The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Depressive/Anxious Symptoms
2.3. Genetic Testing
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics of the Study Groups
3.2. Results Concerning the Assessment of MDD Severity and Evolution
3.3. Results Concerning the Evolution of Anxiety Levels in Patients with MDD
3.4. Longitudinal Changes in CGI Parameters in Group A and B Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinohara, K.; Efthimiou, O.; Ostinelli, E.G.; Tomlinson, A.; Geddes, J.R.; Nierenberg, A.A.; Ruhe, H.G.; Furukawa, T.A.; Cipriani, A. Comparative efficacy and acceptability of antidepressants in the long-term treatment of major depression: Protocol for a systematic review and networkmeta-analysis. BMJ Open 2019, 9, e027574. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Kang, S.; Lee, Y.; Rosenblat, J.D.; Brietzke, E.; Zuckerman, H.; McIntyre, R.S. The long-term effect of bariatric surgery on depression and anxiety. J. Affect. Disord. 2019, 246, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Pastis, I.; Santos, M.G.; Paruchuri, A. Exploring the role of inflammation in major depressive disorder: Beyond the monoamine hypothesis. Front. Behav. Neurosci. 2024, 17, 1282242. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for united action on depression: A Lancet–World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; van Schijndel, M.; van Waarde, J.; van Busschbach, J. Health-economic outcomes in hospital patients with medical-psychiatric comorbidity: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194029. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.; Kim, S.; Rufino, K.A.; Fonagy, P.; Oldham, J.M.; Schanzer, B.; Patriquin, M.A. Time in treatment: Examining mental illness trajectories across inpatient psychiatric treatment. J. Psychiatr. Res. 2020, 130, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Homorogan, C.; Nitusca, D.; Seclaman, E.; Enatescu, V.; Marian, C. Uncovering the Roles of MicroRNAs in Major Depressive Disorder: From Candidate Diagnostic Biomarkers to Treatment Response Indicators. Life 2021, 11, 1073. [Google Scholar] [CrossRef]
- Tudoran, M.; Tudoran, C.; Ciocarlie, T.; Giurgi-Oncu, C. Aspects of diastolic dysfunction in patients with new and recurrent depression. PLoS ONE 2020, 15, e0228449. [Google Scholar] [CrossRef]
- Hankin, B.L.; Griffith, J.M. What Do We Know About Depression Among Youth and How Can We Make Progress toward Improved Understanding and Reducing Distress? A New Hope. Clin. Child Fam. Psychol. Rev. 2023, 10, 919–942. [Google Scholar] [CrossRef]
- Schoevers, R.A.; Deeg, D.J.H.; Van Tilburg, W.; Beekman, A.T.F. Depression and generalized anxiety disorder: Co-occurrence and longitudinal patterns in elderly patients. Am. J. Geriatr. Psychiatry 2005, 13, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, M. Anxiety Symptoms in Patients with Major Depressive Disorder: Commentary on Prevalence and Clinical Implications. Neurol. Ther. 2023, 12 (Suppl. S1), 5–12. [Google Scholar] [CrossRef] [PubMed]
- CHMP. Committee for Medicinal Products for Human Use (CHMP) Guideline on Clinical Investigation of Medicinal Products in the Treatment of Depression Discussion in the Efficacy Working Party. 2013. Available online: www.ema.europa.eu (accessed on 27 March 2024).
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. Hamilton anxiety scale. Group 1959, 1, 10–37. [Google Scholar]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976.
- Depression in Adults: Treatment and Management, NICE Guideline. 2022. Available online: https://www.nice.org.uk/guidance/ng222 (accessed on 8 November 2023).
