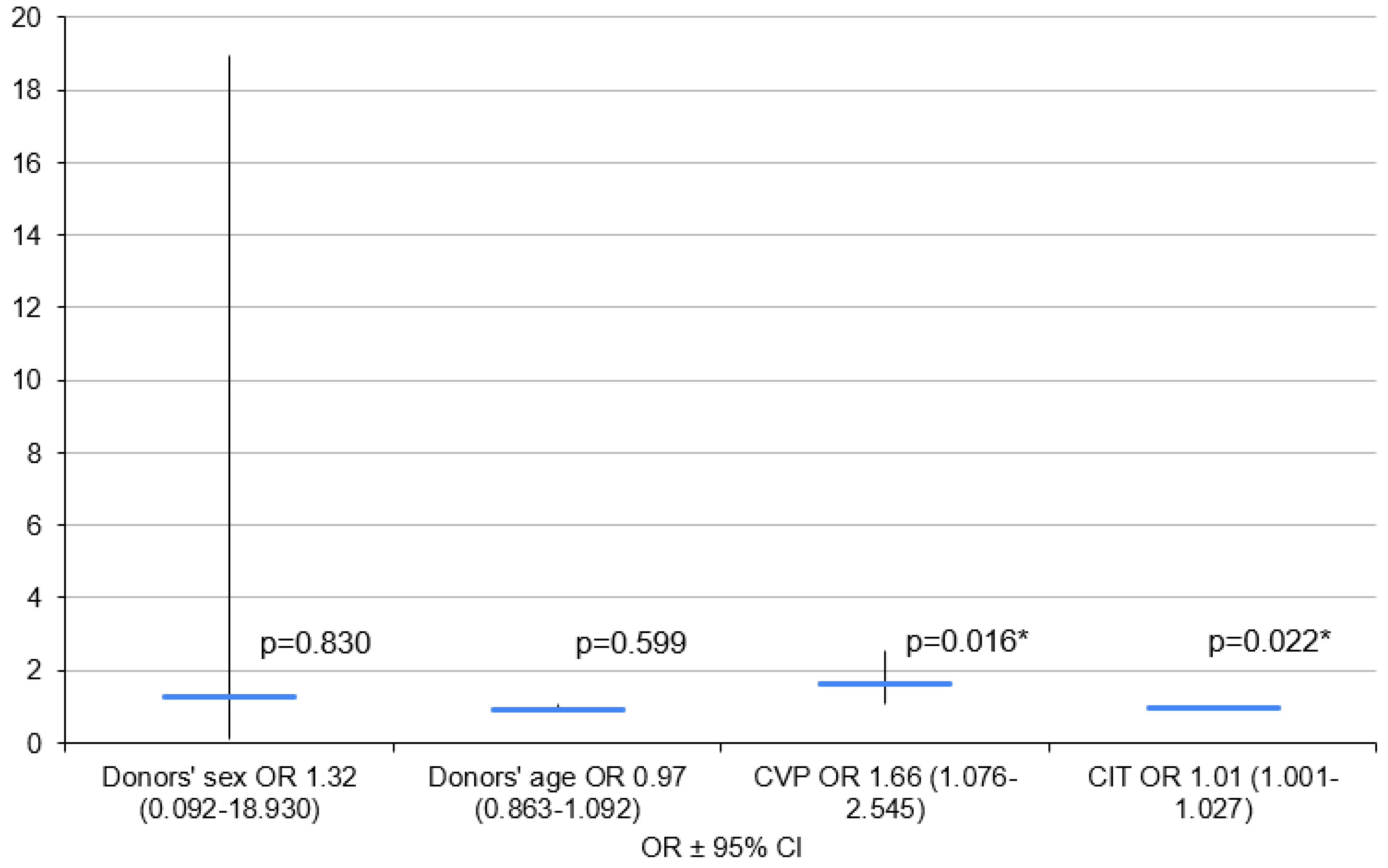
3.2. Variant 2—PGD 3 Group vs. PGD 0 Group
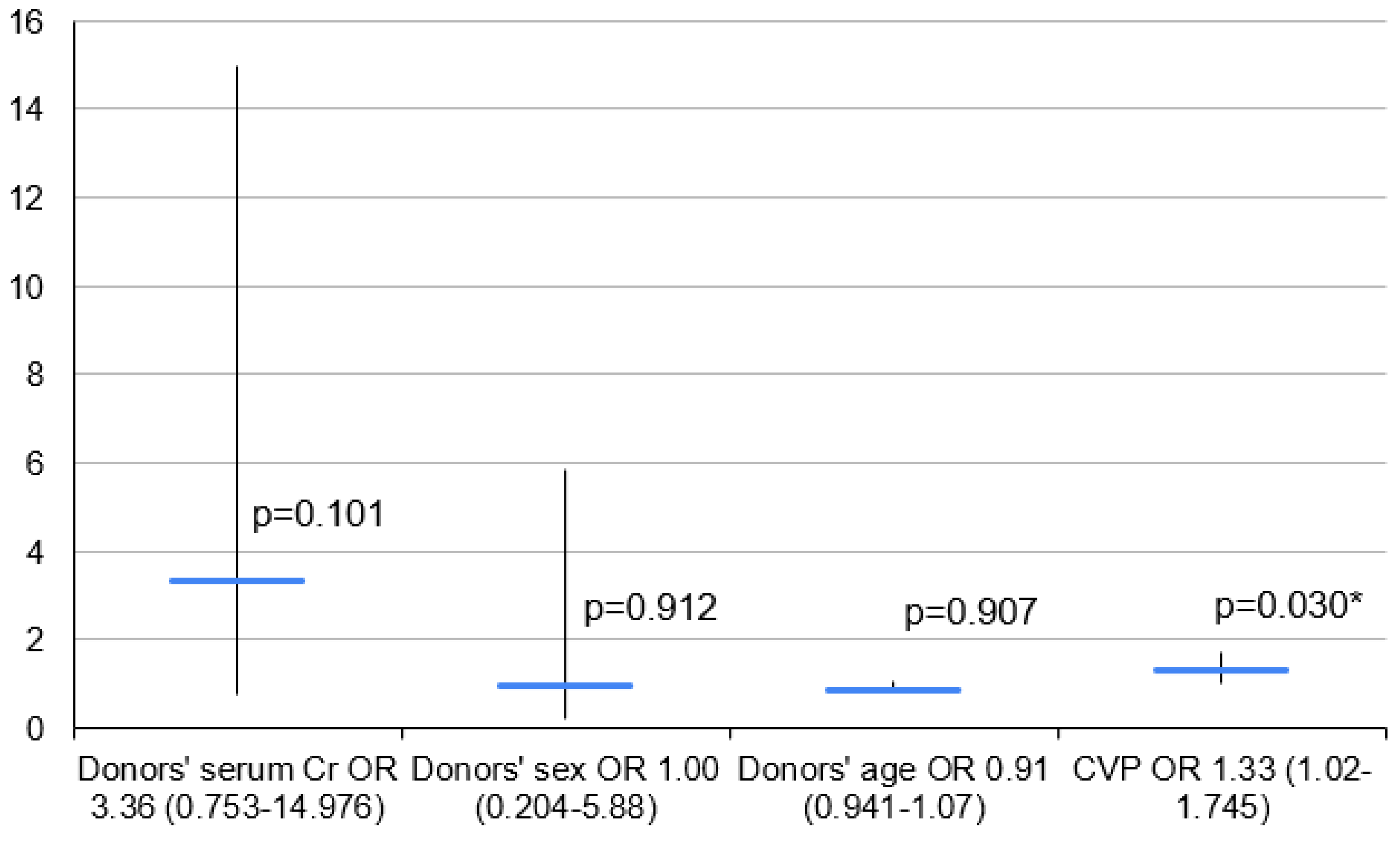
3.3. Variant 3—PGD 3 Group vs. PGD 0–2 Group
4. Discussion
4.1. Key Findings and Explanations
4.2. Comparison with Similar Researches
4.3. Strengths and Limitations
4.4. Implications and Actions Needed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christie, J.D.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden Ch Dipchand, A.I.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Stehlik, J.; Hertz, M.I. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J. Heart Lung Transplant. 2012, 31, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.I.; Pither, T.L.; Fisher, A.J. Pathophysiology and classification of primary graft dysfunction after lung transplantation. J. Thorac. Dis. 2017, 9, 4084–4097. [Google Scholar] [CrossRef] [PubMed]
- Fuehner, T.; Welte, T.; Simon, A.; Gottlieb, J. [Complications after lung transplantation. Part 1: Intensive medical and pneumologic complications]. Dtsch. Med. Wochenschr. 2008, 133, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005, 24, 1454–1459. [Google Scholar] [CrossRef]
- Snell, G.I.; Yusen, R.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part I: Definition and grading—A 2016 Consensus Group statement of The International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Diamond, J.M. Primary Graft Dysfunction (PGD) Following Lung Transplantation. Semin. Respir. Crit. Care Med. 2018, 39, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Porteous, M.K.; Diamond, J.M.; Christie, J.D. Primary graft dysfunction: Lessons learned about the first 72 h after lung transplantation. Curr. Opin. Organ. Transplant. 2015, 20, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Kuo, E.