Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
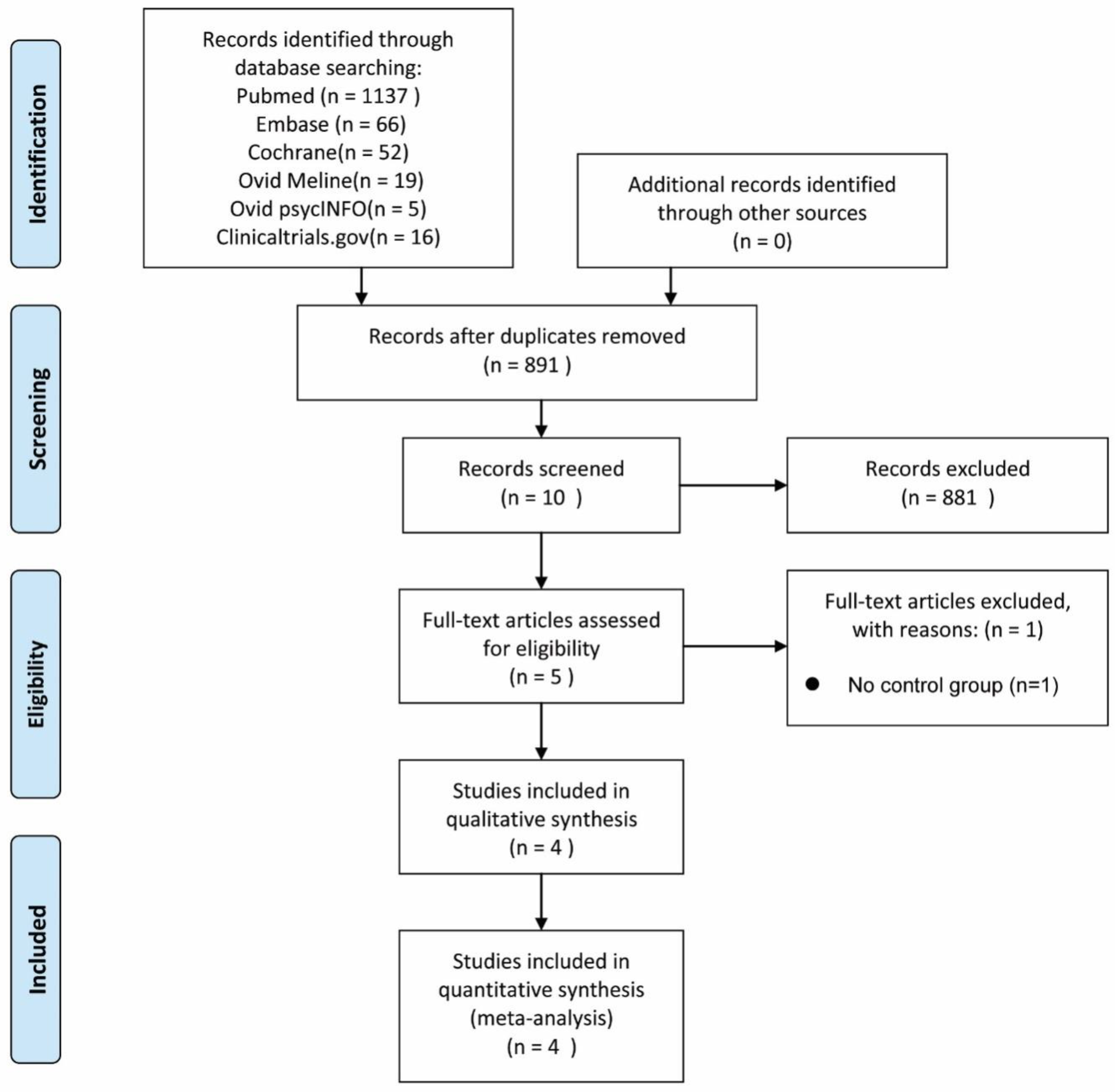
2.1. Study Selection
2.2. Eligibility Criteria
2.3. Data Collection Process
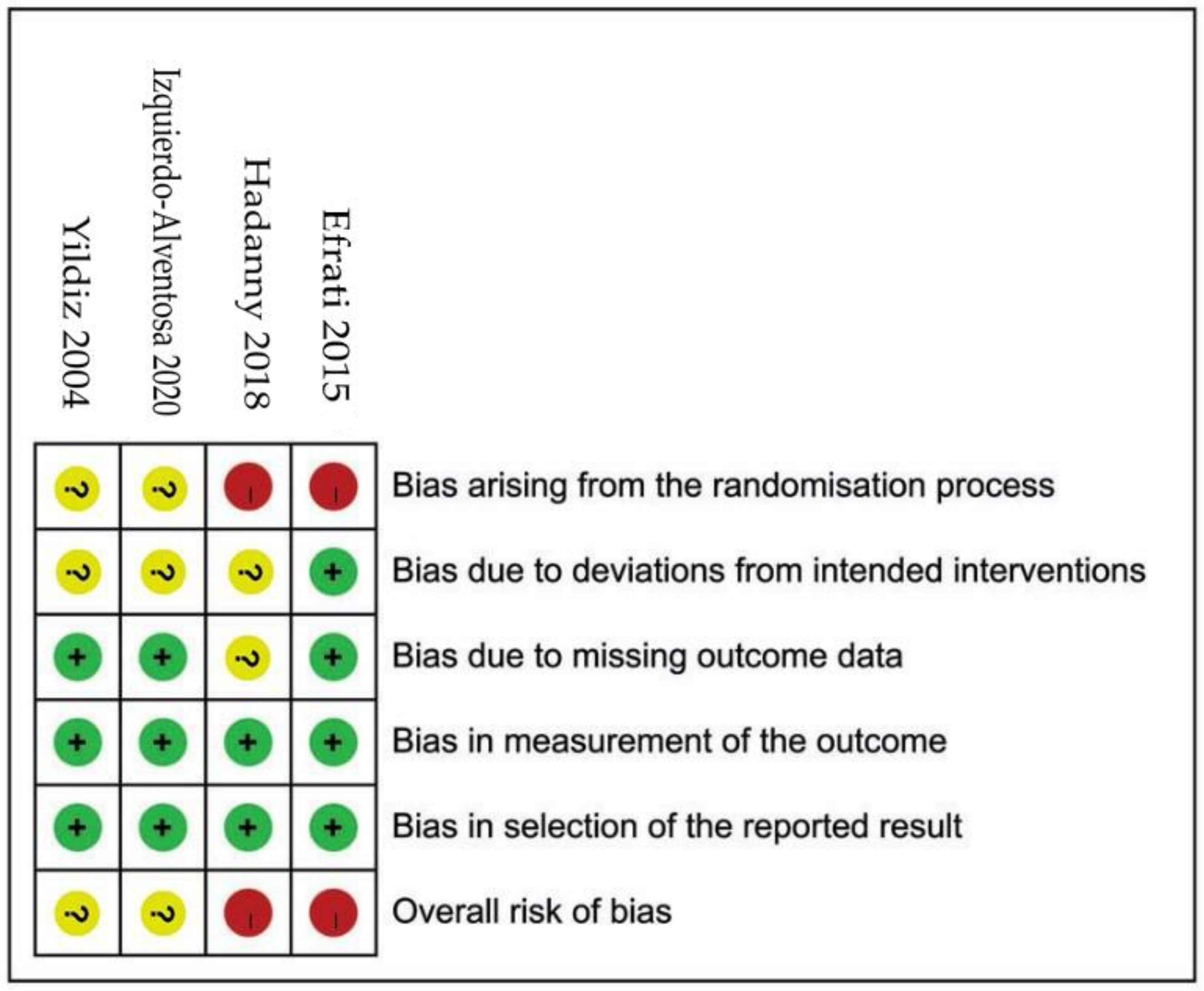
2.4. Rik of Bias (RoB) in Individual Studies
2.5. Assessment of Heterogeneity
2.6. Assessment of Treatment Effect
3. Results
3.1. Hallmarks of Included Studies
3.2. Participant Hallmarks
3.3. ROBs of Included Studies (Figure 2)
3.4. Effects of HBOT Interventions
3.5. Primary Outcome Measures
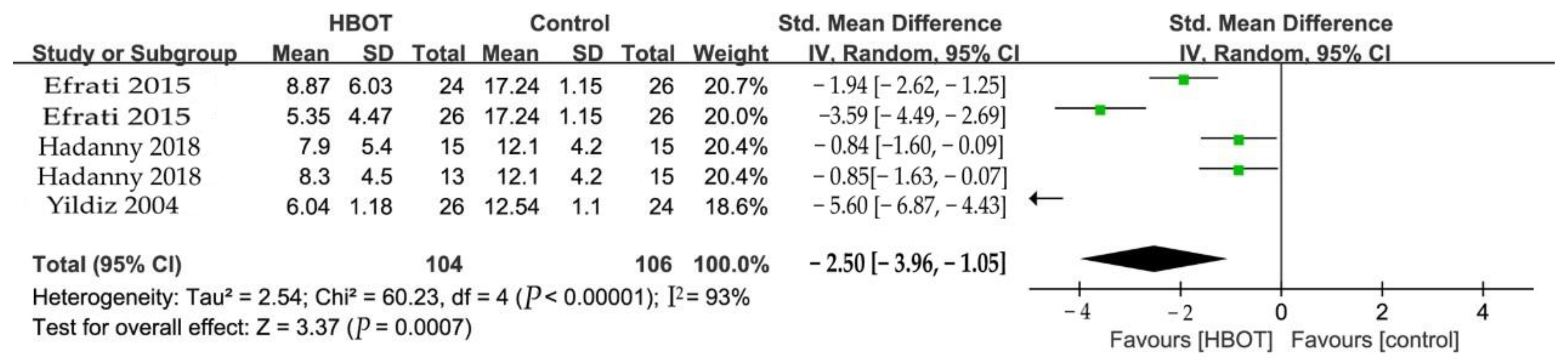
3.5.1. Pain
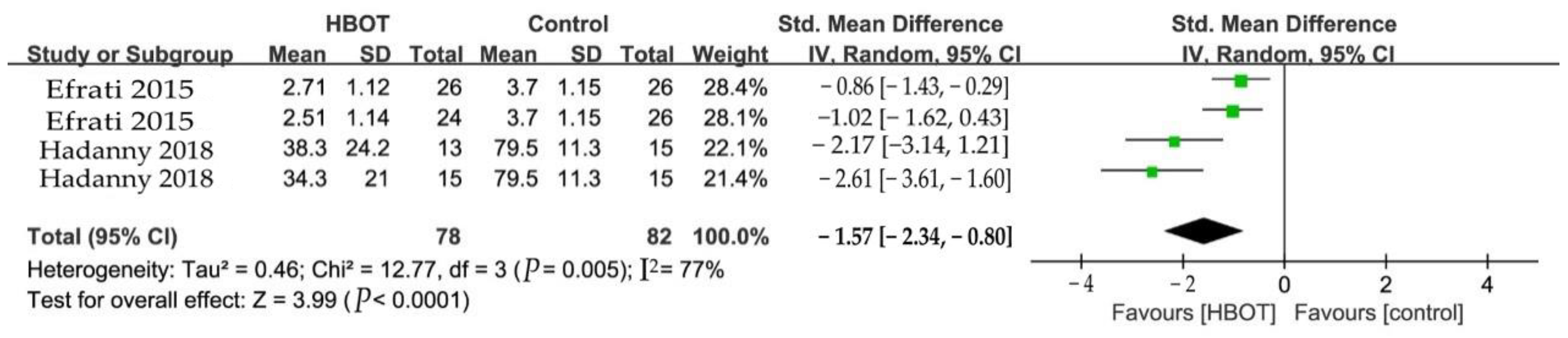
3.5.2. Fibromyalgia Impact Questionnaire (FIQ)
3.5.3. Tender Points Count (TPC)
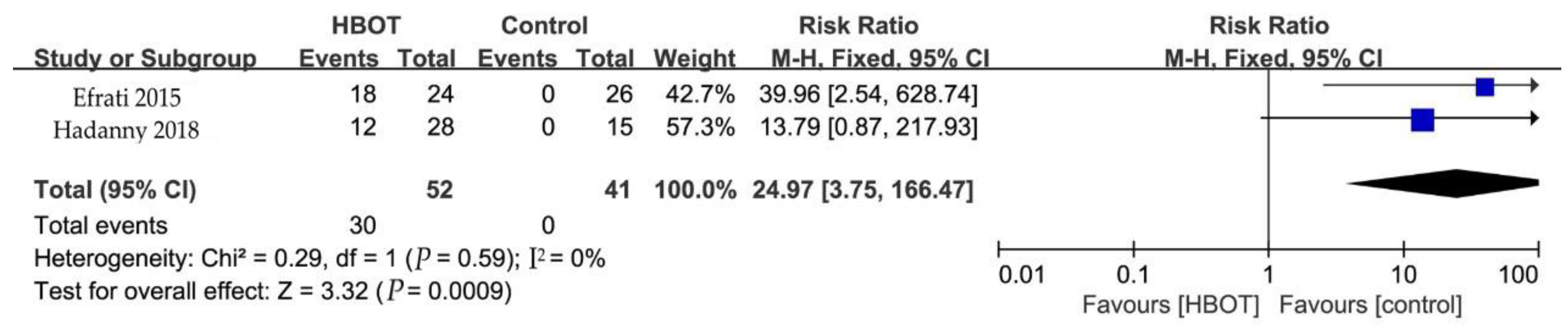
3.5.4. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaeth, M. Epidemiology, costs, and the economic burden of fibromyalgia. Arthritis Res. Ther. 2009, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Paes Leme, M.; Yuan, S.L.K.; Oliveira Magalhaes, M.; Ferreira de Meneses, S.R.; Marques, A.P. Pain and quality of life in knee osteoarthritis, chronic low back pain and fibromyalgia: A comparative cross-sectional study. Reumatismo 2019, 71, 68–74. [Google Scholar] [CrossRef]
- Wolfe, F.; Brahler, E.; Hinz, A.; Hauser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mease, P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. J. Rheumatol. Suppl. 2005, 75, 6–21. [Google Scholar] [PubMed]
- Marques, A.P.; Santo, A.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain. Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Hauser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Whipple, M.O.; McAllister, S.J.; Aleman, K.M.; St Sauver, J.L. A cross-sectional assessment of the prevalence of multiple chronic conditions and medication use in a sample of community-dwelling adults with fibromyalgia in Olmsted County, Minnesota. BMJ Open 2015, 5, e006681. [Google Scholar] [CrossRef]