- Thomas, S.J.; Shin, M.; McInnis, M.G.; Bostwick, J.R. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: Strategies for the management of treatment-resistant depression. Pharmacotherapy 2015, 35, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, A.L.; Roberts, D.; Khawaja, A.; McNair, R. The role of serotonin neurotransmission in rapid antidepressant actions. Psychopharmacology 2022, 239, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Giurgi-Oncu, C.; Tudoran, C.; Enatescu, V.R.; Tudoran, M.; Pop, G.N.; Bredicean, C. Evolution of Heart Rate Variability and Heart Rate Turbulence in Patients with Depressive Illness Treated with Selective Serotonin Reuptake Inhibitors. Medicina 2020, 56, 590. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Michel, S.; Allen, J.D.; Meyer, T.; McGonagle, E.J.; Carpentier, R.; Vecchia, A.; Schlichte, A.; Bishop, J.R.; Dunnenberger, H.M.; et al. Development and Implementation of In-House Pharmacogenomic Testing Program at a Major Academic Health System. Front. Genet. 2021, 12, 712602. [Google Scholar] [CrossRef]
- Krause, D.S.; Dowd, D. Use of a consultation service following pharmacogenetic testing in psychiatry. Pharmacogenomics 2022, 23, 327–333. [Google Scholar] [CrossRef]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Dowd, D.; Fava, M.; Lencz, T.; Krause, D.S. Randomized, controlled, participant- and rater-blind trial of pharmacogenomic test-guided treatment versus treatment as usual for major depressive disorder. Depress. Anxiety 2020, 37, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C.; Shear, M.K.; Klerman, G.L.; Portera, L.; Rosenbaum, J.F.; Goldenberg, I. A comparison of symptom determinants of patient and clinician global ratings in patients with panic disorder and depression. J. Clin. Psychopharmacol. 1993, 13, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Spearing, M.K.; Post, R.M.; Leverich, G.S.; Brandt, D.; Nolen, W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997, 73, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Zaider, T.I.; Heimberg, R.G.; Fresco, D.M.; Schneier, F.R.; Liebowitz, M.R. Evaluation of the clinical global impression scale among individuals with social anxiety disorder. Psychol. Med. 2003, 33, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, T.; Scherer, A.; Boecker, M.; Pawelzik, M.; Jostes, R.; Gauggel, S. The clinical global impression scale and the influence of patient or staff perspective on outcome. BMC Psychiatry 2011, 11, 83. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Manole, F.; Marian, P.; Mekeres, G.M.; Voiţă-Mekereş, F. Systematic Review of the Effect of Aging on Health Costs. Arch. Pharm. Pract. 2023, 14, 58–61. [Google Scholar] [CrossRef]
- Hek, K.; Demirkan, A.; Lahti, J.; Terracciano, A.; Teumer, A.; Cornelis, M.C.; Amin, N.; Bakshis, E.; Baumert, J.; Ding, J.; et al. A genome-wide association study of depressive symptoms. Biol. Psychiatry 2013, 73, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Lee, S.H.; Mehta, D.; Vinkhuyzen, A.A.; Dudbridge, F.; Middeldorp, C.M. Research review: Polygenic methods and their application to psychiatric traits. J. Child Psychol. Psychiatry 2014, 55, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.; Lewis, C.M. Genetics of depression: Progress at last. Curr. Psychiatry Rep. 2017, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Hettema, J.M. What is the genetic relationship between anxiety and depression? Am. J. Med. Genet. Part C Semin. Med. Genet. 2008, 148, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Cole, D.P.; Chengappa, K.R. Anxiety disorders and major depression, together or apart. Depress. Anxiety 2001, 14, 94–104. [Google Scholar] [CrossRef]
- Brown, L.C.; Stanton, J.D.; Bharthi, K.; Maruf, A.A.; Müller, D.J.; Bousman, C.A. Pharmacogenomic Testing and Depressive Symptom Remission: A Systematic Review and Meta-Analysis of Prospective, Controlled Clinical Trials. Clin. Pharmacol. Ther. 2022, 112, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Mehta, R.; Edwards, A.M.; Tiwari, A.; Imbens, G.W. Pharmacogenetic testing among patients with mood and anxiety disorders is associated with decreased utilization and cost: A propensity-score matched study. Depress. Anxiety 2018, 35, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.E.; Agrawal, D.; Deem, A.P.; Knoper, T.L.D.; Merino, R.F.; Molzof, H.E.; Maus, L.E.; Kim, F.; Lodin, Z.; Lim, S. Medication optimization using pharmacogenomic testing in a complex mental health population prescribed psychiatric polypharmacy. J. Clin. Pharmacol. 2022, 62, 898–904. [Google Scholar] [CrossRef]
