Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
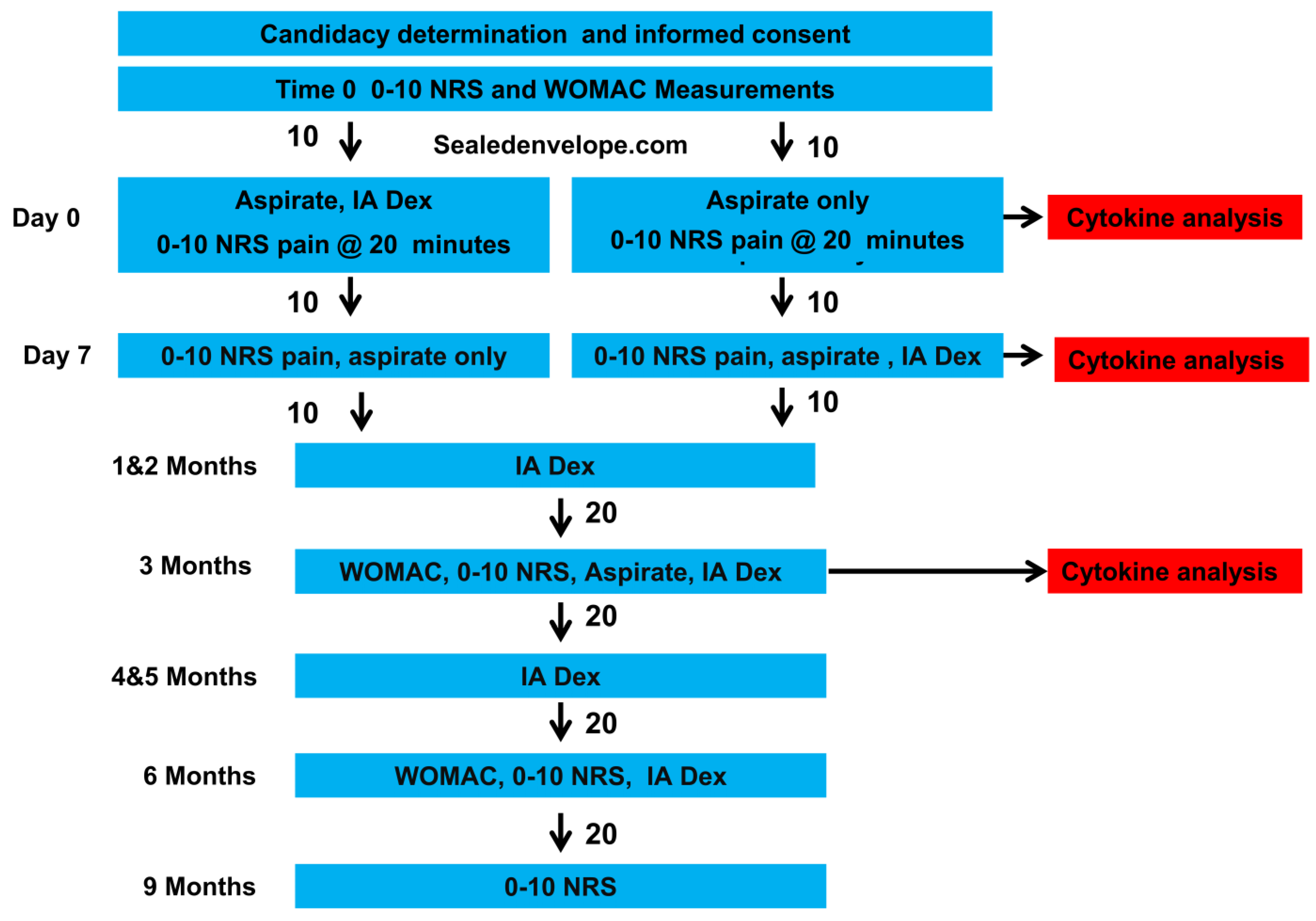
2.2. Screening, Group Assignment, Data Gathering, and Group Allocation
2.3. Synovial-Fluid Aspiration and Injection Method
2.4. Primary and Secondary Measures
2.5. ELISA and Total Protein Testing
2.6. Analysis
3. Results
3.1. Analgesic Effects of Dextrose
3.2. Effects of Dextrose Injection and Aspiration Only on Synovial-Fluid Cytokine Levels by 1 Week
3.3. Effect of Dextrose Injection on Cytokine Levels at 3 Months
3.4. Effect of Dextrose Injection on the WOMAC Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Kloppenburg, M.; Schett, G.; Bijlsma, J.W. Osteoarthritis research priorities: A report from a EULAR ad hoc expert committee. Ann. Rheum. Dis. 2014, 73, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.D.; Hassanein, K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Alt. Ther. Health Med. 2000, 6, 68–80. [Google Scholar]
- Mallik, A.K.; Chanu, K.P.; Mandal, H.; Singh, Y.N.; Das, R. Study to Compare the Effectiveness of 25% Dextrose Prolotherapy and Methylprednisolone in Primary Osteoarthritis of Knee in Terms of Pain and Improvement of Knee Function. Int. J. Sci. Res. 2018, 7, 74–75. Available online: https://www.researchgate.net/publication/340885410 (accessed on 1 November 2022).
- Sert, A.T.; Sen, E.I.; Esmaeilzadeh, S.; Ozcan, E. The effects of dextrose prolotherapy in symptomatic knee osteoarthritis: A randomized controlled study. J. Altern. Complement. Med. 2020, 26, 409–417. [Google Scholar] [CrossRef]
- Sit, R.W.S.; Wu, R.W.K.; Rabago, D.; Reeves, K.D.; Chan, D.C.C.; Yip, B.H.K.; Chung, V.C.H.; Wong, S.Y.S. Efficacy of intra-articular hypertonic dextrose (prolotherapy) for knee osteoarthritis: A randomized controlled trial. Ann. Fam. Med. 2020, 18, 235–242. [Google Scholar] [CrossRef]
