Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrollment
2.2. Ethics
2.3. Procedure
End Points
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, A.M.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, A.E.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Munro, A.P.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. COV-BOOST study group. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Reimann, P.; Ulmer, H.; Mutschlechner, B.; Benda, M.; Severgnini, L.; Volgger, A.; Lang, T.; Atzl, M.; Huynh, M.; Gasser, K.; et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br. J. Haematol. 2022, 196, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; Mrak, D.; Tobudic, S.; Sieghart, D.; Koblischke, M.; Mandl, P.; Kornek, B.; Simader, E.; Radner, H.; Perkmann, T.; et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: A randomised controlled trial. Ann. Rheum. Dis. 2022, 81. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.N.; Roberts, D.J.; Robertson, H.L.; Kramer, A.H.; Laupland, K.B.; Ousman, S.S.; Kubes, P.; Zygun, D.A. Incidence, prevalence, and occurrence rate of infection among adults hospitalized after traumatic brain injury: Study protocol for asystematic review and meta-analysis. Syst. Rev. 2013, 2, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharfi, I.M.; Charyk Stewart, T.; Al Helali, I.; Daoud, H.; Fraser, D.D. Infection rates, fevers, and associated factors in pediatricsevere traumatic brain injury. J. Neurotrauma 2014, 31, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Lord, J.M.; Belli, A. Traumatic brain injury and peripheral immune suppression: Primer and prospectus. Front. Neurol. 2015, 6, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Munno, I.; Damiani, S.; Lacedra, G.; Mastropasqua, V.; Megna, G.F. Impairment of non-specific immunity in patients in persistentvegetative state. Immunopharmacol. Immunotoxicol. 