- King, C.D.; Yip, A.G.; Cao, Y.A.; Rodriguez-Villa, F.; Krause, D.S.; Dowd, D.; Ressler, K.J. Open-label pilot study of psychiatric pharmacogenetic testing in an adult psychiatric inpatient population. Pers. Med. Psychiatry 2020, 21, 100060. [Google Scholar] [CrossRef]
- Brouwer, J.M.J.L.; Nijenhuis, M.; Soree, B.; Guchelaar, H.-J.; Swen, J.J.; van Schaik, R.H.N.; van der Weide, J.; Rongen, G.A.P.J.M.; Buunk, A.-M.; de Boer-Veger, N.J.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur. J. Hum. Genet. 2022, 30, 1114–1120. [Google Scholar] [CrossRef]
- Kendler, K.S.; Gardner, C. A longitudinal etiologic model for symptoms of anxiety and depression in women. Psychol. Med. 2011, 41, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Patel, K.; Moscovici, M.; McMaster, R.; Glancy, G.; Simpson, A.I. Adaptation of the clinical global impression for use in correctional settings: The CGI-C. Front. Psychiatry 2019, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Giurgi-Oncu, C.; Tudoran, C.; Pop, G.N.; Bredicean, C.; Pescariu, S.A.; Giurgiuca, A.; Tudoran, M. Cardiovascular Abnormalities and Mental Health Difficulties Result in a Reduced Quality of Life in the Post-Acute COVID-19 Syndrome. Brain Sci. 2021, 11, 1456. [Google Scholar] [CrossRef]

| Characteristics | Total Sample = 76 Subjects (No./Percent) | Group A = 37 Subjects Genetically Tested (No./Percent) | Group B = 39 Subjects Not Tested (No./Percent) | Chi2 | p | |
|---|---|---|---|---|---|---|
| Gender: | Men | 26 (34.2%) | 16/43.2% | 10/25.6% | 0.791 | 0.373 |
| Women | 50 (65.8%) | 21/56.8% | 29/74.4% | 1.672 | 0.195 | |
| Education: | ˂4 grades | 0 | 0 | 0 | 0 | 0 |
| ≥8 grades | 1 (1.3%) | 0 | 1/2.6% | 0 | 0 | |
| High school | 46 (60.5%) | 14/37.8% | 32/82.1% | 8.683 | 0.003 * | |
| College | 29 (38.2%) | 23/62.2% | 6/15.4% | 4.035 | 0.04 * | |
| Civil status: | Single | 35 (46.1%) | 21/56.8% | 14/35.9% | 1.427 | 0.232 |
| Married | 33 (43.4%) | 14/37.8% | 19/48.7% | 0.377 | 0.539 | |
| Divorced | 3 (3.9%) | 1/2.7% | 2/5.1% | 0.006 | 0.937 | |
