Glomangioma Supply from Profunda Femoris Artery in Peripheral Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
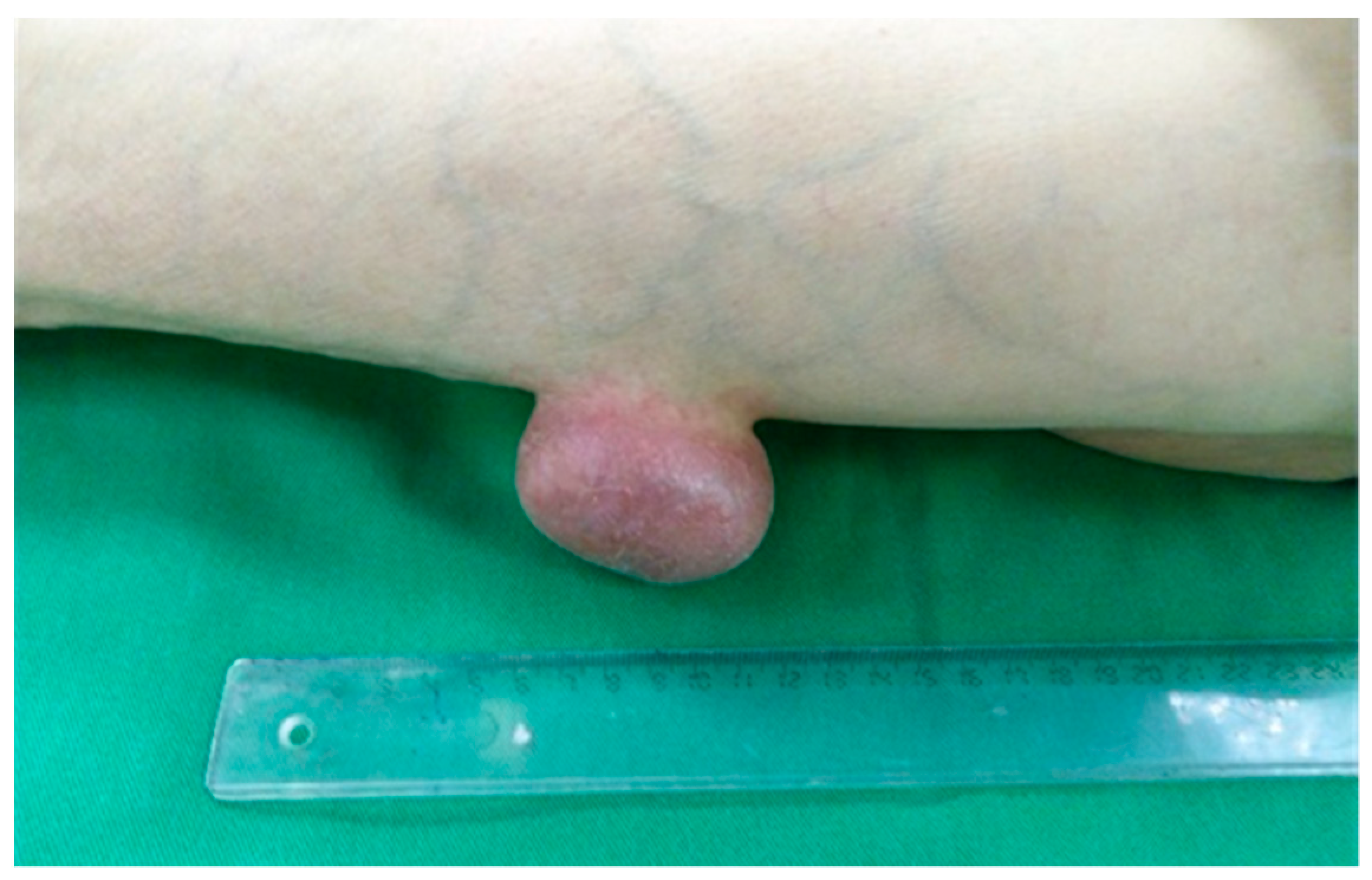
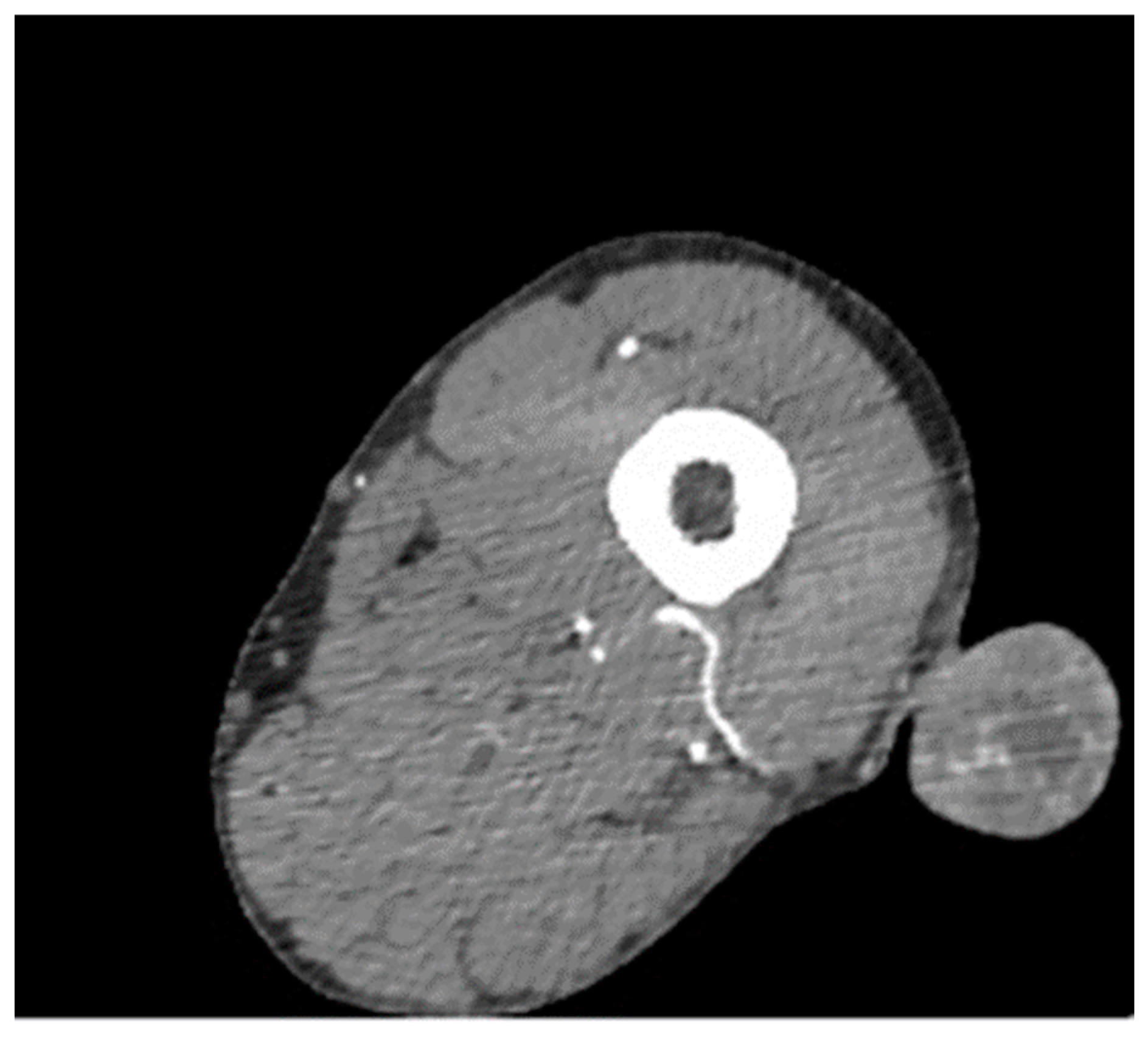
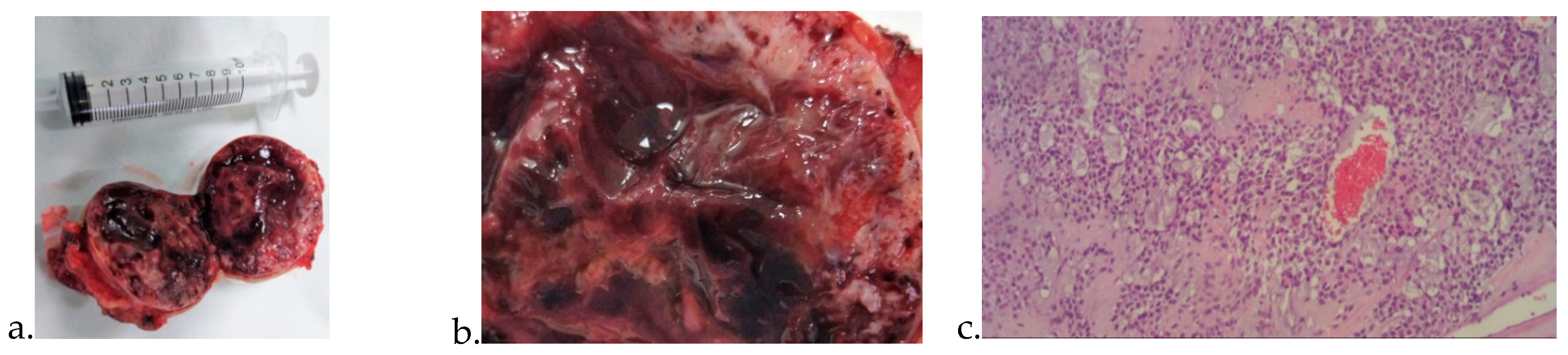
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDermott, E.M.; Weiss, A.C. Glomus tumors. J. Hand Surg. Am. 2006, 31, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Gombos, Z.; Zhang, P.J. Glomus tumor. Arch. Pathol. Lab. Med. 2008, 132, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Tokgöz, S.A.; Saylam, G.; Bayır, Ö.; Keseroğlu, K.; Toptaş, G.; Tatar, E.Ç.; Akın, İ.; Korkmaz, M.H. Glomus tumors of the head and neck: Thirteen years’ institutional experience and management. Acta Otolaryngol. 2019, 139, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Eady, J.L. Vascular tumors. Hand Clin. 2004, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Patel, U.; Mandal, R.; Walters, R.; Cook, K.; Haimovic, A.; Andrew, G.F., Jr. Glomangioma. Dermatol. Online J. 2010, 16, 11. [Google Scholar] [CrossRef]
- Folpe, A.L.; Fanburg-Smith, J.C.; Miettinen, M.; Weiss, S.W. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am. J. Surg. Pathol. 2001, 250, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cullen, R.D.; Hanna, E.Y. Intranasal glomangioma. Am. J. Otolaryngol. 2000, 21, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Kenn, W.; Klein, I.; Gassel, H.; Gattenloehner, S.; Gassel, A.M.; Hahn, D. Primary glomangioma of the liver: Imaging findings. Abdom. Imaging 2002, 27, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.; Santiago, F.; Tellechea, O. Multiple glomus tumors and segmental neurofibromatosis: There are no coincidences. Dermatol. Online J. 2011, 17, 4. [Google Scholar] [CrossRef]