1996, 18, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Wolach, B.; Sazbon, L.; Gavrieli, R.; Ben-Tovim, T.; Zagreba, F.; Schlesinger, M. Some aspects of the humoral and neutrophilfunctions in post-comatose unawareness patients. Brain Inj. 1993, 7, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Doran, S.J.; Barrett, J.P.; Henry, R.J.; Ma, E.L.; Faden, A.I.; Loane, D.J. Chronic Alterations in Systemic ImmuneFunction after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.E.; Battaglia, R.; Cerasa, A.; Raso, M.G.; Coschignano, F.; Pagliuso, A.; Bruschetta, R.; Pugliese, G.; Scola, P.; Tonin, P. Anti-SARS-CoV-2 S-RBD IgG Antibody Responses after COVID-19 mRNA Vaccine in the Chronic Disorder of Consciousness: A Pilot Study. J. Clin. Med. 2021, 10, 5830. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, S.J.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
| DOC_heter (n = 24) | HCW_heter (n = 14) | HCW_homol (n = 28) | p-Level | |
|---|---|---|---|---|
| Age | 54.1 ± 16.5 | 44.3 ± 11.1 | 52.9 ± 12.5 | n.s. |
| Gender, (%) male | 55% | 50% | 53% | n.s. |
| Hypertension (Yes; %) | 46% | 7% | 7% | <0.05 |
| Diabetes mellitus (Yes; %) | 0% | 0% | 3.50% | n.s. |
| Heart disease (Yes; %) | 16% | 3.50% | 3% | n.s. |
| Renal insufficiency (Yes; %) | 0% | 0% | 7% | n.s. |
| Obstructive pulmonary disease (Yes; %) | 8% | 0% | 7% | n.s. |
| Liver disease (Yes; %) | 8% | 0% | 0% | n.s. |
| Endocrinopathies (Yes; %) | 12.50% | 7% | 7% | n.s. |
| Tumor (Yes; %) | 2% | 0% | 0% | n.s. |
| DOC-related Clinical Data | ||||
| CRS-r at enrollment | 9.2 ± 4.7 | |||
| Time from injury (years) | 3.9 ± 3.3 | |||
| Etiology n (%) | ||||
| Vascular | 11 (45.9%) | |||
| Traumatic | 6 (25%) | |||
| Anoxic | 4 (16.6%) | |||
| Others | 3 (12.5%) | |||
| (Dementia, infections/post-surgery) | ||||
| Diagnosis n (%) | ||||
| VS | 10 (41.6%) | |||
