Efficacy of Touch Imprint Cytology in Intraoperative Diagnosis of Invasive Mucinous Adenocarcinoma of the Lung: A Case Report and Literature Review
Abstract
1. Introduction
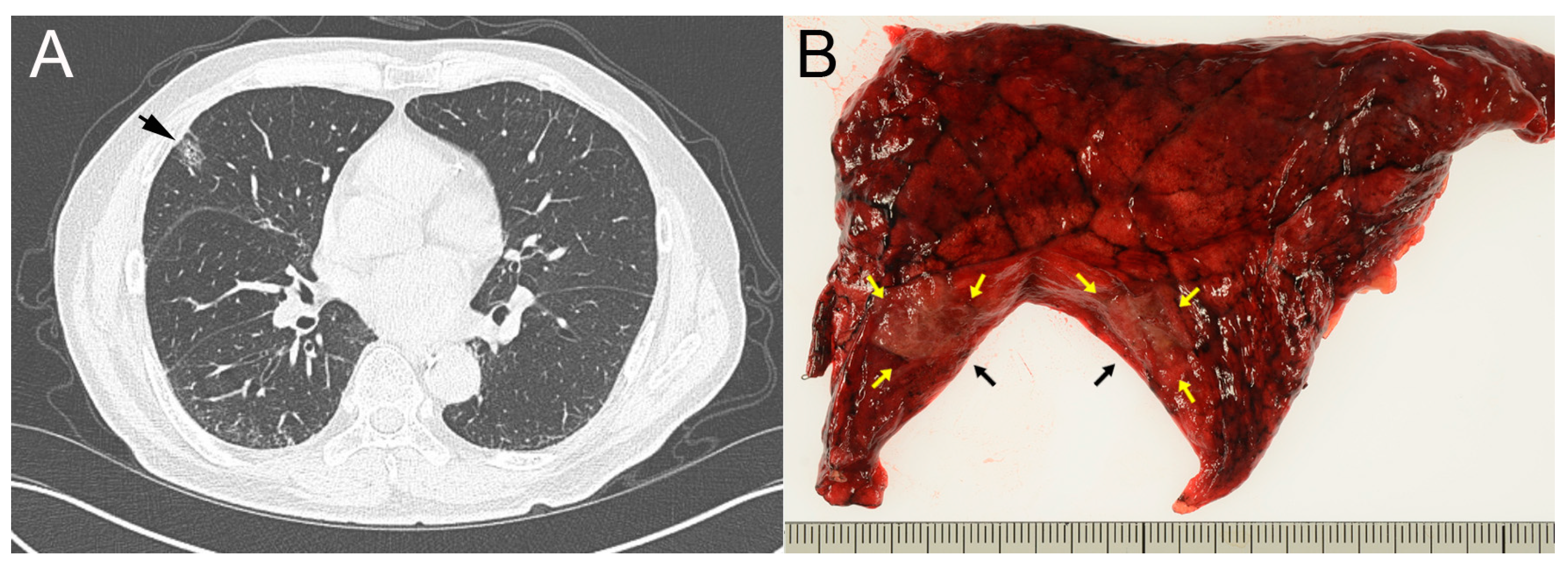
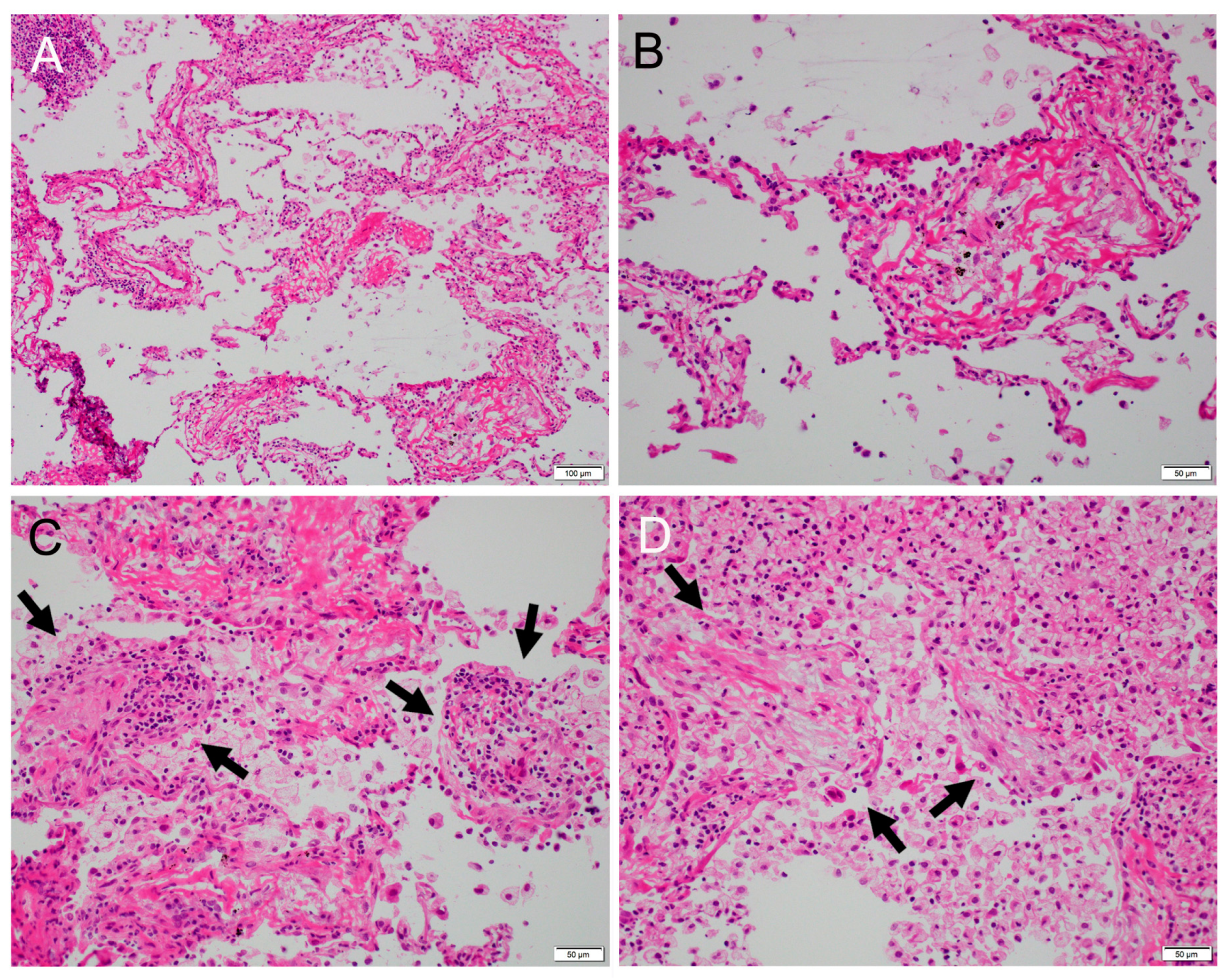
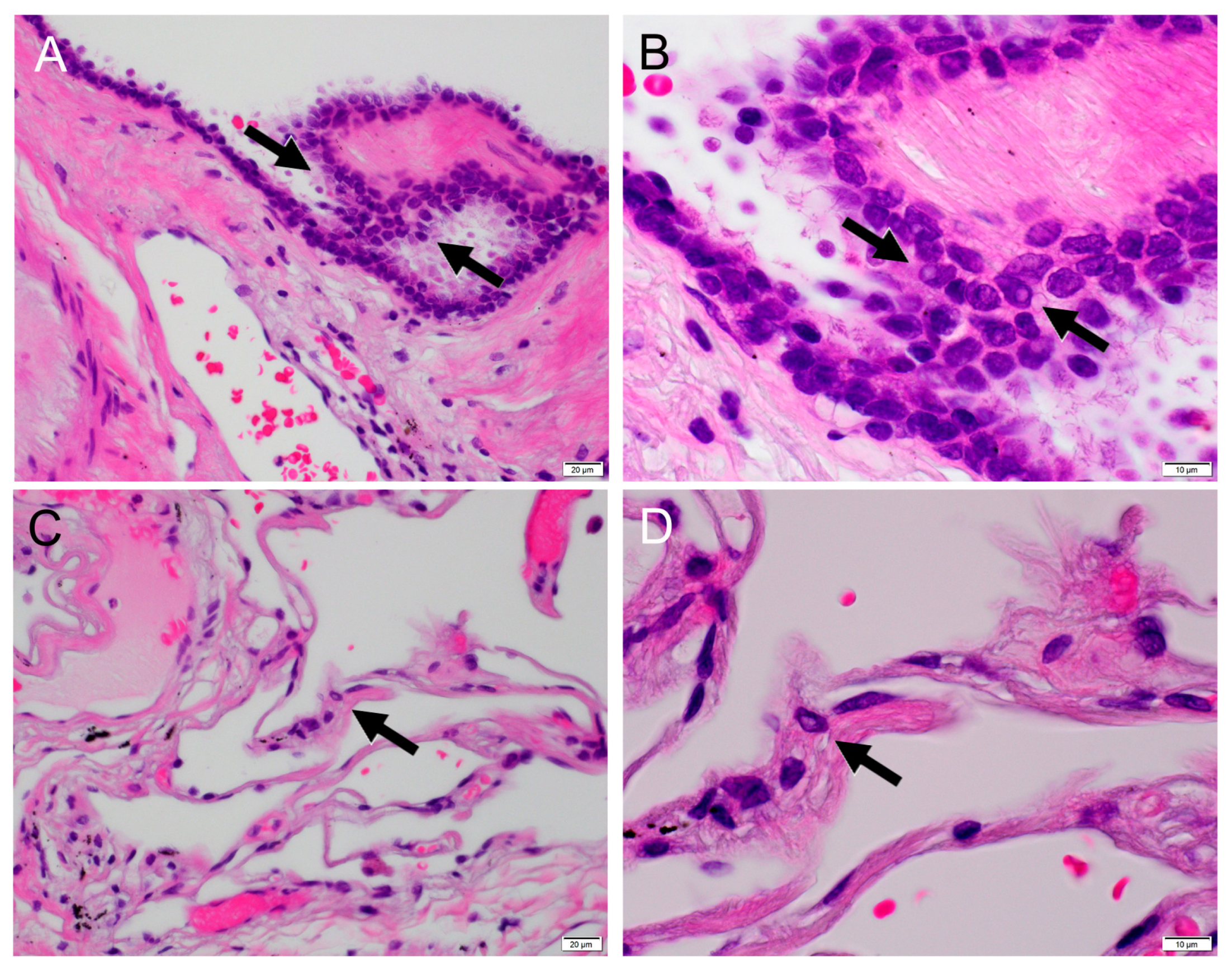
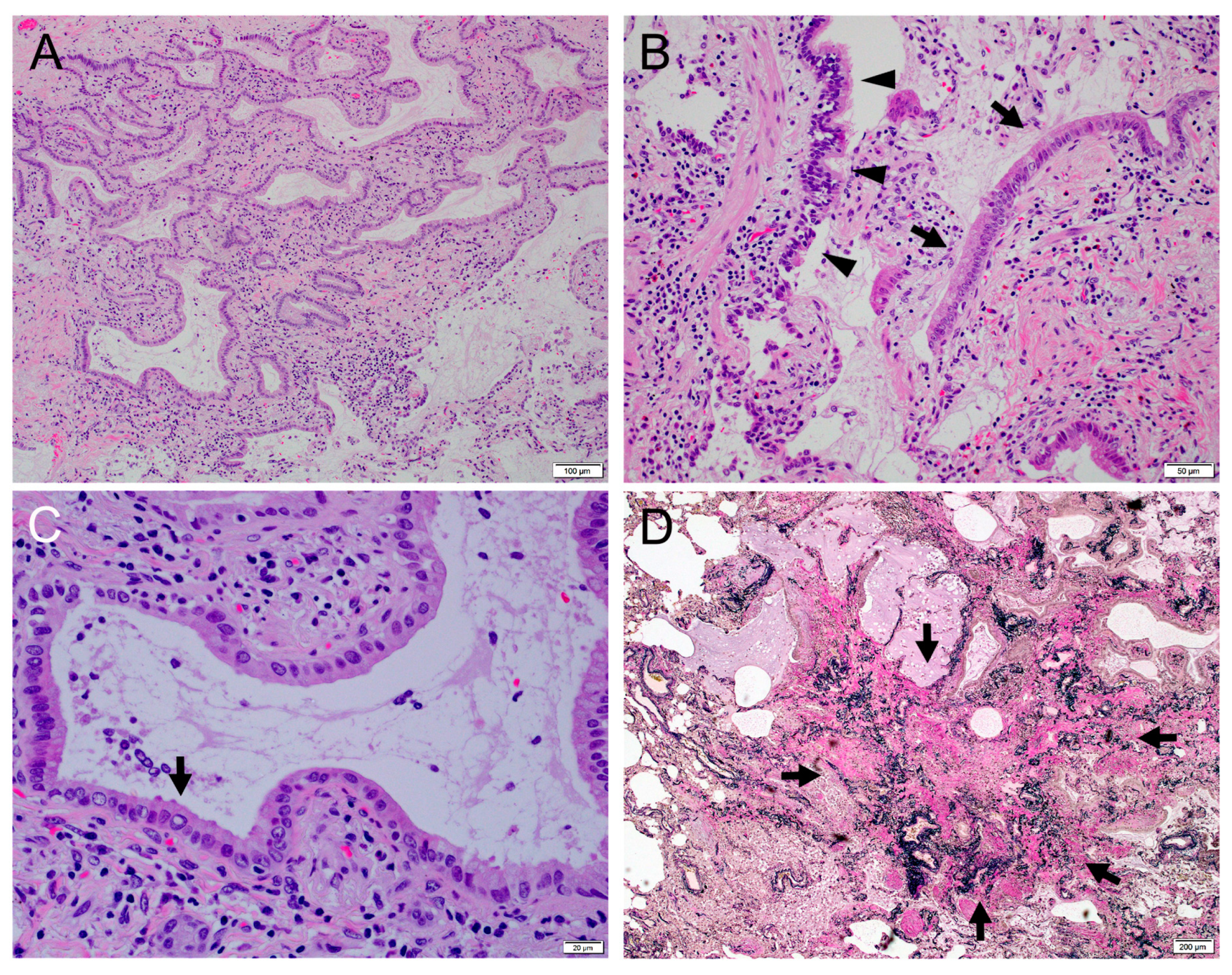
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization classification of lung tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Cooper, W.A.; Chang, J.C.; Chou, T.Y.; MacMahon, H.; Warth, A.; Yoshizawa, A. Invasive mucinous adenocarcinoma of the lung. In WHO Classification of Tumours, Thoracic Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2021; pp. 75–78. [Google Scholar]
- Tsuta, K.; Kawago, M.; Inoue, E.; Yoshida, A.; Takahashi, F.; Sakurai, H.; Watanabe, S.I.; Takeuchi, M.; Furuta, K.; Asamura, H.; et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013, 81, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Motoi, N.; Riely, G.J.; Sima, C.S.; Gerald, W.L.; Kris, M.G.; Park, B.J.; Rusch, V.W.; Travis, W.D. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod. Pathol. 2011, 24, 653–664. [Google Scholar] [CrossRef]
- Warth, A.; Muley, T.; Meister, M.; Stenzinger, A.; Thomas, M.; Schirmacher, P.; Schnabel, P.A.; Budczies, J.; Hoffmann, H.; Weichert, W. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J. Clin. Oncol. 2012, 30, 1438–1446. [Google Scholar] [CrossRef]