- Sbai, M.A.; Benzarti, S.; Gharbi, W.; Khoffi, W.; Maalla, R. Glomus tumor of the leg: A case report. Pan Afr. Med. J. 2018, 31, 186. [Google Scholar] [CrossRef] [PubMed]
- Frith, R.; Alicia, Z.; Harket, A.; Ghfir, M.; Sedrati, O. Glomus tumors: Anatomoclinical study of 14 cases with literature review. Ann. Chir. Plast. Esthet. 2009, 54, 51–56. [Google Scholar]
- Robert, L.B.S., 3rd; Sangueza, O.P.; Schwartz, G.A. Glomus Tumor of the Toe. J. Am. Podiatr. Med. Assoc. 2017, 107, 257–260. [Google Scholar]
- Kapur, U.; Hobbs, C.M.; McDermott, E.; Mooney, E.E. Gastric glomus tumor. Ann. Diagn. Pathol. 2004, 8, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.H.; Bhattacharyya, I.; Cohen, D.M.; Hinze, S.R.; Islam, M.N. Glomus tumor: A comprehensive review of the clinical and histopathologic features with report of two intraoral cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nazerani, S.; Motamedi, M.H.K.; Keramati, M.R. Diagnosis and management of glomus tumors of the hand. Technol. Hand Up. Extrem. Surg. 2010, 14, 8–13. [Google Scholar] [CrossRef] [PubMed]
- CerronI, J.B.J.S.L. Dermatology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 22 October 2017; ISBN 9780702062759. [Google Scholar]
