Myoclonus Secondary to Amantadine: Case Report and Literature Review
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef]
- Chevalier, J.F.; Renier, E.; Brion, S. Edema and myoclonus in a patient with Parkinson’s disease treated by amantadine. L’encephale 1980, 6, 381–384. [Google Scholar]
- Marmol, S.; Feldman, M.; Singer, C.; Margolesky, J. Amantadine Revisited: A Contender for Initial Treatment in Parkinson’s Disease? CNS Drugs 2021, 35, 1141–1152. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Amantadine-induced “vocal” myoclonus. Mov. Disord. 1996, 11, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Uozumi, T.; Qingrui, L.; Hashimoto, T.; Tsuji, S. Amantadine-induced cortical myoclonus. Neurology 2001, 56, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Ito, S.; Shirai, W.; Hattori, T. Severe reversible neurological complications following amantadine treatment in three elderly patients with renal insufficiency. Eur. Neurol. 2006, 56, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.L.; Hung, S.W.; Lin, L.W.; Chong, C.F.; Lau, C.L. Amantadine-induced serotonin syndrome in a patient with renal failure. Am. J. Emerg. Med. 2008, 26, 112.e5-6. [Google Scholar] [CrossRef]
- Hong, C.T.; Sun, Y.; Lu, C.J. Fatal intoxication using amantadine and pramipexole in a uremic patient. Acta Neurol. Taiwan. 2008, 17, 109–111. [Google Scholar]
- Nishikawa, N.; Nagai, M.; Moritoyo, T.; Yabe, H.; Nomoto, M. Plasma amantadine concentrations in patients with Parkinson’s disease. Park. Relat. Disord. 2009, 15, 351–353. [Google Scholar] [CrossRef]
- Gupta, A.; Lang, A.E. Drug-induced cranial myoclonus. Mov. Disord. 2010, 25, 2264–2265. [Google Scholar] [CrossRef]
- Hardwick, A.; Devereaux, M.; Walter, B. A Case of Subacute Encephalopathy, Ataxia and Myoclonus Due to Amantadine Toxicity in Chronic Renal Insufficiency. Mov. Disord. 2010, 25, 493. [Google Scholar]
- Yarnall, A.J.; Burn, D.J. Amantadine-induced myoclonus in a patient with progressive supranuclear palsy. Age Ageing 2012, 41, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Arii, Y.; Inui, T.; Mitsui, T. A case of progressive supranuclear palsy with cortical myoclonus. Tokushima 2013, 4, 62–63. [Google Scholar]