- Rabago, D.; Patterson, J.J.; Mundt, M.; Kijowski, R.; Grettie, J.; Segal, N.A.; Zgierska, A. Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Ann. Fam. Med. 2013, 11, 229–237. [Google Scholar] [CrossRef]
- Dumais, R.; Benoit, C.; Dumais, A.; Babin, L.; Bordage, R.; de Arcos, C.; Allard, J.; Bélanger, M. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: A randomized crossover study. Pain Med. 2012, 13, 990–999. [Google Scholar] [CrossRef][Green Version]
- Maniquis-Smigel, L.; Reeves, K.D.; Rosen, H.J.; Lyftogt, J.; Graham-Coleman, C.; Cheng, A.L.; Rabago, D. Short term analgesic effects of 5% dextrose epidural injection for chronic low back pain. A randomized controlled trial. Anesth Pain Med. 2017, 7, e42550. [Google Scholar] [CrossRef]
- Lam, S.K.H.; Reeves, K.D.; Cheng, A.L. Transition from Deep Regional Blocks toward Deep Nerve Hydrodissection in the Upper Body and Torso: Method Description and Results from a Retrospective Chart Review of the Analgesic Effect of 5% Dextrose Water as the Primary Hydrodissection Injectate to Enhance Safety. BioMed Res. Int. 2017, 2017, 7920438. [Google Scholar]
- Lyftogt, J. Subcutaneous prolotherapy treatment of refractory knee, shoulder and lateral elbow pain. Australas. Musculoskelet. Med. 2007, 12, 110–112. [Google Scholar]
- Lyftogt, J. Prolotherapy for recalcitrant lumbago. Australas. Musculoskelet. Med. 2008, 13, 18–20. [Google Scholar]
- Lyftogt, J. Subcutaneous prolotherapy for Achilles tendinopathy: The best solution. Australas. Musculoskelet. Med. 2007, 12, 107–109. [Google Scholar]
- Lyftogt, J. Pain conundrums: Which hypothesis? Central nervous system sensitization versus peripheral nervous system autonomy. Australas. Musculoskelet. Med. 2008, 13, 72–74. [Google Scholar]
- Pinto, L.G.; Pinho-Ribeiro, F.A.; Verri, W.A., Jr. Editorial: Cytokines and Pain. Front. Immunol. 2021, 12, 788578. [Google Scholar] [CrossRef]