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 2008, 86, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.; Yusen, R.D.; Meyers, B.F.; Chakinala, M.M.; Walter, M.J.; Aloush, A.A.; Patterson, A.G.; Trulock, E.P.; Hachemet, R.R. Impact of Immediate Primary Lung Allograft Dysfunction on Bronchiolitis Obliterans Syndrome. Am. J. Respir. Crit. Care Med. 2007, 175, 507–513. [Google Scholar] [CrossRef]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan Ch DeMissie, E.; Kimmel, S.E. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, C.L.; Hadjiliadis, D.; Ahya, V.N.; Kotloff, R.M.; Pochettino, A.; Lewis, J.; Christie, J.D. Risk factors for early primary graft dysfunction after lung transplantation: A registry study. Clin. Transplant. 2009, 23, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Nath, D.S.; Johnson, A.C.; Walker, A.R.; Prekker, M.E.; Radosevich, D.M.; Herrington, C.S.; Dahlberg, P.S. Risk factors for primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2006, 131, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Levvey, B.J.; Harkess, M.; Hopkins, P.; Chambers, D.; Merry, C.; Glanville, A.R.; Snell, G.I. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am. J. Transplant. 2012, 12, 2406–2413. [Google Scholar] [CrossRef]
- Lowery, E.M.; Kuhlmann, E.A.; Mahoney, E.L.; Dilling, D.F.; Kliethermes, S.A.; Kovacs, E.J. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol. Clin. Exp. Res. 2014, 38, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Su, L.; Jiang, S. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: A systematic review and meta-analysis. PLoS ONE 2014, 9, e92773. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Kawut, S.M.; Wickersham, N.; Winterbottom, C.; Bhorade, S.; Palmer, S.M.; Lee, J.; Diamond, J.M.; Wille, K.M.; Weinacker, A.; et al. Obesity and primary graft dysfunction after lung transplantation: The Lung Transplant Outcomes Group Obesity Study. Am. J. Respir. Crit. Care Med. 2011, 184, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Porteous, M.K.; Ky, B.; Kirkpatrick, J.N.; Shinohara, R.; Diamond, J.M.; Shah, R.J.; Lee, J.C.; Christie, J.D.; Kawut, S.M. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am. J. Respir. Crit. Care Med. 2016, 193, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Kotloff, R.M.; Pochettino, A.; Arcasoy, S.M.; Rosengard, B.R.; Landis, J.R.; Kimmel, S.E. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003, 124, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, N.; Bhama, J.; Gries, C.J.; Kawamura, T.; Crespo, M.; Johnson, B.; Zaldonis, D.; Pilewski, J.; Toyoda, Y.; Bermudez, C. Lung transplantation in patients with prior cardiothoracic surgical procedures. Am. J. Transplant. 2012, 12, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Aeba, R.; Griffith, B.P.; Kormos, R.L.; Armitage, J.M.; Gasior, T.A.; Fuhrman, C.R.; Yousem, S.A.; Hardesty, R.L. Effect of cardiopulmonary bypass on early graft dysfunction in clinical lung transplantation. Ann. Thorac. Surg. 1994, 57, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Porteous, M.K.; Lee, J.C.; Lederer, D.J.; Palmer, S.M.; Cantu, E.; Shah, R.J.; Bellamy, S.L.; Lama, V.N.; Bhorade, S.M.; Crespo, M.M.; et al. Clinical Risk Factors and Prognostic Model for Primary Graft Dysfunction after Lung Transplantation in Patients with Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2017, 14, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Prekker, M.E.; Nath, D.S.; Walker, A.R.; Johnson, A.C.; Hertz, M.I.; Herrington, C.S.; Radosevich, D.M.; Dahlberg, P.S. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 2006, 25, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Louis, M.A. Physiology, Central Venous Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519493 (accessed on 4 October 2023).