- Gouvinhas, C.; Veiga, D.; Mendonca, L.; Sampaio, R.; Azevedo, L.F.; Castro-Lopes, J.M. Interventional Pain Management in Multidisciplinary Chronic Pain Clinics: A Prospective Multicenter Cohort Study with One-Year Follow-Up. Pain. Res. Treat. 2017, 2017, 8402413. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Edwards, M.L. Hyperbaric oxygen therapy. Part 2: Application in disease. J. Vet. Emerg. Crit. Care 2010, 20, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Kiralp, M.Z.; Akin, A.; Keskin, I.; Ay, H.; Dursun, H.; Cimsit, M. A new treatment modality for fibromyalgia syndrome: Hyperbaric oxygen therapy. J. Int. Med. Res. 2004, 32, 263–267. [Google Scholar] [CrossRef]
- Kiralp, M.Z.; Yildiz, S.; Vural, D.; Keskin, I.; Ay, H.; Dursun, H. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J. Int. Med. Res. 2004, 32, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Niu, J.Y.; Liu, W.T.; Sun, Y.Y.; Liu, S.; Lv, Y.; Dong, H.L.; Song, X.J.; Xiong, L.Z. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur. J. Pain 2012, 16, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Uzun, G.; Kiralp, M.Z. Hyperbaric oxygen therapy in chronic pain management. Curr. Pain Headache Rep. 2006, 10, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.H.; French, C.; Schnabel, A.; Wasiak, J.; Kranke, P. Normobaric and hyperbaric oxygen therapy for migraine and cluster headache. Cochrane Database Syst. Rev. 2008, 16, 1–33. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, Z.J.; Zhang, X.; Shen, Y.; Gao, Y.J. Repetitive hyperbaric oxygen treatment attenuates complete Freund’s adjuvant-induced pain and reduces glia-mediated neuroinflammation in the spinal cord. J. Pain 2013, 14, 747–758. [Google Scholar] [CrossRef]
- Efrati, S.; Fishlev, G.; Bechor, Y.; Volkov, O.; Bergan, J.; Kliakhandler, K.; Kamiager, I.; Gal, N.; Friedman, M.; Ben-Jacob, E.; et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients--randomized, prospective trial. PLoS ONE 2013, 8, e53716. [Google Scholar] [CrossRef]