- Pan, P.J.; Wang, J.C.; Tsai, C.C.; Kuo, H.C. Identification of early response to hypertonic dextrose prolotherapy markers in knee osteoarthritis patients by an inflammation-related cytokine array. J. Chin. Med. Assoc. 2022, 85, 525–531. [Google Scholar] [CrossRef]
- Grimsholm, O.; Rantapaa-Dahlqvist, S.; Dalen, T.; Forsgren, S. Observations favouring the occurrence of local production and marked effects of bombesin/gastrin-releasing peptide in the synovial tissue of the human knee joint--comparisons with substance P and the NK-1 receptor. Neuropeptides 2008, 42, 133–145. [Google Scholar] [CrossRef]
- Aikawa, J.; Uchida, K.; Takano, S.; Inoue, G.; Minatani, A.; Miyagi, M.; Iwase, D.; Sekiguchi, H.; Mukai, M.; Takaso, M. Expression of calcitonin gene-related peptide in the infrapatellar fat pad in knee osteoarthritis patients. J. Orthop. Surg. Res. 2017, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Pan, H.; Peng, S.; Lv, M.; Lu, W.W. Levels of neuropeptide Y in synovial fluid relate to pain in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 319. [Google Scholar] [CrossRef]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, M.; Safaie, A.; Mirfazaelian, H.; Jalili, M. How much change in pain score does really matter to patients? Am. J. Emerg. Med. 2020, 38, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Aeschlimann, A.; Stucki, G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001, 45, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Maniquis-Smigel, L.; Reeves, K.D.; Rosen, J.H.; Coleman, C.; Lyftogt, J.; Cheng, A.L.; Rabago, D. Analgesic effect and potential cumulative benefit from caudal epidural D5W in consecutive participants with chronic low back and buttock/leg pain. J. Alt. Complement. Med. 2018, 12, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sasaki, M.; Sanai, K.; Kuwahata, H.; Sakurada, C.; Tsuzuki, M.; Iwata, Y.; Sakurada, S.; Sakurada, T. Intrathecal substance P augments morphine-induced antinociception: Possible relevance in the production of substance P N-terminal fragments. Peptides 2009, 30, 1689–1696. [Google Scholar] [CrossRef]
