Multiple Adenocarcinomas of the Small Bowel in a Patient with Brunner’s Glands Agenesia: A Previously Unreported Association
Abstract
1. Introduction
2. Case Report
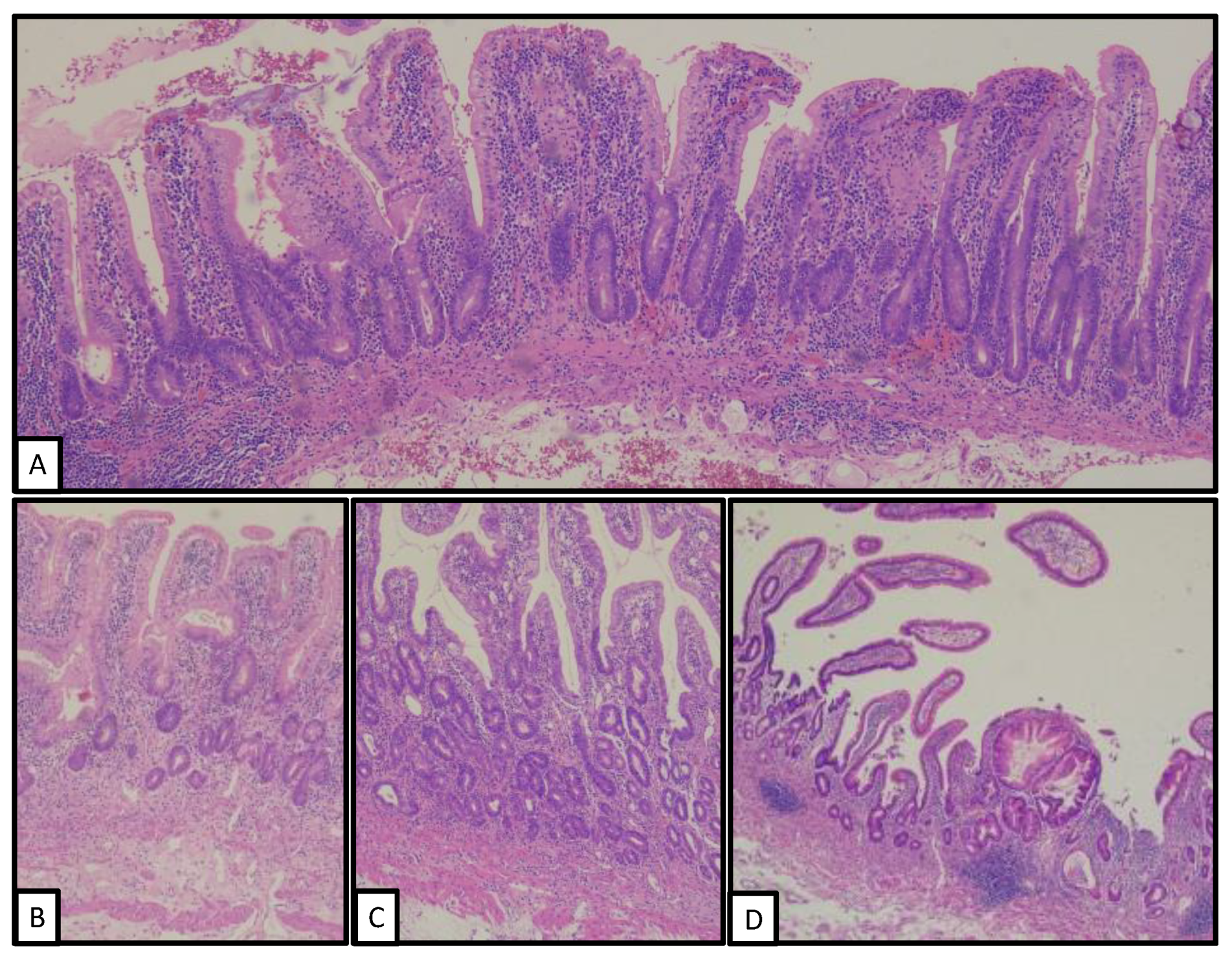
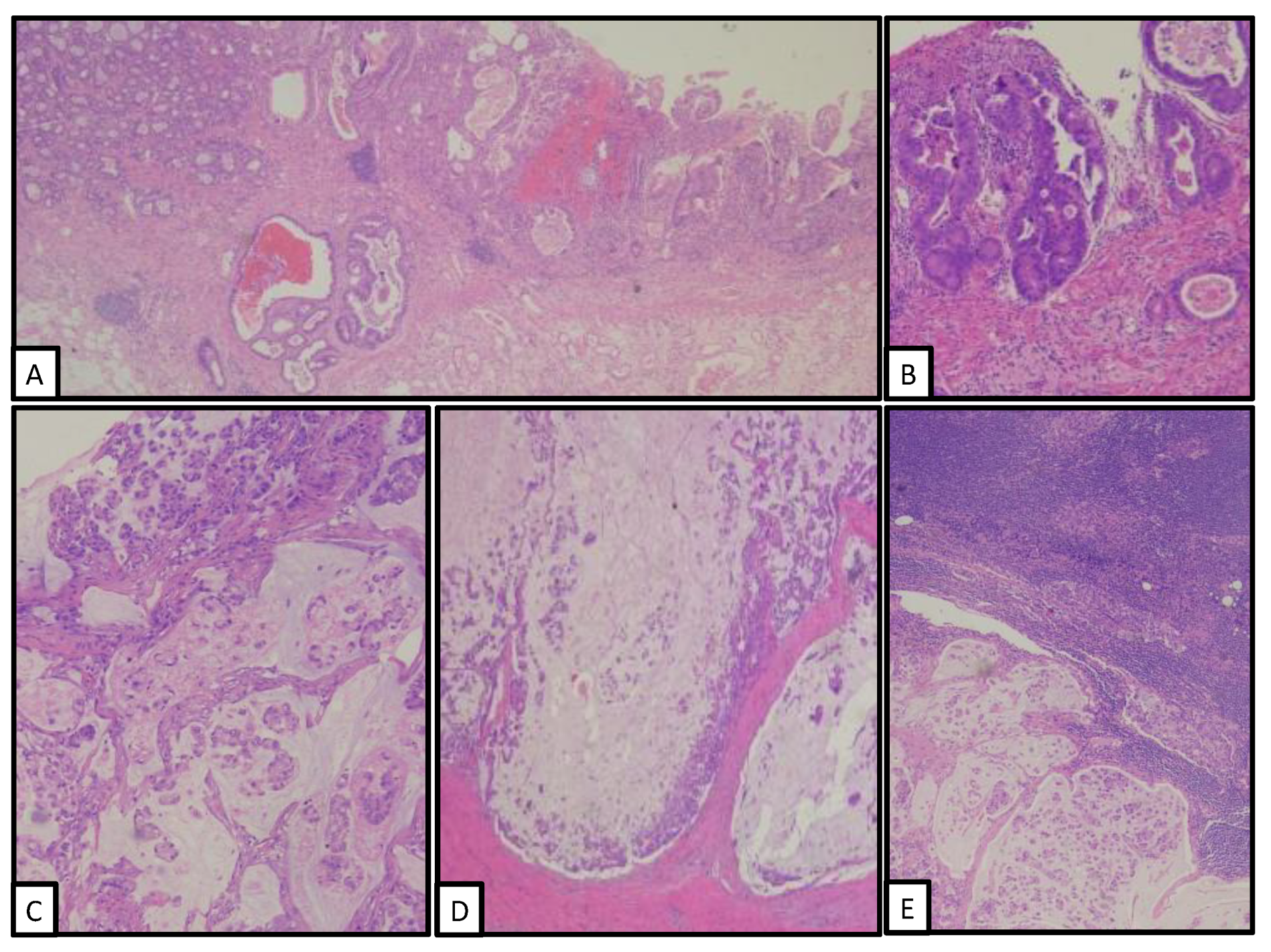
3. Pathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gill, S.S.; Heuman, D.M.; Mihas, A.A. Small intestinal neoplasms. J. Clin. Gastroenterol. 2001, 33, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Struck, A.; Howard, T.; Chiorean, E.G.; Clarke, J.M.; Riffenburgh, R.; Cardenes, H.R. Non-ampullary duodenal adenocarcinoma: Factors important for relapse and survival. J. Surg. Oncol. 2009, 100, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.H.; Inam, Z.S.; Farrell, T.J. Patient with Lynch syndrome with subsequent development of small bowel adenocarcinoma. BMJ Case Rep. 2018, 2018, bcr2018225003. [Google Scholar] [CrossRef] [PubMed]
- Weren, R.D.A.; Ligtenberg, M.J.L.; Kets, C.M.; de Voer, R.M.; Verwiel, E.T.P.; Spruijt, L.; van Zelst-Stams, W.A.G.; Jongmans, M.C.; Gilisen, C.; Hehir-Kwa, J.Y.; et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015, 47, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.A. Migration of the ductular elements of gut associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: The origins of “gastric metaplasia” in the duodenum of the specialized mucosa of Barrett’ esophagus and of pseudopyloric metaplasia. Yale J. Biol. Med. 1996, 69, 147–153. [Google Scholar] [PubMed]
- Krause, W.J. Brunner’s glands: A structural, histochemical and pathological profile. Prog. Histochem. Cytochem. 2000, 35, 259–367. [Google Scholar] [CrossRef]