- Moon, S.W.; Choi, S.Y.; Moon, M.H. Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: A population-based study. J. Thorac. Dis. 2018, 10, 3595–3608. [Google Scholar] [CrossRef]
- Xu, L.; Li, C.; Lu, H. Invasive mucinous adenocarcinoma of the lung. Transl. Cancer Res. 2019, 8, 2924–2932. [Google Scholar] [CrossRef]
- Boland, J.M.; Maleszewski, J.J.; Wampfler, J.A.; Voss, J.S.; Kipp, B.R.; Yang, P.; Yi, E.S. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma-a clinicopathological and molecular genetic study with survival analysis. Hum. Pathol. 2018, 71, 8–19. [Google Scholar] [CrossRef]
- Simsir, A.; Wei, X.J.; Yee, H.; Moreira, A.; Cangiarella, J. Differential expression of cytokeratins 7 and 20 and thyroid transcription factor-1 in bronchioloalveolar carcinoma: An immunohistochemical study in fine-needle aspiration biopsy specimens. Am. J. Clin. Pathol. 2004, 121, 350–357. [Google Scholar] [CrossRef]
- Jaafer, H. Intra-operative frozen section consultation: Concepts, applications and limitations. Malays. J. Med. Sci. 2006, 13, 4–12. [Google Scholar]
- Marchevsky, A.M.; Changsri, C.; Gupta, I.; Fuller, C.; Houck, W.; McKenna, R.J., Jr. Frozen section diagnoses of small pulmonary nodules: Accuracy and clinical implication. Ann. Thorac. Surg. 2004, 78, 1755–1759. [Google Scholar] [CrossRef]