- Paschos, N.K.; Giotis, D.; Abuhemoud, K.; Georgoulis, A.D. Effectiveness of aspiration in knee joint effusion management: A prospective randomized controlled study. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 226–232. [Google Scholar] [CrossRef]
- Nakayama, T.; Naono, R.; Ikeda, T.; Nishimori, T. NMDA and AMPA receptors contribute to the maintenance of substance P-induced thermal hyperalgesia. Neurosci. Res. 2010, 67, 18–24. [Google Scholar] [CrossRef]
- Holden, J.E.; Pizzi, J.A.; Jeong, Y. An NK1 receptor antagonist microinjected into the periaqueductal gray blocks lateral hypothalamic-induced antinociception in rats. Neurosci. Lett. 2009, 453, 115–119. [Google Scholar] [CrossRef]
- Parenti, C.; Arico, G.; Ronsisvalle, G.; Scoto, G.M. Supraspinal injection of Substance P attenuates allodynia and hyperalgesia in a rat model of inflammatory pain. Peptides 2012, 34, 412–418. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, W.N.; Chen, C.J.; Lin, Y.W.; Zimmer, A.; Chen, C.C. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc. Natl. Acad. Sci. USA 2012, 109, E76–E83. [Google Scholar] [CrossRef]
- Chung, E.; Yoon, T.G.; Kim, S.; Kang, M.; Kim, H.J.; Son, Y. Intravenous Administration of Substance P Attenuates Mechanical Allodynia Following Nerve Injury by Regulating Neuropathic Pain-Related Factors. Biomol. Ther. 2017, 25, 259–265. [Google Scholar] [CrossRef]
- Kim, M.Y.; Na, Y.M.; Moon, J.H. Comparison on treatment effects of dextrose water, saline and lidocaine for trigger point injection. J. Korean Acad. Rehab Med. 1997, 21, 967–973. [Google Scholar]