- Abdelnour, T.; Rieke, S. Relationship of Hormonal Resuscitation Therapy and Central Venous Pressure on Increasing Organs for Transplant. J. Heart Lung Transplant. 2009, 28, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, D.V.; Scheinkestel, C.D.; Snell, G.I.; Davey-Quinn, A.; Bailey, M.J.; Williamset, T.J. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J. Thorac. Cardiovasc. Surg. 2005, 129, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, M.S.; Lee, J.G.; Jung, J.Y.; Kang, J.A.; Kim, Y.S.; Kim, S.K.; Chang, J.; Paik, H.C.; Kim, S.Y. Risk factors and outcome of primary graft dysfunction after lung transplantation in Korea. J. Thorac. Dis. 2016, 8, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Atchade, E.; De Tymowski, C.; Lepitre, E.; Zappella, N.; Snauwaert, A.; Jean, S.; Tran, A.; Lortat, B.; Messika, J.; Mal, H.; et al. Impact of recipient and donor pretransplantation body mass index on early postosperative complications after lung transplantation. BMC Pulm. Med. 2024, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Gammie, J.S.; Stukus, D.R.; Pham, S.M.; Hattler, B.G.; McGrath, M.F.; Mc Curry, K.R.; Griffith, B.P.; Keenanet, R.J. Effect of ischemic time on survival in clinical lung transplantation. Ann. Thorac. Surg. 1999, 68, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.C.; Valero, V., 3rd; Kilic, A.; Magruder, J.T.; Merlo, C.H.A.; Shah, P.D.; Shah, A. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg. 2015, 150, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D., Jr.; Hartwig, M.G.; Tobias, J.D.; Tumin, D. Lung Transplant Center Volume Ameliorates Adverse Influence of Prolonged Ischemic Time on Mortality. Am. J. Transplant. 2017, 17, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D., Jr.; Black, S.M.; Tobias, J.T.; Higgins, R.S.; Whitson, B.A. Influence of donor and recipient age in lung transplantation. J. Heart Lung Transplant. 2015, 34, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Cerier, E.J.; Manerikar, A.J.; Kandula, V.; Bharat, A.; Kurihara, C. Recipient, donor, and surgical factors leading to primary graft dysfunction after lung transplant. J. Tthorac Dis. 2023, 15, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Altun, G.T.; Arslantaş, M.; Cinel, I. Primary Graft Dysfunction after Lung Transplantation. Turk. J. Anaesthesiol. Reanim. 2015, 43, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.; Romero, L.; Deu, M.; Jauregui, A.; Pérez, J.; Ochoa, J.; Díaz, V.; Sanchez, L.; Ascanio, F.; Solé, J. Obese Recipients of Lung Transplant Have an Increased Risk of Primary Graft Dysfunction. J. Heart Lung Transplant. 2016, 35, 316–317. [Google Scholar] [CrossRef]