- Efrati, S.; Golan, H.; Bechor, Y.; Faran, Y.; Daphna-Tekoah, S.; Sekler, G.; Fishlev, G.; Ablin, J.N.; Bergan, J.; Volkov, O.; et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome--prospective clinical trial. PLoS ONE 2015, 10, e0127012. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Altman, D.G. Chapter 9. Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Atzeni, F.; Casale, R.; Alciati, A.; Masala, I.F.; Batticciotto, A.; Talotta, R.; Gerardi, M.C.; Salaffi, F.; Sarzi-Puttini, P. Hyperbaric oxygen treatment of fibromyalgia: A prospective observational clinical study. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 63–69. [Google Scholar]
- Hadanny, A.; Bechor, Y.; Catalogna, M.; Daphna-Tekoah, S.; Sigal, T.; Cohenpour, M.; Lev-Wiesel, R.; Efrati, S. Hyperbaric Oxygen Therapy Can Induce Neuroplasticity and Significant Clinical Improvement in Patients Suffering From Fibromyalgia with a History of Childhood Sexual Abuse-Randomized Controlled Trial. Front. Psychol. 2018, 9, 2495. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Alventosa, R.; Ingles, M.; Cortes-Amador, S.; Gimeno-Mallench, L.; Sempere-Rubio, N.; Chirivella, J.; Serra-Ano, P. Comparative study of the effectiveness of a low-pressure hyperbaric oxygen treatment and physical exercise in women with fibromyalgia: Randomized clinical trial. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The fibromyalgia impact questionnaire: Development and validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar] [PubMed]
- Warren, J.; Sacksteder, M.R.; Thuning, C.A. Therapeutic effect of prolonged hyperbaric oxygen in adjuvant arthritis of the rat. Arthritis Rheum. 1979, 22, 334–339. [Google Scholar] [CrossRef]