- Louw, W.F.; Reeves, K.D.; Lam, S.K.H.; Cheng, A.L.; Rabago, D. Treatment of Temporomandibular Dysfunction With Hypertonic Dextrose Injection (Prolotherapy): A Randomized Controlled Trial With Long-term Partial Crossover. Mayo Clin. Proc. 2019, 94, 820–832. [Google Scholar] [CrossRef]
- Xiao, W.H.; Bennett, G.J. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain 2008, 135, 262–270. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef]
- MacIver, M.B.; Tanelian, D.L. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology 1992, 76, 617–623. [Google Scholar] [CrossRef]
- Hokfelt, T.; Brumovsky, P.; Shi, T.; Pedrazzini, T.; Villar, M. NPY and pain as seen from the histochemical side. Peptides 2007, 28, 365–372. [Google Scholar] [CrossRef]
- Naveilhan, P.; Hassani, H.; Lucas, G.; Blakeman, K.H.; Hao, J.X.; Xu, X.J.; Wiesenfeld-Hallin, Z.; Thorén, P.; Ernfors, P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 2001, 409, 513–517. [Google Scholar] [CrossRef]
- Topol, G.A.; Podesta, L.A.; Reeves, K.D.; Giraldo, M.M.; Johnson, L.J.; Grasso, R.; Jamín, A.; Clark, T.; Rabago, D. The chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. PMR 2016, 8, 1072–1082. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; Horton, W.A.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J. Bone Miner. Res. 2006, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chen, Y.P.; Lam, K.H.S.; Reeves, K.D.; Lin, J.A.; Kuo, C.Y. Mechanism of glucose water as a neural injection: A perspective on neuroinflammation. Life 2022, 12, 832. [Google Scholar] [CrossRef] [PubMed]
| Measures | Dextrose 1st (n = 10) | Aspiration 1st (n = 10) | p |
|---|---|---|---|
| Age (SD) | 65 (7) | 71 (10) | 0.13 |
| BMI (SD) | 31 (3) | 30 (3) | 0.30 |
| 0–10NRSPain (SD) | 7.5 (1.3) | 8.6 (1.4) | 0.09 |
| WOMAC (SD) | 55 (9) | 54 (25) | 0.92 |
| Measures and Comparisons | Dextrose 1st (n = 10) | Aspiration 1st (n = 10) | Combined (n = 20) |