- Gupta, R.; McKenna, R., Jr.; Marchevsky, A.M. Lessons learned from mistakes and deferrals in the frozen section diagnosis of bronchioloalveolar carcinoma and well-differentiated pulmonary adenocarcinoma: An evidence-based pathology approach. Am. J. Clin. Pathol. 2008, 30, 11–20. [Google Scholar] [CrossRef]
- Dacic, S. Pros: The present classification of mucinous adenocarcinomas of the lung. Transl. Lung Cancer Res. 2017, 6, 230–233. [Google Scholar] [CrossRef]
- Jain, D.; MacMahon, H.; Pelosi, G.; Riely, G.; Rusch, V.W.; Van Schil, P.E.Y. Colloid adenocarcinoma of the lung. In WHO Classification of Tumours, Thoracic Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2021; pp. 79–80. [Google Scholar]
- Chu, P.G.; Schwarz, R.E.; Lau, S.K.; Yen, Y.; Weiss, L.M. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: Application of CDX2, CK17, MUC1, and MUC2. Am. J. Surg. Pathol. 2005, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Gown, A.M.; Wu, L.S.; Barry, T.S.; Wheeler, D.T.; Yemelyanova, A.; Seidman, J.D.; Ronnett, B.M. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: Comparison with CK20 and correlation with coordinate expression of CK7. Mod. Pathol. 2006, 19, 1421–1428. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Zhang, Y.; Li, Y.; Cheng, C.; Pan, Y.; Xiang, J.; Zhang, Y.; Chen, H.; Sun, Y. Precise diagnosis of intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. J. Clin. Oncol. 2016, 34, 307–313. [Google Scholar] [CrossRef]
- Zaman, S.S.; van Hoeven, K.H.; Slott, S.; Gupta, P.K. Distinction between bronchioloalveolar carcinoma and hyperplastic pulmonary proliferations: A cytologic and morphometric analysis. Diagn. Cytopathol. 1997, 16, 396–401. [Google Scholar] [CrossRef]
- Ohori, N.P.; Santa Maria, E.L. Cytopathologic diagnosis of bronchioalveolar carcinoma. Am. J. Clin. Pathol. 2004, 122, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Raz, D.J.; Zell, J.A.; Karnezis, A.N. Misclassification of bronchioloalveolar carcinoma with cytologic diagnosis of lung cancer. J. Thorac. Oncol. 2006, 1, 943–948. [Google Scholar] [CrossRef]
- MacDonald, L.L.; Yazdi, H.M. Fine-needle aspiration biopsy of bronchioloalveolar carcinoma. Cancer 2001, 93, 29–34. [Google Scholar] [CrossRef]
- Komatsu, H.; Tajima, S.; Kawamura, M.; Sato, K.; Yoneda, R. Intranuclear inclusions in adenocarcinoma of the lung. J. Jpn. Soc. Clin. Cytol. 1988, 27, 343–349. (In Japanese) [Google Scholar] [CrossRef]
- Kasai, T.; Miyazaki, R.; Onodera, T.; Sugimoto, K.; Arikura, I.; Haratake, J. Comparison of atypical adenomatous hyperplasia with nonmucinous bronchioloalveolar carcinoma using touch smear cytology. J. Jpn. Soc. Clin. Cytol. 2002, 41, 381–387. (In Japanese) [Google Scholar] [CrossRef]