- Dandalides, S.M.; Carey, W.D.; Petras, R.E.; Achkar, E. Endoscopic small bowel mucosal biopsy: A controlled trial evaluating force size and biopsy location in the diagnosis of normal and abnormal mucosal architecture. Gastrointest. Endosc. 1989, 35, 197. [Google Scholar] [CrossRef]
- Giacosa, A. Morphometry of normal duodenal mucosa. Scand. J. Gastroenterol. 1989, 167, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Kakushima, N.; Ono, H.; Yoshida, M.; Takizawa, K.; Tanaka, M.; Kawata, N.; Ito, S.; Imai, K.; Hotta, K.; Ishiwatari, H.; et al. Characteristics and risk factors for sporadic non-ampullary duodenal adenocarcinoma. Scand. J. Gastroenterol. 2017, 52, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Swinson, C.M.; Slavin, G.; Coles, E.C.; Booth, C.C. Coeliac disease and malignancy. Lancet 1983, 1, 111–115. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Vigneau, F.D. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann. Epidemiol. 2009, 19, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, K.R.; Dasari, B.V. Operative management of small bowel Crohn’s disease. Surg. Clin. N. Am. 2007, 87, 587–610. [Google Scholar] [CrossRef] [PubMed]
- Shaoul, R.; Hong, D.; Okada, Y.; Cutz, E.; Marcon, M.A. Lineage development in a patient without goblet, Paneth, and enteroendocrine cells: A clue for intestinal epithelial differentiation. Pediatr. Res. 2005, 58, 492–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salva, M.N.; Gupta, C.; Pandey, A.K.; Kumar, N.; Kotian, S.R.; Kalthur, S.G. Histogenesis and histomorphometric study of human fetal small intestine. Ethiop. J. Health Sci. 2019, 29, 689–696. [Google Scholar] [PubMed]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: General conclusions on the kinetics in the oxyntic epithelium. Anat. Rec. 1993, 236, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Dasari, B.V.; Gardiner, K.R. Management of adenocarcinoma of the small intestine. Gastrointest. Cancer Res. 2009, 3, 121–122. [Google Scholar] [PubMed]