- Estraneo, A.; Pascarella, A.; Moretta, P.; Loreto, V.; Trojano, L. Clinical and electroencephalographic on-off effect of amantadine in chronic non-traumatic minimally conscious state. J. Neurol. 2015, 262, 1584–1586. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Bloem, B.R.; Warrenburg, B.P. The clinical heterogeneity of drug-induced myoclonus: An illustrated review. J. Neurol. 2017, 264, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, K.; Shigematsu, T.; Fujishima, I. Case Reports Describing Amantadine Intoxication in a Rehabilitation Hospital. Prog. Rehabil. Med. 2017, 2, 20170017. [Google Scholar] [CrossRef]
- Dames, B.; Karl, J.A.; Metman, L.V. High dose amantadine therapy may cause increased falling in patients with Parkinson’s disease: A case report. Clin. Park. Relat. Disord. 2020, 3, 100045. [Google Scholar] [CrossRef]
- Poon, L.H.; Lee, A.J.; Vuong, M.; Zuzuarregui, J.R. Amantadine Associated Myoclonus: Case Report and Review of the Literature. J. Pharm. Pract. 2021, 34, 814–817. [Google Scholar] [CrossRef]
- Raupp-Barcaro, I.F.M.; Dias, I.C.S.; Meyer, E.; Vieira, J.C.F.; Pereira, G.S.; Petkowicz, A.R.; Oliveira, R.M.W.; Andreatini, R. Involvement of dopamine D(2) and glutamate NMDA receptors in the antidepressant-like effect of amantadine in mice. Behav. Brain Res. 2021, 413, 113443. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, U.; Svensson, T.H. Further studies on the mode of action of amantadine. Acta Pharmacol. Toxicol. 1971, 30, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Romieu, P.; Maurice, T.; Su, T.P.; Maloteaux, J.M.; Hermans, E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci. 2004, 19, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Otton, H.J.; McLean, A.L.; Pannozzo, M.A.; Davies, C.H.; Wyllie, D.J.A. Quantification of the Mg2+-induced potency shift of amantadine and memantine voltage-dependent block in human recombinant GluN1/GluN2A NMDARs. Neuropharmacology 2011, 60, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Pałasz, A. Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study. Pharmaceuticals 2020, 13, 326. [Google Scholar] [CrossRef]
- Lemmer, B. Effects of amantadine and amphetamine on serotonin uptake and release by human blood platelets. Eur. J. Pharmacol. 1973, 21, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.P.; Placantonakis, D.G.; Warsetsky, S.I.; Marquez, R.G.; Bernstein, L.; Aicher, S.A. The serotonin hypothesis of myoclonus from the perspective of neuronal rhythmicity. Adv. Neurol. 2002, 89, 307–329. [Google Scholar] [PubMed]