- Shah, R.J.; Diamond, J.M.; Cantu, E.; Flesch, J.; Lee, J.C.; Lederer, D.J.; Lama, V.N.; Orens, J.; Weinacker, A.; Wilkes, D.S.; et al. Objective Estimates Improve Risk Stratification for Primary Graft Dysfunction after Lung Transplantation. Am. J. Transplant. 2015, 15, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Gainsford, T.; Willson, T.A.; Metcalf, D.; Handman, E.; McFarlane, C.; Ng, A.; Nicola, N.A.; Alexander, W.S.; Hilton, D.J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 14564–14568. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Jones-Carson, J.; Reed, D.A.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C.; Fantuzzi, G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: Role of tumor necrosis factor alpha and IL-18. Proc. Natl. Acad. Sci. USA 2000, 97, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
| PGD Grade | PaO2/FiO2 Ratio | Pulmonary Edema on Chest X-ray | Other |
|---|---|---|---|
| 0 | Any | No | - |
| 1 | >300 | Yes | - |
| 2 | 200–300 | Yes | - |
| 3 | <200 | Yes | Or ECMO; or FiO2 > 0.5 |
| Group | Risk Factors |
|---|---|
| Donor-Dependent | Brain injury, cardiac arrest before organ collection, alcoholism, nicotinism, donor age, female sex, classification into African-American population |
| Recipient-Dependent | Obesity, LuTx indicator diseases, cardiac diseases, female sex, classification into African-American population |
| Perioperative | Single lung transplant, prior pleurodesis, intraoperative use of CBP, extended CIT, numerous transfusions, higher FiO2 during the reperfusion phase, delayed thorax closure |
| PGD 0 Group n = 22 | PGD 1–3 Group n = 37 | PGD 3 Group n = 17 | PGD 0–2 Group n = 42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Avg. ± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | n | Avg.± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | |
| Age (Years) | 22 | 56.5 (±7.4) | 58 (56–62) | 37 | 46.1 (±14.1) | 50 (36–59) | 17 | 45.5 ± 15.1 | 55 (39–59) | 42 | 51.1 ± 12.0 | 56.5 (43–59) |
| BMI (kg/m2) | 21 | 22.6 (±2.5) | 23.2 (20.9–24.2) | 36 | 21.8 (±3.6) | 21.3 (19.5–24.6) | 16 | 22.7 ± 3.9 | 21.4 (20.1–24.6) | 41 | 21.9 ± 3.00 | 22.4 (19.9–24.2) |
| Sex (Female) | 10/22 (45.5%) | - | - | 14 (37.8%) | - | - | 5 (29.4%) | - | - | 19 (45.2%) | - | - |
| PGD 0 Group n = 22 | PGD 1–3 Group n = 37 | PGD 3 Group n = 17 | PGD 0–2 Group n = 42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Avg. ± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | n | Avg.± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | |
| Donor Data | ||||||||||||
| Age (Years) | 22 | 39.8 ± 13.9 | 44 (33–55) | 37 | 40.5 ± 12.4 | 43 (31–50) | 17 | 41.5 ± 10.3 | 40 (32–48) | 42 | 39.7 ± 13.9 | 44 (30–50) |
| Sex (Female) | 13 (59.1%) | 13 (59.1%) | - | 15 (40.5%) | - | - | 7(41.2%) | - | - | 21 | - | - |
| BMI (kg/m2) | 22 | 22.5 ± 3.5 | 22.0 (21.5–22.6) | 37 | 24.6 ± 3.0 | 24.5 (22.6–25.8) | 17 | 24.4 ± 2.8 | 24.5 (22.3–25.1) | 42 | 23.6 ± 3.6 | 22.6 (21.6–24.8) |