- Kumar, A.; Wilke, B.K.; Folpe, A.L.; Murray, P.M. Glomangiomatosis of the Upper Extremity. J. Hand Surg. Am. 2020, 6, 716.e1–716.e3. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Cormack, G.C.; Scerri, G. Hereditary multiple glomangiomas. Br. J. Plast. Surg. 1998, 51, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ardeleanu, V.; Jecan, C.R.; Tatu, A.L.; Motoc, A.G.M. A recurrent solitary glomus tumor of the forearm. Rom. J. Morphol. Embryol. 2019, 60, 1019–1023. [Google Scholar] [PubMed]
- Kumar, T.; Jamal, I.; Nigam, J.S.; Pandey, J.K. Malignant glomus tumor of the index finger. Autops. Case Rep. 2020, 10, e2020184. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Takami, Y.; Wada, Y.; Ryu, T.; Imamura, H.; Ureshino, H.; Fujiwara, M.; Saitsu, H. Glomus tumor of the duodenum: A rare case report. Surg. Case Rep. 2020, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Maselli, A.M.; Jambhekar, A.V.; Hunter, J.G. Glomangiosarcoma Arising from a Prior Biopsy Site. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1219. [Google Scholar] [CrossRef] [PubMed]
- Vikkula, M.; Boon, L.M.; Mulliken, J.B. Molecular genetics of vascular malformations. Matrix Biol. 2001, 20, 327–335. [Google Scholar] [CrossRef]
- Chen, P.G.; Nguyen, J.H.; Payne, S.C.; Sheehan, J.P.; Hashisaki, G.T. Treatment of glomus jugulare tumors with gamma knife radiosurgery. Laryngoscope 2010, 120, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.F.; Jones, N.F. Malignant Glomus Tumors of the Hand. Hand 2016, 11, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Gencosmanoglu, R.; Inceoglu, R.; Kurtkaya-Yapicier, O. Glomangioma of the hip. Dermatol. Surg. 2003, 29, 1244–1247. [Google Scholar] [PubMed]