- Rissardo, J.P.; Caprara, A.L.; Durante, Í.; Rauber, A. Lithium-associated movement disorder: A literature review. Brain Circ. 2022, 8, 76–86. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines 2023, 10, 33. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, I.S.; Lim, H.K.; Choi, H.S.; Oh, S.; Park, W.K.; Jang, C.G.; Kim, S.H.; Chang, M.J. N-Methyl-D-aspartate receptor antagonists enhance the head-twitch response, a 5-hydroxytryptamine2 receptor-mediated behaviour, in reserpine-treated mice. J. Pharm. Pharmacol. 2000, 52, 717–722. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Pena, A.B.; Caviness, J.N. Physiology-Based Treatment of Myoclonus. Neurotherapeutics 2020, 17, 1665–1680. [Google Scholar] [CrossRef]
- Horadam, V.W.; Sharp, J.G.; Smilack, J.D.; McAnalley, B.H.; Garriott, J.C.; Stephens, M.K.; Prati, R.C.; Brater, D.C. Pharmacokinetics of amantadine hydrochloride in subjects with normal and impaired renal function. Ann. Intern. Med. 1981, 94, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Minocha, A.; Spyker, D.A.; Hoffman, H.E. Comparative single-dose pharmacokinetics of amantadine hydrochloride and rimantadine hydrochloride in young and elderly adults. Antimicrob. Agents Chemother. 1985, 28, 216–221. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, P.J.; Ing, T.S.; Daugirdas, J.T.; Jeevanandhan, R.; Soung, L.S.; Galinis, S. In vitro binding of amantadine to plasma proteins. Clin. Neuropharmacol. 1984, 7, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Haass, A.; Jacobi, P.; Schimrigk, K. Amantadine sulphate in treating Parkinson’s disease: Clinical effects, psychometric tests and serum concentrations. J. Neurol. 1989, 236, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Köppel, C.; Tenczer, J. A revision of the metabolic disposition of amantadine. Biomed. Mass. Spectrom. 1985, 12, 499–501. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Puertas, I.; Toledo-Heras, M. Drug-induced myoclonus: Frequency, mechanisms and management. CNS Drugs 2004, 18, 93–104. [Google Scholar] [CrossRef]
- Degelau, J.; Somani, S.; Cooper, S.L.; Irvine, P.W. Occurrence of adverse effects and high amantadine concentrations with influenza prophylaxis in the nursing home. J. Am. Geriatr. Soc. 1990, 38, 428–432. [Google Scholar] [CrossRef]
- Factor, S.A.; Molho, E.S.; Brown, D.L. Acute delirium after withdrawal of amantadine in Parkinson’s disease. Neurology 1998, 50, 1456–1458. [Google Scholar] [CrossRef]
- Oertel, W.; Eggert, K.; Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Trenkwalder, C.; Ehret, R.; Azulay, J.P.; Isaacson, S.; Felt, L.; et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov. Disord. 2017, 32, 1701–1709. [Google Scholar] [CrossRef]