| PGD 0 Group n = 22 | PGD 1–3 Group n = 37 | PGD 3 Group n = 17 | PGD 0–2 Group n = 42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Avg. ± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | n | Avg.± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | |
| Blood Type A | 15 (68.2%) | - | - | 14 (37.8%) | - | - | 8 (47.1%) | - | - | 21 (50%) | - | - |
| Blood Type B | 6 (27.3%) | - | - | 3 (18.9%) | - | - | 3 (17.6%) | - | - | 10 (23.8%) | - | - |
| Blood Type AB | 0 (0%) | - | - | 6 (16.2%) | - | - | 4 (23.5%) | - | - | 2 (4.8%) | - | - |
| Blood Type 0 | 1 (4.5%) | - | - | 10 (27.0%) | - | - | 2 (11.8%) | - | - | 9 (21.4%) | - | - |
| RhD (+) | 20 (90.9%) | - | - | 29 (78.4%) | - | - | 14 (82.4%) | - | - | 35 (83.3%) | - | - |
| COPD | 11 (50.0%) | - | - | 11 (21.6%) | - | - | 4 (23.5%) | - | - | 15 (35.7%) | - | - |
| ILD | 9 (40.1%) | - | - | 9 (35.1%) | - | - | 6 (35.2%) | - | - | 16 (38.1%) | - | - |
| CF | 2 (9.1%) | - | - | 12 (32.4%) | - | - | 5 (29.4%) | - | - | 9 (21.4%) | - | - |
| Hypertension | 6 (27.3%) | - | - | 14 (37.8%) | - | - | 6 (35.3%) | - | - | 14 (33.3%) | - | - |
| CHD | 0 (0.0%) | - | - | 4 (10.8%) | - | - | 2 (11.8%) | - | - | 2 (4.8%) | - | - |
| Diabetes | 4 (18.2%) | - | - | 8 (21.6%) | - | - | 5 (29.4%) | - | - | 7 (16.7%) | - | - |
| Secondary PAH | 14 (63.6%) | - | - | 23 (62.2%) | - | - | 11 (64.7%) | - | - | 23 (54.8%) | - | - |
| Pregnancy History | 7/10 (70.0%) | - | - | 10/14 (71.4%) | - | - | 4/5 (80.0%) | - | - | 13/19 (68.4%) | - | - |
| Nicotinism >10 y | 9 (40.1%) | - | - | 13 (35.1%) | - | - | 5 (29.4%) | - | - | 17 (40.5%) | - | - |
| BLuTx | 12 (54.5%) | - | - | 30 (81.0%) | - | - | 13 (76.5%) | - | - | 29 (69.0%) | - | - |
| Circulatory Support | 4 (18.2%) | - | - | 23 (62.2%) | - | - | 11 (64.7%) | - | - | 16 (38.1%) | - | - |
| CPB | 0 (0.0%) | - | - | 5 (13.5%) | - | - | 3 (17.6%) | - | - | 2 (4.8%) | - | - |
| ECMO | 4 (18.2%) | - | - | 18 (48.6%) | - | - | 8 (47.1%) | - | - | 14 (33.3%) | - | - |
| CIT (min) | 22 | 467 (±109) | 485 (350–540) | 35 | 534 (±123) | 534 (440–600) | 15 | 562 ± 118 | 540 (470–660) | 42 | 490 ± 118 | 490 (380–580) |
| Intraoperative Fluids (mL) | 19 | 2347 (±1150) | 2200 (1700–2850) | 34 | 3081 (±1753) | 2450 (2000–3850) | 15 | 2898 ± 1023 | 2500 (2300–3850) | 38 | 2786 ± 1703 | 2275 (1700–3400) |
| RBCC (Units) | 19 | 2.8 (±1.3) | 2 (2–4) | 34 | 4.4 (±3.4) | 4 (2–6) | 15 | 4.9 (±4.2) | 4 (2–6) | 38 | 3.4 ± 2.2 | 3 (2–4) |
| PGD 0 Group n = 22 | PGD 1–3 Group n = 37 | PGD 3 Group n = 17 | PGD 0–2 Group n = 42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Avg. ± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | n | Avg.± SD | Med. ± (IQR) | n | Avg. ± SD | Med. ± (IQR) | |