- Iyoda, A.; Baba, M.; Saitoh, H.; Hoshino, H.; Shibuya, K.; Nomoto, Y.; Horiuchi, F.; Hiroshima, K.; Ohwada, H.; Fujisawa, T. Imprint cytologic features of pulmonary sclerosing hemangioma: Comparison with well-differentiated papillary adenocarcinoma. Cancer 2002, 96, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Maleki, Z.; Muller, S.; Layfield, L.; Siddiqui, M.T.; Rekhtman, N.; Pantanowitz, L. Pulmonary sclerosing pneumocytoma: Cytomorphology and immunoprofile. Cancer Cytopathol. 2020, 128, 414–423. [Google Scholar] [CrossRef] [PubMed]
- McNary, W.F., Jr.; Gaensler, E.A. Intranuclear inclusion bodies in desquamative interstitial pneumonia. Ann. Int. Med. 1971, 74, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, O.; Ferrans, V.J.; Fulmer, J.D.; Crystal, R.G. Nuclear inclusions in alveolar epithelium of patients with fibrotic lung disorders. Am. J. Pathol. 1979, 94, 301–322. [Google Scholar]
- Kawanami, O.; Tanaka, M.; Mitsui, K.; Kawai, T. Bronchiolo-fiberscopic, histological and ultrastructural studies of peripheral lung tissue in bronchial asthma patients. Nihon Kyobu Shikkan Zasshi 1989, 27, 925–932. (In