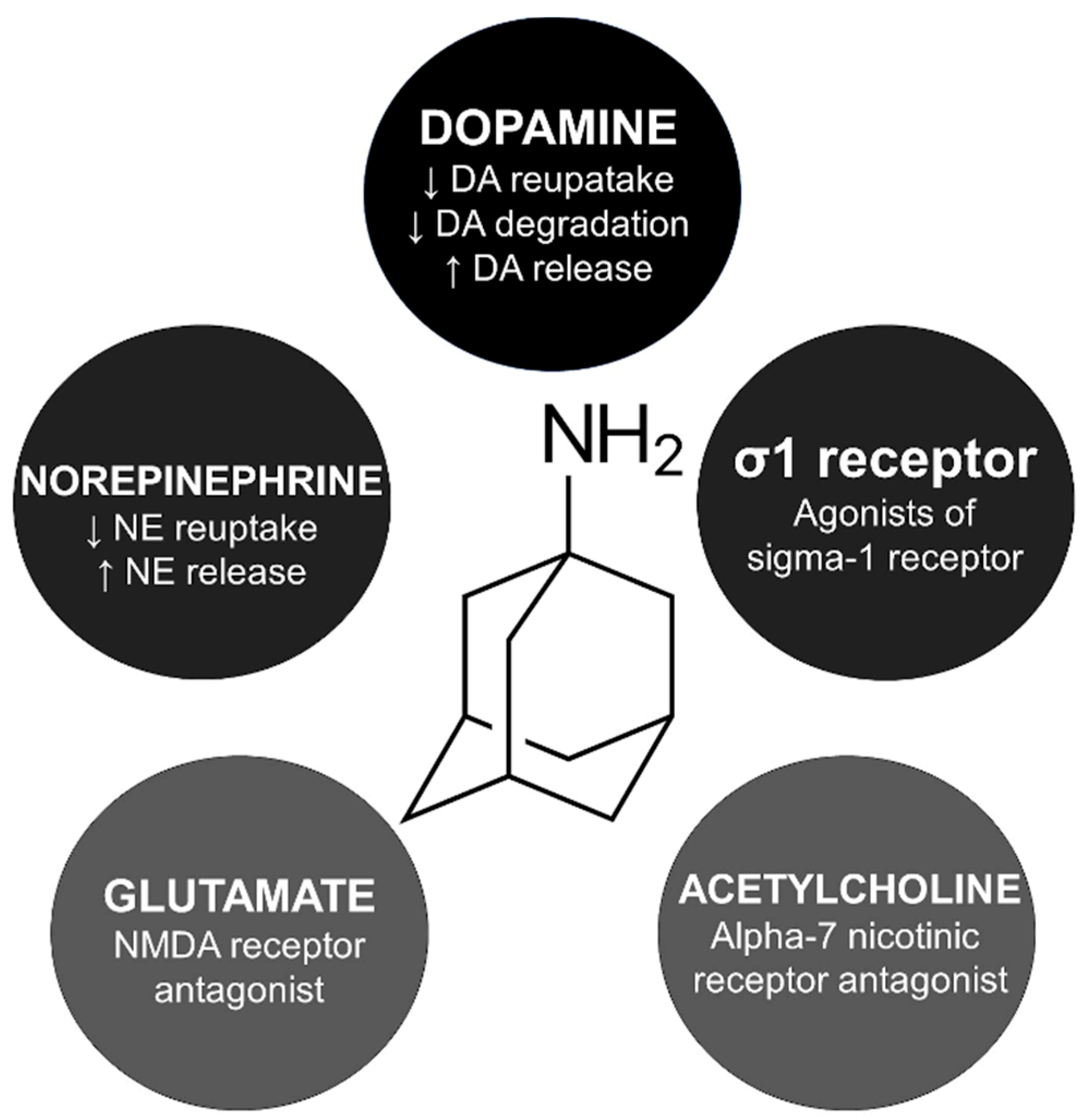
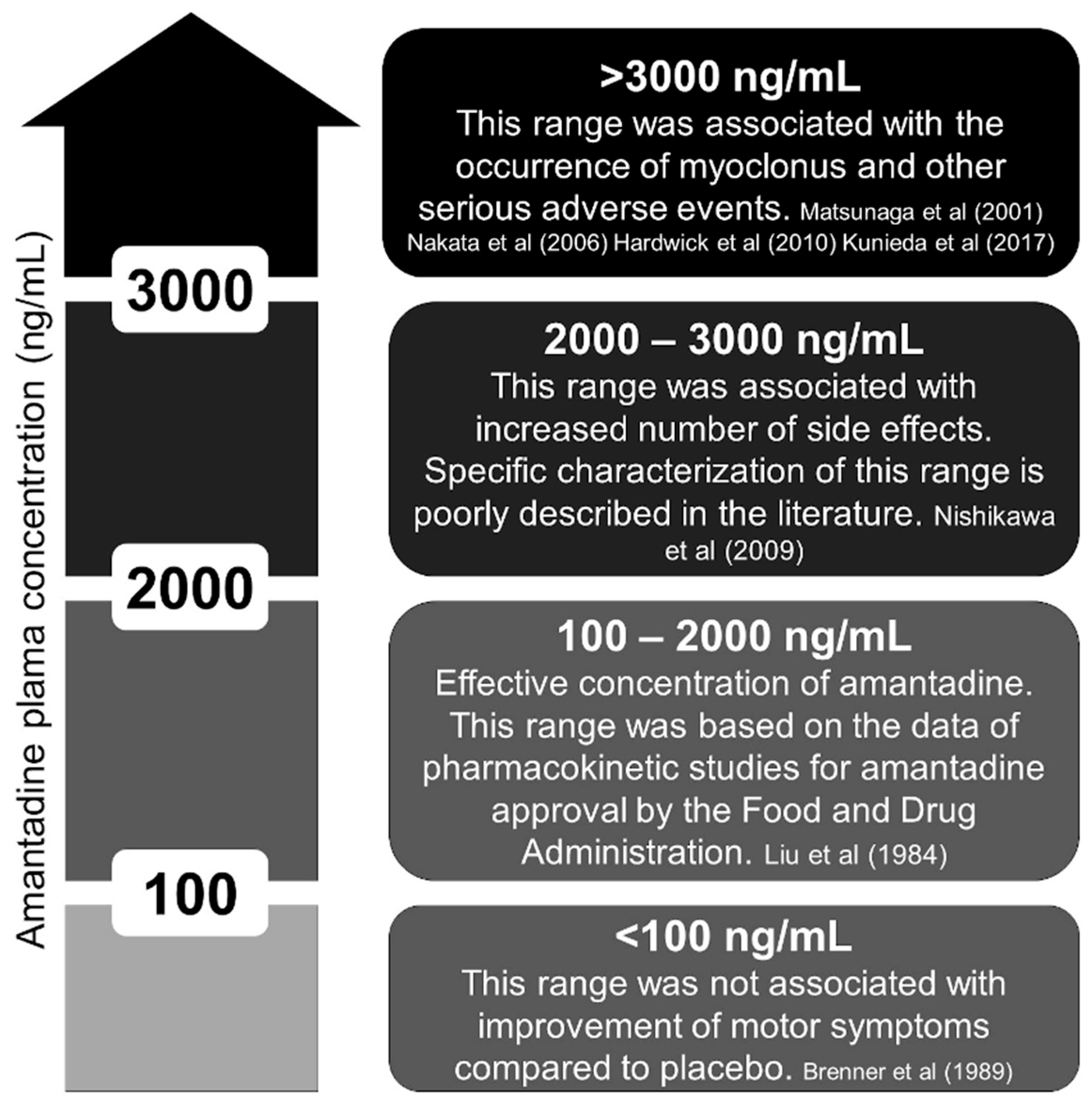
| Reference (Year) | Age/Sex | AMT Dosing (mg/Daily) and Indication | MCL Presentation | KF | MCL Onset a | Management | MCL Recovery b | EEG c | F/U | Considerations |
|---|---|---|---|---|---|---|---|---|---|---|
| Chevalier et al. (1980) [2] | 64, M | NA; PD | Generalized MCL. | N | NA | AMT withdrawal | NA | NA | NA | First report of AMT-induced MCL. Diuretics increased the intoxication by AMT. |
| Pfeiffer et al. (1996) [4] | 64, F | 200; PD | Focal (cranial) MCL. | N | NA | AMT withdrawal. Clonazepam was attempted. | NA | NA | NA | First report of vocal (cranial) MCL. Misdiagnosed with stuttering. Videotape. |
| Matsunaga et al. (2001) [5] | 87, F | 100; NA | Generalized (multifocal) MCL. Cortical MCL. | Y | 30 days | AMT withdrawal | 14 days | Abnormal | CR | Plasma AMT concentration. |
| 78, F | 200; PD | Generalized (multifocal) MCL. Cortical MCL. | N | 90 days | AMT withdrawal | 8 days | Abnormal | CR | Dose-dependent MCL. AMT-dose increase was associated with a rise in MCL frequency. | |
| 79, F | 200; PD | Generalized (multifocal) MCL. Cortical MCL. | Y | 9 days after worsening of renal function | AMT withdrawal | 7 days | Abnormal | Plasma AMT concentration. MCL appeared with worsening renal function. | ||
| Nakata et al. (2006) [6] | 70, F | 150; PD | Generalized MCL. | Y | NA (y) | AMT withdrawal | 21 days | Normal | CR | Plasma AMT concentration. |
| 74, F | 200; Depression | Generalized MCL | N | NA (y) | AMT withdrawal | 21 days | Normal | CR | Possible serotonin syndrome. | |
| 73, F | 300; PD | Generalized MCL. | Y | 7 days | AMT withdrawal | NA | Abnormal | No | Possible serotonin syndrome. | |
| Cheng et al. (2008) [7] | 78, M | 100; PD | Generalized MCL | Y | 3 days | AMT withdrawal | 12 days | Abnormal | CR | Serotonin syndrome. |
| Hong et al. (2008) [8] | 59, F | 200; PD | Generalized MCL | Y | 11 days | AMT withdrawal | NA | NA | NA | Possible interaction with pramipexole. |
| Nishikawa et al. (2009) [9] | 62, F | 200; PD | Generalized MCL | Y | NA | AMT withdrawal | NA | NA | CR | Plasma AMT concentration. |
| 55, F | 150; PD | Generalized MCL | Y | NA | AMT withdrawal | NA | NA | CR | Plasma AMT concentration. | |
| Gupta et al. (2010) [10] | 63, M | 300; parkinsonism with postural instability | Focal (cranial) MCL. Resting and action MCL of lower face. | N | NA (several months) | AMT withdrawal | NA | NA | CR | Videotape. Misdiagnosed as stuttering. |