- Ren, P.T.; Fu, H. Primary segmental and multiple adenocarcinomas of the small bowel. Intern. Med. 2012, 51, 877–880. [Google Scholar] [CrossRef] [PubMed]
| Location | Histology | Grade | Stage | |
|---|---|---|---|---|
| Tumor 1 | Duodenum (2nd part) | Well-diff. adenocarcinoma | 1 | pT1 |
| Tumor 2 | Duodenum (3rd part) | Signet-ring adenocarcinoma | 3 | pT4 |
| Tumor 3 | Duodenum (4th part) | Well-diff. adenocarcinoma | 1 | pT1 |
| Tumor 4 | Jejunum (1st loop) | Well-diff. adenocarcinoma | 1 | pT1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coverlizza, S.; Masu, L.; Manini, C. Multiple Adenocarcinomas of the Small Bowel in a Patient with Brunner’s Glands Agenesia: A Previously Unreported Association. Clin. Pract. 2022, 12, 672-676. https://doi.org/10.3390/clinpract12050069
Coverlizza S, Masu L, Manini C. Multiple Adenocarcinomas of the Small Bowel in a Patient with Brunner’s Glands Agenesia: A Previously Unreported Association. Clinics and Practice. 2022; 12(5):672-676. https://doi.org/10.3390/clinpract12050069
Chicago/Turabian StyleCoverlizza, Sergio, Lavinia Masu, and Claudia Manini. 2022. "Multiple Adenocarcinomas of the Small Bowel in a Patient with Brunner’s Glands Agenesia: A Previously Unreported Association" Clinics and Practice 12, no. 5: 672-676. https://doi.org/10.3390/clinpract12050069
APA StyleCoverlizza, S., Masu, L., & Manini, C. (2022). Multiple Adenocarcinomas of the Small Bowel in a Patient with Brunner’s Glands Agenesia: A Previously Unreported Association. Clinics and Practice, 12(5), 672-676. https://doi.org/10.3390/clinpract12050069