| Stable relationship | 4 (5.3%) | 1/2.7% | 3/7.7% | 0.023 | 0.878 | |
| Widowed | 1 (1.3%) | 0 | 1/2.6% | 0 | 0 | |
| Occupation: | Employed | 41 (53.9%) | 21/56.8% | 20/51.3% | 0.122 | 0.727 |
| Retired | 26 (34.2%) | 9/24.3% | 17/43.6% | 0.905 | 0.341 | |
| Unemployed | 2 (2.6%) | 0 | 2/5.1% | 0 | 0 | |
| Student | 7 (9.2%) | 7/18.9% | 0 | 0 | 0 | |
| Provenience: | Urban | 26 (34.2%) | 30/81.1% | 20/51.3% | 4.898 | 0.02 * |
| Rural | 50 (65.8%) | 7/18.9% | 19/48.7% | 1.810 | 0.178 | |
| Scales Employed | Group A = 37 Subjects with Genetic Testing (m/SD) | Group B = 39 Subjects Not Tested (m/SD) | T | p | |
|---|---|---|---|---|---|
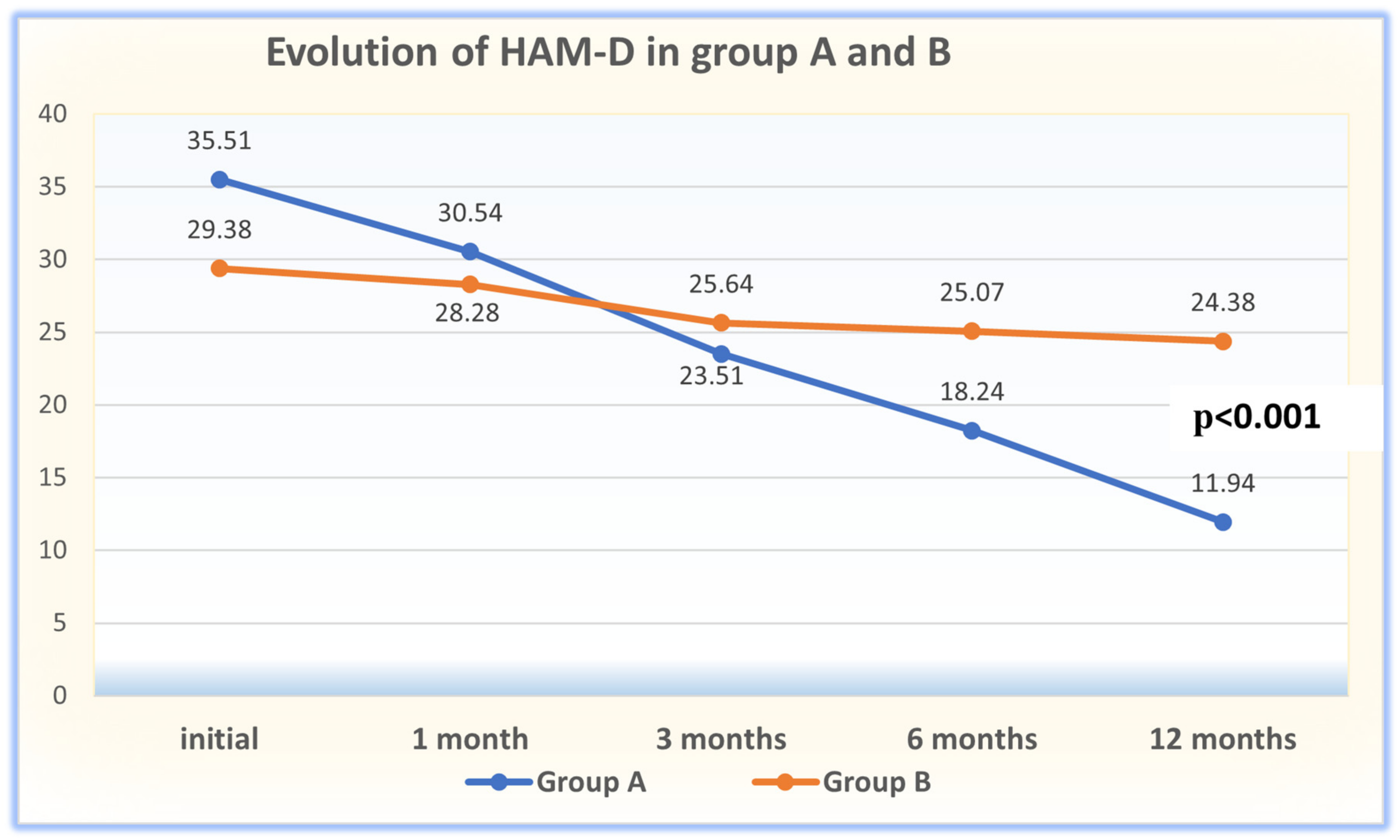
| HAM-D: | initial (T1) | 35.51 ± 6.87 | 29.38 ± 3.87 | 4.821 | 0.001 * |
| 1 month (T2) | 30.54 ± 11.88 | 28.28 ± 3.47 | 1.137 | 0.259 | |
| 3 months (T3) | 23.51 ± 11.93 | 25.64 ± 3.68 | −1.062 | 0.292 | |
| 6 months (T4) | 18.24 ± 11.94 | 25.07 ± 3.01 | −3.460 | 0.001 * | |
| 12 months (T5) | 11.94 ± 8.87 | 24.38 ± 3.94 | −7.962 | 0.001 * | |
| HAM-A: | initial (T1) | 31.81 ± 5.71 | 27.41 ± 4.10 | 3.872 | 0.001 * |
| 1 month (T2) | 26.45 ± 5.01 | 26.84 ± 4.68 | −0.347 | 0.729 | |
| 3 months (T3) | 21.16 ± 4.30 | 24.43 ± 4.19 | −3.356 | 0.001 * | |
| 6 months (T4) | 16.00 ± 4.36 | 24.00 ± 3.19 | −9.150 | 0.001 * | |
| 12 months (T5) | 11.35 ± 4.95 | 23.30 ± 4.01 | −11.592 | 0.001 * | |
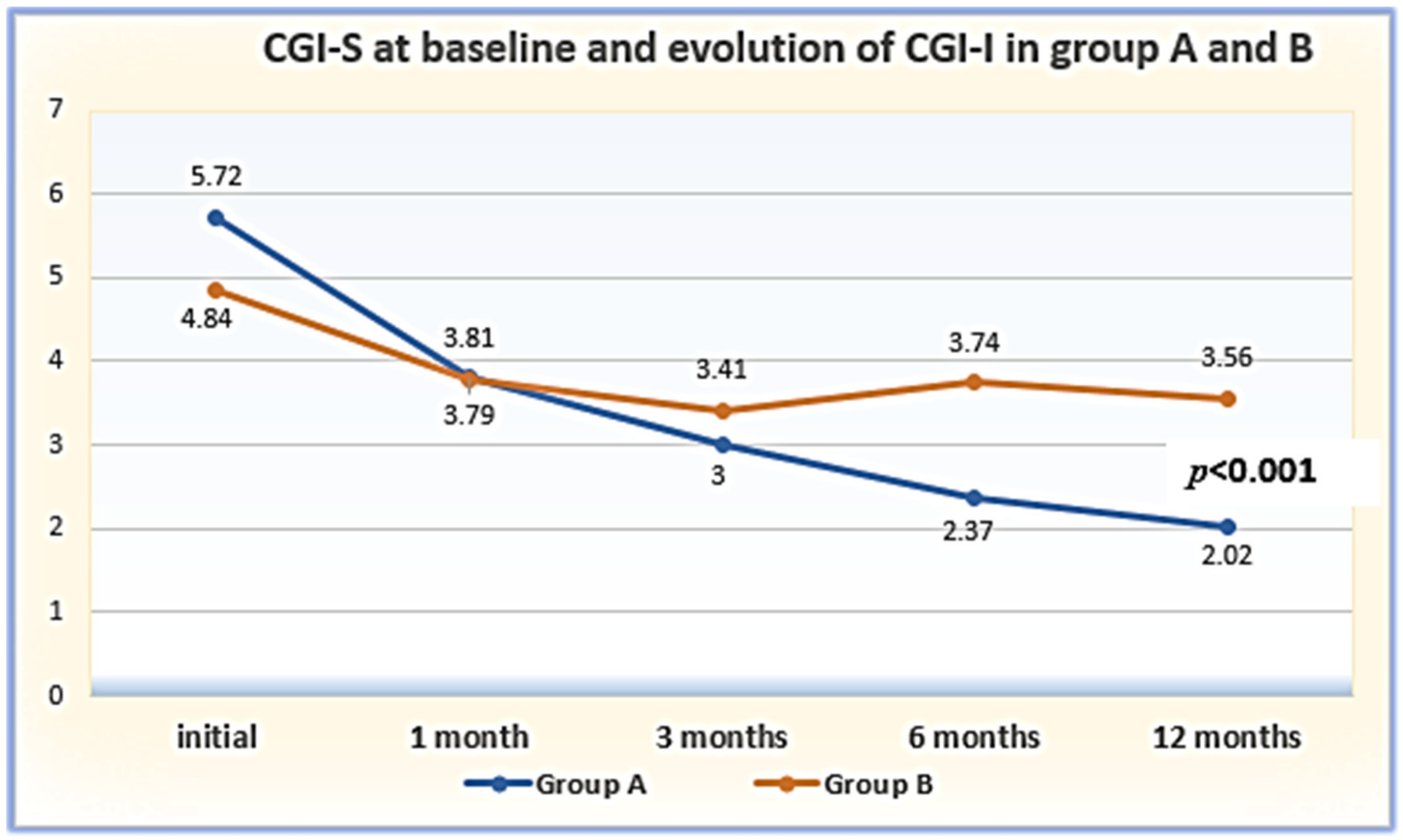
| CGI–S: | baseline (T1) | 5.72 ± 0.60 | 4.84 ± 0.74 | 5.649 | 0.001 * |
| CGI-I: | 1 month (T2) | 3.81 ± 0.46 | 3.79 ± 0.40 | 0.159 | 0.874 |
| 3 months (T3) | 3.00 ± 0.47 | 3.41 ± 0.49 | −3.683 | 0.001 * | |
| 6 months (T4) | 2.37 ± 0.49 | 3.74 ± 2.39 | −3.403 | 0.001 * | |
| 12 months (T5) | 2.02 ± 0.55 | 3.56 ± 0.71 | −10.422 | 0.001 * | |
| Disease Type | Patient Group | Sum of Squares | Df | Squared Mean Value | F | p | η2 |
|---|---|---|---|---|---|---|---|
| RDD | Group A Genetically tested | 13,098.184 | 4 | 3274.546 | 74.334 | 0.001 | 0.674 |
| Group B Not tested | 730.082 | 4 | 182.521 | 21.218 | 0.001 | 0.358 |
| Disease Type | Patient Group | (A) HAM-D | (B) HAM-D | Mean of Differences (A–B) | Standard Error | p |
|---|---|---|---|---|---|---|