- Davis, T.R.; Griffiths, I.D.; Stevens, J. Hyperbaric oxygen treatment for rheumatoid arthritis; failure to show worthwhile benefit. Br. J. Rheumatol. 1988, 27, 72. [Google Scholar] [CrossRef]
- Koo, S.T.; Lee, C.H.; Choi, H.; Shin, Y.I.; Ha, K.T.; Ye, H.; Shim, H.B. The effects of pressure on arthritic knees in a rat model of CFA-induced arthritis. Pain Physician 2013, 16, E95–E102. [Google Scholar]
- Zelinski, L.M.; Ohgami, Y.; Chung, E.; Shirachi, D.Y.; Quock, R.M. A prolonged nitric oxide-dependent, opioid-mediated antinociceptive effect of hyperbaric oxygen in mice. J. Pain 2009, 10, 167–172. [Google Scholar] [CrossRef]
- Ohgami, Y.; Zylstra, C.C.; Quock, L.P.; Chung, E.; Shirachi, D.Y.; Quock, R.M. Nitric oxide in hyperbaric oxygen-induced acute antinociception in mice. Neuroreport 2009, 20, 1325–1329. [Google Scholar] [CrossRef]
- Sumen, G.; Cimsit, M.; Eroglu, L. Hyperbaric oxygen treatment reduces carrageenan-induced acute inflammation in rats. Eur. J. Pharmacol. 2001, 431, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Zelinski, L.M.; Ohgami, Y.; Shirachi, D.Y.; Quock, R.M. Hyperbaric oxygen treatment induces a 2-phase antinociceptive response of unusually long duration in mice. J. Pain 2010, 11, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Lund, N.; Bengtsson, A.; Thorborg, P. Muscle tissue oxygen pressure in primary fibromyalgia. Scand. J. Rheumatol. 1986, 15, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Leach, R.M.; Rees, P.J.; Wilmshurst, P. Hyperbaric oxygen therapy. BMJ 1998, 317, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Applbaum, E.; Lichtbroun, A. Novel Sjogren’s autoantibodies found in fibromyalgia patients with sicca and/or xerostomia. Autoimmun. Rev. 2019, 18, 199–202. [Google Scholar] [CrossRef]