Japanese) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Higuchi, Y.; Oshima, M.; Endo, F.; Sato, F.; Sugihara, S.; Yamamoto, M.; Imai, Y. Efficacy of Touch Imprint Cytology in Intraoperative Diagnosis of Invasive Mucinous Adenocarcinoma of the Lung: A Case Report and Literature Review. Clin. Pract. 2024, 14, 242-249. https://doi.org/10.3390/clinpract14010019
Kato T, Higuchi Y, Oshima M, Endo F, Sato F, Sugihara S, Yamamoto M, Imai Y. Efficacy of Touch Imprint Cytology in Intraoperative Diagnosis of Invasive Mucinous Adenocarcinoma of the Lung: A Case Report and Literature Review. Clinics and Practice. 2024; 14(1):242-249. https://doi.org/10.3390/clinpract14010019
Chicago/Turabian StyleKato, Toshihiko, Yumiko Higuchi, Mei Oshima, Fuki Endo, Fuminori Sato, Shiro Sugihara, Manabu Yamamoto, and Yasuo Imai. 2024. "Efficacy of Touch Imprint Cytology in Intraoperative Diagnosis of Invasive Mucinous Adenocarcinoma of the Lung: A Case Report and Literature Review" Clinics and Practice 14, no. 1: 242-249. https://doi.org/10.3390/clinpract14010019
APA StyleKato, T., Higuchi, Y., Oshima, M., Endo, F., Sato, F., Sugihara, S., Yamamoto, M., & Imai, Y. (2024). Efficacy of Touch Imprint Cytology in Intraoperative Diagnosis of Invasive Mucinous Adenocarcinoma of the Lung: A Case Report and Literature Review. Clinics and Practice, 14(1), 242-249. https://doi.org/10.3390/clinpract14010019