| RDD | Group A Genetically tested | Inclusion | 1 month | 4.97 | 2.23 | 0.321 |
| 3 months | 12.00 * | 2.21 | 0.001 | |||
| 6 months | 17.27 * | 2.21 | 0.001 | |||
| 12 months | 23.56 * | 1.82 | 0.001 | |||
| Group B Not tested | Inclusion | 1 month | 1.10 | 0.50 | 0.366 | |
| 3 months | 3.74 * | 0.72 | 0.001 | |||
| 6 months | 4.30 * | 0.64 | 0.001 | |||
| 12 months | 5.00 * | 0.82 | 0.001 |
| Disease Type | Patient Group | Sum of Squares | Df | Squared Mean Value | F | p | η2 |
|---|---|---|---|---|---|---|---|
| RDD | Group A Genetically tested | 9774.13 | 4 | 2443.532 | 160.621 | 0.001 | 0.817 |
| Group B Not tested | 514.79 | 4 | 128.697 | 18.875 | 0.001 | 0.332 |
| Disease Type | Patient Group | (A) HAM-A | (B) HAM-A | Mean of Differences (A–B) | Standard Error | p |
|---|---|---|---|---|---|---|
| RDD | Group A Genetically tested | Inclusion | 1 month | 5.35 * | 0.71 | 0.001 |
| 3 months | 10.64 * | 0.95 | 0.001 | |||
| 6 months | 15.81 * | 1.05 | 0.001 | |||
| 12 months | 20.45 * | 1.25 | 0.001 | |||
| Group B Not tested | Inclusion | 1 month | 0.56 | 0.43 | 1.000 | |
| 3 months | 2.97 * | 0.62 | 0.001 | |||
| 6 months | 3.41 * | 0.50 | 0.001 | |||
| 12 months | 4.10 * | 0.64 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platona, R.I.; Voiță-Mekeres, F.; Tudoran, C.; Tudoran, M.; Enătescu, V.R. The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder. Clin. Pract. 2024, 14, 703-717. https://doi.org/10.3390/clinpract14030056
Platona RI, Voiță-Mekeres F, Tudoran C, Tudoran M, Enătescu VR. The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder. Clinics and Practice. 2024; 14(3):703-717. https://doi.org/10.3390/clinpract14030056
Chicago/Turabian StylePlatona, Rita Ioana, Florica Voiță-Mekeres, Cristina Tudoran, Mariana Tudoran, and Virgil Radu Enătescu. 2024. "The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder" Clinics and Practice 14, no. 3: 703-717. https://doi.org/10.3390/clinpract14030056
APA StylePlatona, R. I., Voiță-Mekeres, F., Tudoran, C., Tudoran, M., & Enătescu, V. R. (2024). The Contribution of Genetic Testing in Optimizing Therapy for Patients with Recurrent Depressive Disorder. Clinics and Practice, 14(3), 703-717. https://doi.org/10.3390/clinpract14030056