- Guggino, G.; Schinocca, C.; Lo Pizzo, M.; Di Liberto, D.; Garbo, D.; Raimondo, S.; Alessandro, R.; Brighina, F.; Ruscitti, P.; Giacomelli, R.; et al. T helper 1 response is correlated with widespread pain, fatigue, sleeping disorders and the quality of life in patients with fibromyalgia and is modulated by hyperbaric oxygen therapy. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 81–89. [Google Scholar]

| Database | Step | Search Algorithm | Items |
|---|---|---|---|
| PubMed | #1 | “Fibromyalgia” [Mesh Terms] | 8800 |
| #2 | “Musculoskeletal Disease” [ Mesh Terms] | 1849 | |
| #3 | “Chronic Pain Syndrome” [All Fields] | 705 | |
| #4 | “FMS“ [All Fields] | 9726 | |
| #5 | (((#1) OR (#2) OR (#3) OR #4) | 1,139,600 | |
| #6 | “oxygen therapies, hyperbaric” [ Mesh Terms] | 12,098 | |
| #7 | “Hyperbaric Oxygenation” [All Fields] | 12,467 | |
| #8 | “Hyperbaric Oxygenations” [All Fields] | 12,468 | |
| #9 | “Hyperbaric Oxygen Therapies” [All Fields] | 12,661 | |
| #10 | “Hyperbaric Oxygen Therapy” [All Fields] | 14,330 | |
| #11 | “HBOT” [All Fields] | 907 | |
| #12 | (((((#6) OR #7) OR #8) OR #9)OR #10) OR #11) | 619 | |
| #13 | #5 AND #12 | 1137 | |
| Embase | #1 | ‘fibromyalgia’/exp | 21,930 |
| #2 | ‘musculoskeletal disease’ | 38,748 | |
| #3 | ‘chronic pain syndrome’ | 1223 | |
| #4 | ‘FMS’ | 13,022 | |
| #5 | #1 OR #2 OR #3 OR #4 | 72,195 | |
| #6 | ‘Hyperbaric Oxygen Therapy’ | 19,726 | |
| #7 | ‘HBOT’ | 1220 | |
| #8 | #6 OR #7 | 19,743 | |
| #9 | #5 AND #8 | 66 | |
| Cochrane | #1 | MeSH descriptor: “Fibromyalgia” explode all trees | 1429 |
| #2 | MeSH descriptor: “Musculoskeletal Diseases” explode all trees | 42,086 | |
| #3 | Chronic Pain Syndrome: ti, ab, kw (Word variations have been searched) | 303 | |
| #4 | FMS: ti, ab, kw (Word variations have been searched) | 704 | |
| #5 | #1 or #2 or #3 or #4 | 45,416 | |
| #6 | MeSH descriptor: [Balneotherapy] explode all trees | 261 | |
| #7 | Spa therapy:ti,ab,kw (Word variations have been searched) | 122 | |
| #8 | Thermal water:ti,ab,kw (Word variations have been searched) | 79 | |
| #9 | Balneology:ti,ab,kw (Word variations have been searched) | 211 | |