|---|---|---|---|
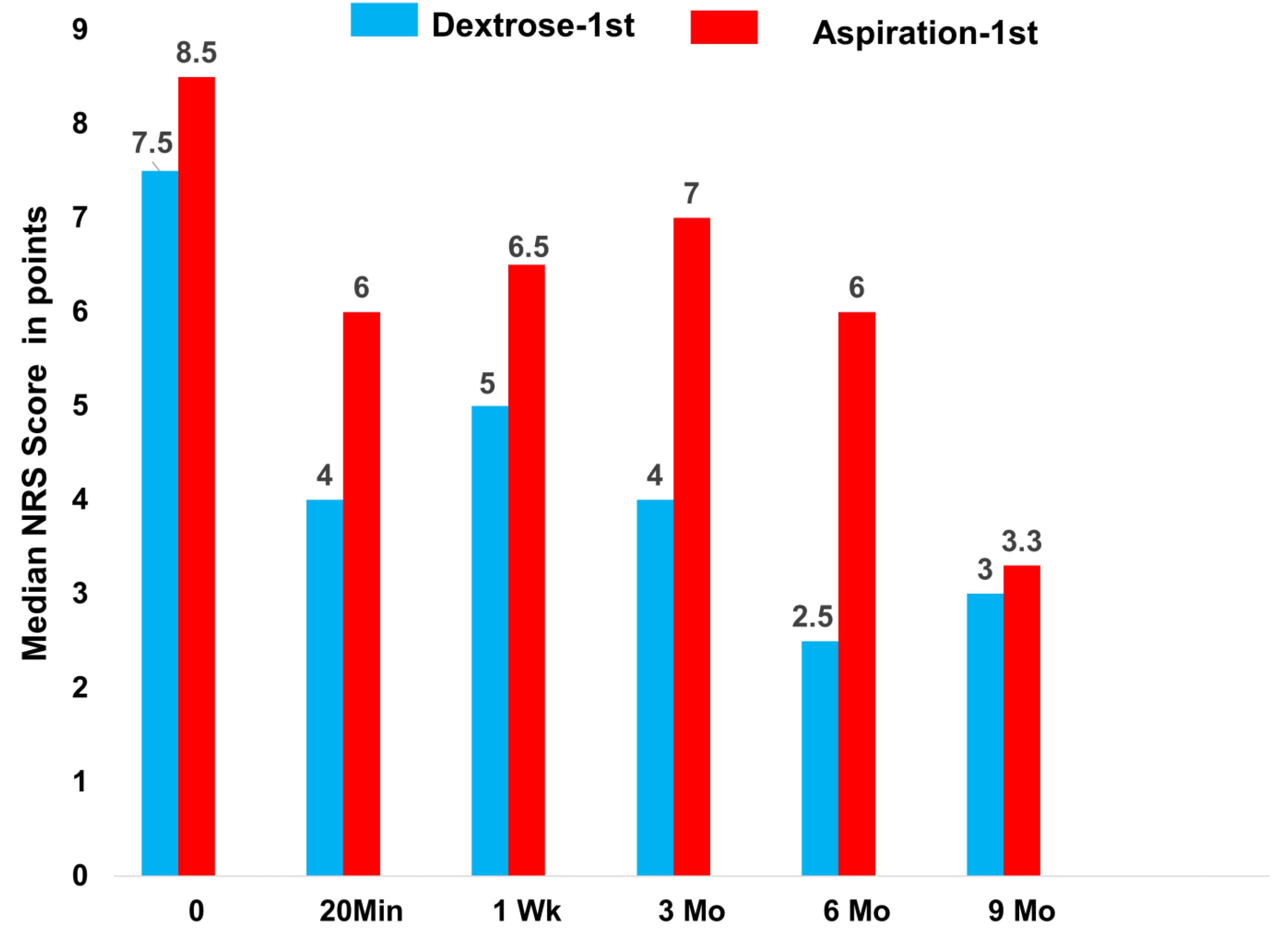
| NRS 0 ME (IQR) | 7.5 (3.0) | 8.5 (2.0) | 8.0 (2.0) |
| NRS 20 min ME (IQR) | 4.0 (4.0) | 6.0 (4.0) | |
| NRS 0–20 min within group (raw score) ME (IQR); p | 4.0 (3.5); <0.005 | 2.0 (5.3); 0.011 | |
| NRS 0–20 min between groups (raw score) OR; p | OR = 9.03 1; 0.05 1 | ||
| NRS 1wk ME (IQR) | 5.0 (4.0) | 6.5 (3.0) | |
| NRS 0–1wk within group ME (IQR); p | 2.0 (2.0); 0.005 | 1.0 (3.25); 0.041 | |
| NRS 0–1wk between groups OR; p | OR = 4.48 1; 0.14 1 | ||
| NRS 3 months ME (IQR) | 4.0 (5.0) | 7.0 (7.0) | 5.0 (6.0) |
| NRS 0–3 months within group ME (IQR); p | 2.5 (5.5); 0.005 | 2.5 (5.3); 0.018 | 2.5 (4.5); <0.001 |
| NRS 0–3 months between groups OR; p | OR = 2.36 1; 0.37 1 | ||
| NRS 6 months ME (IQR) | 2.5 (5.0) | 6.0 (4.0) | 4.5 (5.0) |
| NRS 0–6 months within group ME (IQR); p | 5.5 (4.8); 0.008 | 2.5 (3.5); 0.008 | 3.0 (4.0); <0.001 |
| NRS 0–6 months between groups OR; p | OR = 4.06 1; 0.19 1 | ||
| NRS 9 months ME (IQR) | 3.0 (6.0) | 5.5 (5.0) | 4.0 (5.0) |
| NRS 0–9 months within group ME (IQR); p | 4.5 (3.0); 0.007 | 3.0 ± 2.25; 0.005 | 3.5 (3.5); <0.001 |
| NRS 0–9 months between groups OR; p | OR = 2.03 1; 0.44 1 | ||
| Cytokine | Time | Parameters | Dextrose 1st, (n = 10) | Aspiration 1st (n = 10) | Between Groups |
|---|---|---|---|---|---|
| SP | 0 | Mean (SD) | 51 (50) | 40 (29) | |
| 1 Wk | Mean (SD) | 108 (124) | 56 (82) | ||
| 0 to 1 Wk | MD 1 (SE); p | +57 (28); p = 0.028 2 | +16 (22); p = 0.58 | 41 (35); p = 0.07 2 | |
| CGRP | 0 | Mean (SD) | 2.0 (0.9) | 81 (99) | |
| 1 Wk | Mean (SD) | 4.1 (3.5) | 94 (77) | ||
| 0 to 1 Wk | MD (SE); p | +2.1 (1.0); p = 0.14 | +13 (34); p = 0.29 | 10.9 (34); p = 0.36 | |
| NPY | 0 | Mean (SD) | 8.3 (5.2) | 6.6 (8.0) | |
| 1 Wk | Mean (SD) | 8.2 (3.7) | 4.5 (4.7) | ||
| 0 to 1 Wk | MD (SE); p | −0.1 (1.4); p = 0.80 | −2.1 (2.9); p = 0.45 | 2.0 (2.3); p = 0.71 | |