| Trauma | 8 (36.3%) | 8 (36.3%) | - | 16 (43.2%) | - | - | 5 (29.4%) | - | - | 19 (45.2%) | - | - |
| SAH | 10 | 10 (45.5%) | - | 18 (48.6%) | - | - | 9 (52.9%) | - | - | 19 (45.2%) | - | - |
| Alcoholism | 7 | 7 (31.8%) | - | 14 (37.8%) | - | - | 7 (41.2%) | - | - | 14 (33.3%) | - | - |
| CVP (mm H2O) | 19 | 7.3 (±3.5) | 7 (5–11) | 25 | 9.4 ± 3.9 | 9 (7–12) | 11 | 11.1 ± 3.7 | 10 (8–14) | 33 | 7.7 ± 3.5 | 7 (5–11) |
| WBCs (103/mm3) | 21 | 14.1 ± 5.3 | 12.5 (10.9–16.4) | 35 | 14.8 ± 9.5 | 12.5 (9.7–18) | 16 | 18.0 ± 12.4 | 15.8 (10.3–19.0) | 40 | 13.1 ± 5.3 | 12.1 (10.4–15.2) |
| PLTs (103/mm3) | 21 | 161 ± 124 | 136 (79–175) | 35 | 169 ± 102 | 160 (76–222) | 16 | 181 ± 97 | 192 (76.5–270.5) | 40 | 160 ± 116 | 136 (76.5–193.5) |
| Serum Cr (mg/dL) | 22 | 1.24 ± 1.03 | 0.95 (0.73–1.30) | 36 | 1.51 ± 1.14 | 1.04 (0.80–1.90) | 17 | 1.96 ± 1.44 | 1.63 (1.03–2.17) | 41 | 1.18 ± 0.84 | 0.89 (0.78–1.20) |
| INR | 21 | 1.25 ± 0.23 | 1.23 (1.10–1.40) | 35 | 1.29 ± 0.29 | 1.21 (1.08–1.45) | 16 | 1.39 ± 0.35 | 1.29 (1.15–1.69) | 40 | 1.23 ± 0.22 | 1.20 (1.08–1.37) |
| Sex Mismatch | 7 (31.8%) | - | - | 7 (29.7%) | - | - | 6 (35.3%) | - | - | 12/42 (28.6%) | - | - |
| ABO Mismatch | 4 (18.2%) | - | - | 17 (45.9%) | - | - | 8 (47.1%) | - | - | 13/42 (31.0%) | - | - |
| Rh Mismatch | 7 (31.8%) | - | - | 8 (21.6%) | - | - | 2 (11.8%) | - | - | 13/42 (31.0%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubisa, M.J.; Wojtyś, M.E.; Lisowski, P.; Kordykiewicz, D.; Piotrowska, M.; Wójcik, J.; Pieróg, J.; Safranow, K.; Grodzki, T.; Kubisa, B. Analysis of Primary Graft Dysfunction (PGD) Risk Factors in Lung Transplantation (LuTx) Patients. Clin. Pract. 2024, 14, 1571-1583. https://doi.org/10.3390/clinpract14040127
Kubisa MJ, Wojtyś ME, Lisowski P, Kordykiewicz D, Piotrowska M, Wójcik J, Pieróg J, Safranow K, Grodzki T, Kubisa B. Analysis of Primary Graft Dysfunction (PGD) Risk Factors in Lung Transplantation (LuTx) Patients. Clinics and Practice. 2024; 14(4):1571-1583. https://doi.org/10.3390/clinpract14040127
Chicago/Turabian StyleKubisa, Michał Jan, Małgorzata Edyta Wojtyś, Piotr Lisowski, Dawid Kordykiewicz, Maria Piotrowska, Janusz Wójcik, Jarosław Pieróg, Krzysztof Safranow, Tomasz Grodzki, and Bartosz Kubisa. 2024. "Analysis of Primary Graft Dysfunction (PGD) Risk Factors in Lung Transplantation (LuTx) Patients" Clinics and Practice 14, no. 4: 1571-1583. https://doi.org/10.3390/clinpract14040127
APA StyleKubisa, M. J., Wojtyś, M. E., Lisowski, P., Kordykiewicz, D., Piotrowska, M., Wójcik, J., Pieróg, J., Safranow, K., Grodzki, T., & Kubisa, B. (2024). Analysis of Primary Graft Dysfunction (PGD) Risk Factors in Lung Transplantation (LuTx) Patients. Clinics and Practice, 14(4), 1571-1583. https://doi.org/10.3390/clinpract14040127