| Hardwick et al. (2010) [11] | 63, M | NA; pruritus | Generalized MCL | Y | NA | AMT withdrawal | 56 days | Normal | CR | Plasma AMT concentration. |
| Yarnall et al. (2012) [12] | 74, M | 200; PSP | Generalized MCL | N | 26 days | AMT withdrawal | 5 days | NA | CR | PSP diagnosis supported by abnormal DaTSCAN. |
| Kawamura et al. (2013) [13] | 58, M | NA; PSP | Generalized MCL | N | NA | Clonazepam was attempted | NA | NA | NA | Giant potential was found in somatosensory evoked potential of the median nerve. |
| Estraneo et al. (2015) [14] | 57, F | 200; coma state | Focal (cranial) MCL | N | 21 days | AMT withdrawal | 21 days | Abnormal | NA | Three attempts of AMT rechallenge. |
| Janssen et al. (2017) [15] | 66, M | 300; PD with Levodopa-induced dyskinesias | Generalized MCL | N | 30 days | AMT withdrawal | 14 days | NA | CR | Videotape. |
| Kunieda et al. (2017) [16] | 83, M | 150; PD | Generalized MCL. | Y | 5 days | AMT withdrawal | 29 days | NA | CR | Plasma AMT concentration |
| 53, M | 100; spontaneity | Generalized MCL. | Y | 21 days | AMT withdrawal. AMT rechallenge. | NA | NA | CR | AMT rechallenge without symptoms occurrence. | |
| Dames et al. (2020) [17] | 55, M | 400; PD | Generalized MCL. | Y | NA (y) | AMT withdrawal | 7 days | NA | No | Videotape. |
| Poon et al. (2021) [18] | 80, M | PD with Levodopa-induced dyskinesias | Generalized MCL. Asterixis. | N | 9 days | AMT withdrawal | 3 days | NA | CR | Subcortical MCL. |
| Present report | 64, M | PD | Generalized MCL. Asterixis. | N | 7 days | AMT withdrawal | 3 days | Normal | CR | Subcortical MCL. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Fornari Caprara, A.L. Myoclonus Secondary to Amantadine: Case Report and Literature Review. Clin. Pract. 2023, 13, 830-837. https://doi.org/10.3390/clinpract13040075
Rissardo JP, Fornari Caprara AL. Myoclonus Secondary to Amantadine: Case Report and Literature Review. Clinics and Practice. 2023; 13(4):830-837. https://doi.org/10.3390/clinpract13040075
Chicago/Turabian StyleRissardo, Jamir Pitton, and Ana Letícia Fornari Caprara. 2023. "Myoclonus Secondary to Amantadine: Case Report and Literature Review" Clinics and Practice 13, no. 4: 830-837. https://doi.org/10.3390/clinpract13040075
APA StyleRissardo, J. P., & Fornari Caprara, A. L. (2023). Myoclonus Secondary to Amantadine: Case Report and Literature Review. Clinics and Practice, 13(4), 830-837. https://doi.org/10.3390/clinpract13040075