| MMP-3 | 0 | Mean (SD) | 652 (694) | 576 (609) | |
| 1 Wk | Mean (SD) | 589 (345) | 411 (339) | ||
| 0 to 1 Wk | MD (SE); p | −63 (214); p = 0.24 | −165 (131); p = 0.20 | 102 (251); p = 0.08 2 | |
| TIMP-1 | 0 | Mean (SD) | 171 (122) | 152 (60) | |
| 1 Wk | Mean (SD) | 191 (79) | 177 (95) | ||
| 0 to 1 Wk | MD (SE); p | +20 (42); p = 0.29 | +25 (34); p = 0.58 | 5 (54): p = 0.55 | |
| IL-6 | 0 | Mean (SD) | 20 (19) | 21 (24) | |
| 1 Wk | Mean (SD) | 26 (21) | 34 (51) | ||
| 0 to 1 Wk | MD (SE); p | +5.9 (7.5); p = 0.88 | +13 (10); p = 0.28 | 6.8 (12.5); p = 0.33 | |
| IGF | 0 | Mean (SD) | 5.4 (3.1) | 5.3 (4.0) | |
| 1 Wk | Mean (SD) | 5.0 (3.0) | 5.5 (3.0) | ||
| 0 to 1 Wk | MD (SE); p | −0.4 (0.8); p = 0.96 | +0.2 (0.8): p = 0.80 | 0.6 (1.2); p = 0.71 | |
| TGFβ | 0 | Mean (SD) | 91 (67) | 135 (102) | |
| 1 Wk | Mean (SD) | 105 (48) | 140 (73) | ||
| 0 to 1 Wk | MD (SE); p | +14 (22); p = 0.58 | +5 (37); p = 0.96 | 9 (44); p = 0.94 |
| Cytokine 1 | Time | Parameters | Glucose n = 20 |
|---|---|---|---|
| SP | 0 | Mean (SD) | 46 (40) |
| 3 Mo | Mean (SD) | 39 (46) | |
| 0 to 3 Mo | MD 1 (SE); p | −7 (13); p = 0.41 | |
| CGRP | 0 | Mean (SD) | 42 (79) |
| 3 Mo | Mean (SD) | 65 (121) | |
| 0 to 3 Mo | MD (SE); p | +23 (27); p = 0.30 | |
| NPY | 0 | Mean (SD) | 7.5 (6.6) |
| 3 Mo | Mean (SD) | 2.6 (4.2) | |
| 0 to 3 Mo | MD (SE); p | −4.9 (5.6); p < 0.0012 | |
| MMP-3 | 0 | Mean (SD) | 614 (637) |
| 3 Mo | Mean (SD | 395 (211) | |
| 0 to 3 Mo | MD (SE); p | −219 (129); p = 0.15 | |
| TIMP-1 | 0 | Mean (SD) | 161 (94) |
| 3 Mo | Mean (SD) | 178 (63) | |
| 0 to 3 Mo | MD (SE); p | +17 (28); p = 0.19 | |
| IL-6 | 0 | Mean (SD) | 21 (21) |
| 3 Mo | Mean (SD) | 34 (32) | |
| 0 to 3 Mo | MD (SE); p | +14 (7); p = 0.033 2 | |
| IGF | 0 | Mean (SD) | 5.4 (3.5) |
| 3 Mo | Mean (SD) | 8.4 (4.7) | |