| #10 | BT:ti,ab,kw (Word variations have been searched) | 1373 | |
| #11 | #6 or #7 or #8 or #9 or #10 | 1848 | |
| #12 | (#5 and #11) restricted as clinical trials | 52 | |
| Ovid Medline | #1 | Fibromyalgia. mp. | 10,642 |
| #2 | Musculoskeletal disease. mp. | 896 | |
| #3 | Chronic Pain/ | 16,300 | |
| #4 | #1 or #2 or #3 | 27,075 | |
| #5 | oxygen therapies, hyperbaric. mp. | 0 | |
| #6 | Hyperbaric Oxygenation/ | 12,083 | |
| #7 | Hyperbaric Oxygen Therapy. mp. | 3382 | |
| #8 | #5 or #6 or #7 | 12,614 | |
| #9 | #4 and #8 | 19 | |
| Ovid PsycINFO | #1 | Fibromyalgia. mp. | 3596 |
| #2 | exp Musculoskeletal Disorders/ | 18,206 | |
| #3 | exp Chronic Pain/ | 13,747 | |
| #4 | #1 or #2 or #3 | 31,384 | |
| #5 | Hyperbaric Oxygen Therapy. mp. | 122 | |
| #6 | exp Oxygenation/ | 962 | |
| #7 | #5 or #6 | 1082 | |
| #8 | #4 and #7 | 5 | |
| Clinicaltrials.gov | #1 | Fibromyalgia | 1088 |
| #2 | Musculoskeletal Diseases | 18,733 | |
| #3 | Diseases | 273,674 | |
| #4 | Musculoskeletal | 19,065 | |
| #5 | Chronic Pain Syndrome | 45 | |
| #6 | Pain Syndrome | 1612 | |
| #7 | Chronic Pain | 2003 | |
| #8 | Syndrome | 28,720 | |
| #9 | Chronic | 25,425 | |
| #10 | Pain | 21,325 | |
| #11 | FMS | 1167 | |
| #12 | Hyperbaric Oxygenation | 171 | |
| #13 | Oxygenation | 1136 | |
| #14 | Hyperbaric | 410 | |
| #15 | Hyperbaric Oxygen Therapy | 171 | |
| #16 | Oxygen Therapy | 3596 | |
| #17 | Hyperbaric Oxygen | 208 | |
| #18 | Therapy | 140,000 | |
| #19 | Oxygen | 3833 | |
| #20 | HBOT | 91 | |
| #21 | Musculoskeletal Diseases OR Fibromyalgia OR Chronic Pain Syndrome OR FMS|Hyperbaric Oxygenation OR Hyperbaric Oxygen Therapy OR HBOT | 16 |
| Studies | Country | Mean Age (Y) | Female/ Male | Sample Size (n) | Duration (Y) | Intervention | Outcomes | Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBOT | con | HBOT | con | HBOT | con | HBOT | con | HBOT | con | ||||