- Wolter, N.E.; Adil, E.; Irace, A.L.; Werger, A.; Perez-Atayde, A.R.; Weldon, C.; Orbach, D.B.; Rodriguez-Galindo, C.; Rahbar, R. Malignant glomus tumors of the head and neck in children and adults: Evaluation and management. Laryngoscope 2017, 127, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N.G.A.; Nai, G.A.; Wedy, G.F.; de Abreu, M.A.M.M. Congenital plaque-like glomangioma: Report of two cases. An. Bras. Dermatol. 2017, 92, 43–46. [Google Scholar] [CrossRef]
- Schopp, J.G.; Sra, K.K.; Wilkerson, M.G. Glomangioma: A case report and review of the literature. Cutis 2009, 83, 24–27. [Google Scholar] [PubMed]
- Lee, C.H.; Trifiletti, D.M.; Sheehan, J.P. Radiosurgery for Glomus Tumors. Prog. Neurol. Surg. 2019, 34, 215–222. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.N.; Creteanu, M.; Olteanu, G.; Romila, A. Glomangioma Supply from Profunda Femoris Artery in Peripheral Artery Disease. Clin. Pract. 2022, 12, 755-759. https://doi.org/10.3390/clinpract12050078
Lungu CN, Creteanu M, Olteanu G, Romila A. Glomangioma Supply from Profunda Femoris Artery in Peripheral Artery Disease. Clinics and Practice. 2022; 12(5):755-759. https://doi.org/10.3390/clinpract12050078
Chicago/Turabian StyleLungu, Claudiu N., Mihai Creteanu, Gabriel Olteanu, and Aurelia Romila. 2022. "Glomangioma Supply from Profunda Femoris Artery in Peripheral Artery Disease" Clinics and Practice 12, no. 5: 755-759. https://doi.org/10.3390/clinpract12050078
APA StyleLungu, C. N., Creteanu, M., Olteanu, G., & Romila, A. (2022). Glomangioma Supply from Profunda Femoris Artery in Peripheral Artery Disease. Clinics and Practice, 12(5), 755-759. https://doi.org/10.3390/clinpract12050078