| 0 to 3 Mo | MD (SE); p | +3.0 (0.9); p = 0.003 2 | |
| TGFβ | 0 | Mean (SD) | 113 (87) |
| 3 Mo | Mean (SD) | 98 (68) | |
| 0 to 3 Mo | MD (SE); p | −15 (22); p = 0.60 |
| Measures and Comparisons | Dextrose 1st (n = 10) | Aspiration 1st (n = 10) | Combined (n = 20) |
|---|---|---|---|
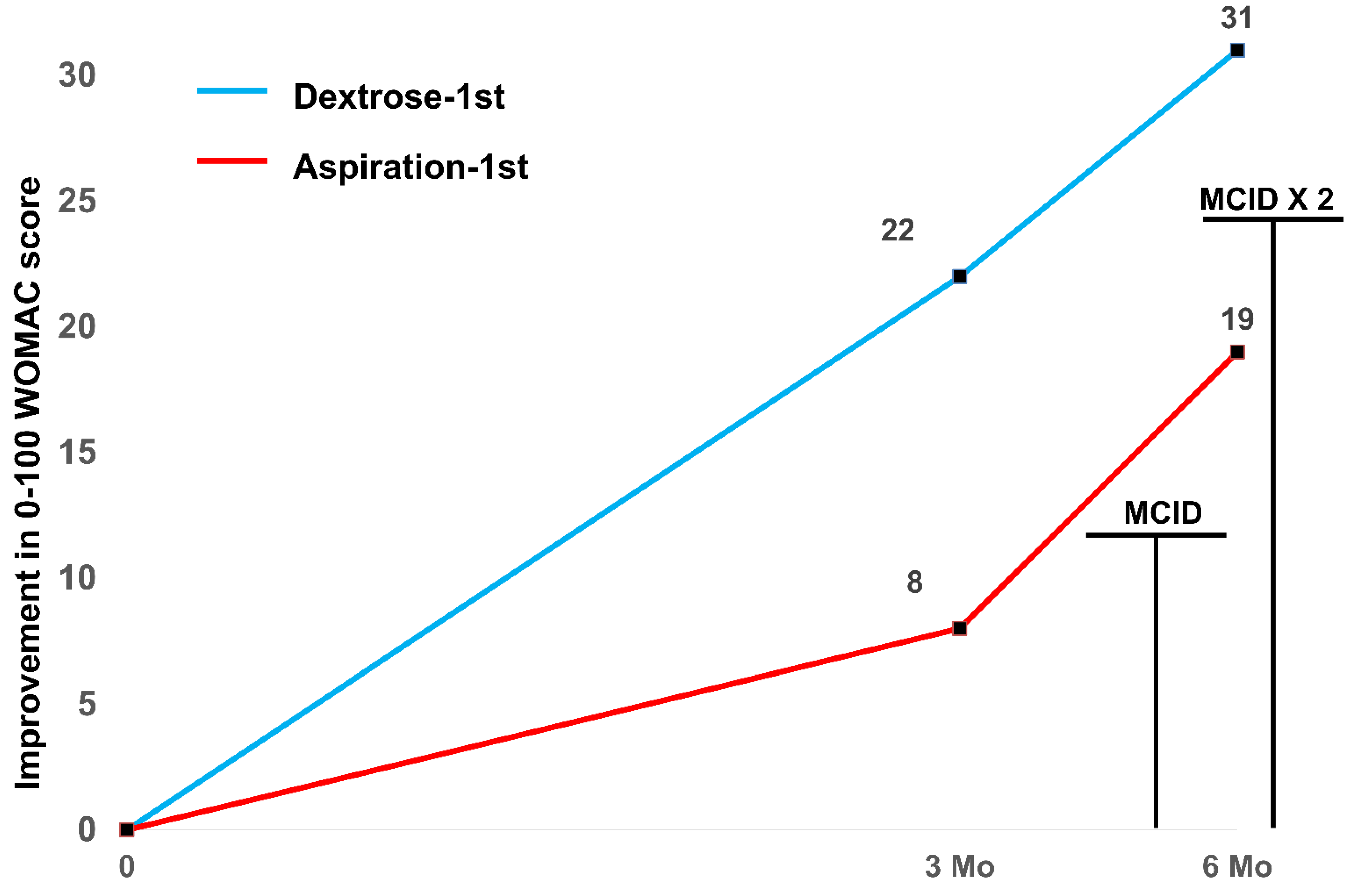
| WOMAC 0 months ± SD | 55 ± 9 | 54 ± 25 | 54 ± 18 |
| WOMAC 3 months ± SD | 33 ± 14 | 46 ± 27 | 40 ± 22 |
| WOMAC 0–3 months within group MD ± SE; p | 22 ± 6; 0.004 | 8 ± 4; 0.09 | 14 ± 3.7; 0.001 |
| WOMAC 0–3 months between groups MD ± SE; p | 17 ± 7 1; 0.031 1 | ||
| WOMAC 6 months ± SD | 23 ± 15 | 35 ± 25 | 29 ± 21 |
| WOMAC 0–6 months within group MD ± SE; p | 31 ± 4; <0.001 | 18 ± 4; 0.001 | 25 ± 3.1; <0.001 |
| WOMAC 0–6 months between groups MD ± SE; p | 11 ± 6 1; 0.09 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topol, G.A.; Pestalardo, I.G.; Reeves, K.D.; Elias, F.; Steinmetz, N.J.; Cheng, A.-L.; Rabago, D. Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations. Clin. Pract. 2022, 12, 926-938. https://doi.org/10.3390/clinpract12060097
Topol GA, Pestalardo IG, Reeves KD, Elias F, Steinmetz NJ, Cheng A-L, Rabago D. Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations. Clinics and Practice. 2022; 12(6):926-938. https://doi.org/10.3390/clinpract12060097
Chicago/Turabian StyleTopol, Gastón Andrés, Ines Guerrero Pestalardo, Kenneth Dean Reeves, Fernando Elias, Neven J. Steinmetz, An-Lin Cheng, and David Rabago. 2022. "Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations" Clinics and Practice 12, no. 6: 926-938. https://doi.org/10.3390/clinpract12060097
APA StyleTopol, G. A., Pestalardo, I. G., Reeves, K. D., Elias, F., Steinmetz, N. J., Cheng, A.-L., & Rabago, D. (2022). Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations. Clinics and Practice, 12(6), 926-938. https://doi.org/10.3390/clinpract12060097