| Efrati 2015 [19] | Israel | 50.4 ± 10.9 | 48.1 ± 11.1 | NA | NA | 24 | 26 | 6.75 ± 5.9 | 6.2 ± 5.1 | hyperbaric oxygen therapy at 2.0 ATA. 40 daily sessions, 5 days/week, 90 min each. | no treatment | TPC (of 18),Pain threshold, FIQ, SF-36 | 2M |
| Hadanny 2018 [24] | Israel | 48.3 ± 10.6 | 43.1 ± 10.6 | 30 | 0 | 15 | 15 | NA | NA | hyperbaric oxygen therapy at 2.0 ATA and psychological therapy. 60 daily sessions, 5 days a week. 90 min each. | psychological therapy | WPI, TPC (of 18), FIQ, SF-36 | 3M |
| Yildiz 2004 [12] | Turkey | 40.46 ± 4.79 | 39.88 ± 4.71 | 35 | 15 | 26 | 24 | 4 ± 1.1 | hyperbaric oxygen therapy at 2.4 ATA. 15 sessions, one session per day for 5 days of the week, 90 min each. | breathed air at 1 ATA for 90 min | TPC, 6MWT, VAS, Pressure pain threshold | 3W | |
| Izquierdo-Alventosa 2020 [25] | Spain | NA | NA | 33 | 0 | 17 | 16 | NA | NA | hyperbaric oxygen therapy at 1.45 ATA. 40 sessions, five sessions per week, 90 min each. | usual medication | VAS, pressure pain threshold | 10W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Li, Q.; Zhang, X.; Varrassi, G.; Wang, H. Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials. Clin. Pract. 2023, 13, 583-595. https://doi.org/10.3390/clinpract13030053
Cao C, Li Q, Zhang X, Varrassi G, Wang H. Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials. Clinics and Practice. 2023; 13(3):583-595. https://doi.org/10.3390/clinpract13030053
Chicago/Turabian StyleCao, Chunfeng, Qianlu Li, Xinran Zhang, Giustino Varrassi, and Haiqiang Wang. 2023. "Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials" Clinics and Practice 13, no. 3: 583-595. https://doi.org/10.3390/clinpract13030053
APA StyleCao, C., Li, Q., Zhang, X., Varrassi, G., & Wang, H. (2023). Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials. Clinics and Practice, 13(3), 583-595. https://doi.org/10.3390/clinpract13030053