| MCS | 14 (59.4%) | |||
| Pre-Booster Phase | Post-Booster Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| t0 (1 Month) | t1 (6 Months) | t2 (9 Months) | 21 Days | |||||
| Raw | Log2 t0 | Raw | Log2 t1 | Raw | Log2 t2 | Raw | Log2 | |
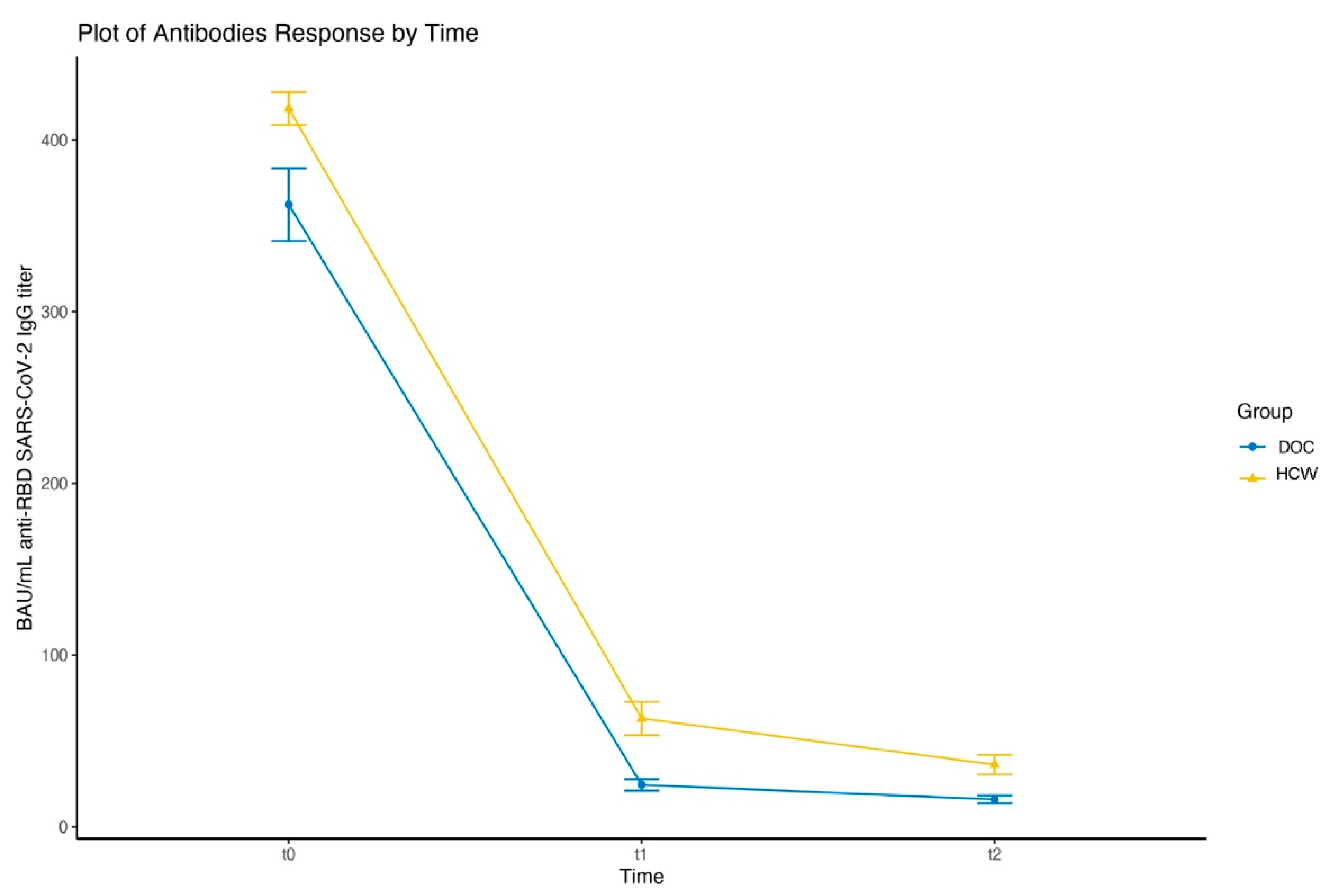
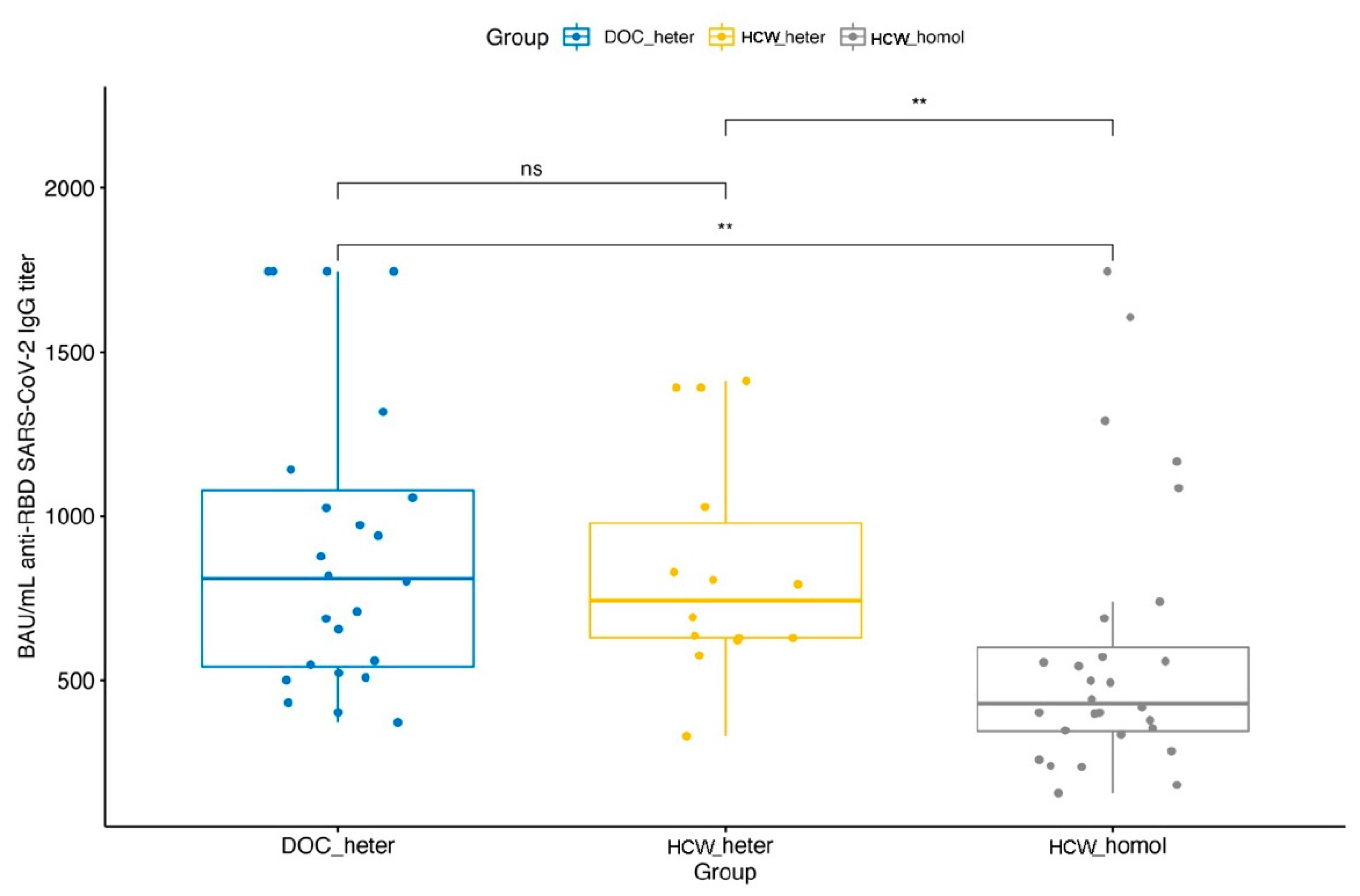
| DOC_heter | 370.7 ± 106.4 | 8.4 ± 0.5 | 24.5 ± 17.8 | 4.2 ± 1.02 | 16 ± 12 | 3.7 ± 0.9 | 909.9 ± 453.9 | 9.6 ± 0.7 |
| HCW_homol | 419 ± 53.1 | 8.7 ± 0.2 | 65 ± 55.7 | 5.6 ± 1.1 | 36 ± 32.1 | 4.7 ± 1.1 | 584.9 ± 416.4 | 8.9 ± 0.9 |
| HCW_heter | 840.4 ± 340.3 | 9.6 ± 0.6 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, M.E.; Battaglia, R.; Raso, M.G.; Chiaravalloti, R.; Coschignano, F.; Pagliuso, A.; Bruschetta, R.; Pugliese, G.; Scola, P.; Tonin, P.; et al. Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study. Clin. Pract. 2022, 12, 318-325. https://doi.org/10.3390/clinpract12030037
Pugliese ME, Battaglia R, Raso MG, Chiaravalloti R, Coschignano F, Pagliuso A, Bruschetta R, Pugliese G, Scola P, Tonin P, et al. Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study. Clinics and Practice. 2022; 12(3):318-325. https://doi.org/10.3390/clinpract12030037
Chicago/Turabian StylePugliese, Maria Elena, Riccardo Battaglia, Maria Girolama Raso, Raffaela Chiaravalloti, Francesco Coschignano, Angela Pagliuso, Roberta Bruschetta, Giovanni Pugliese, Paolo Scola, Paolo Tonin, and et al. 2022. "Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study" Clinics and Practice 12, no. 3: 318-325. https://doi.org/10.3390/clinpract12030037
APA StylePugliese, M. E., Battaglia, R., Raso, M. G., Chiaravalloti, R., Coschignano, F., Pagliuso, A., Bruschetta, R., Pugliese, G., Scola, P., Tonin, P., & Cerasa, A. (2022). Heterologous COVID-19 Booster Vaccination in the Chronic Disorder of Consciousness: A Pilot Study. Clinics and Practice, 12(3), 318-325. https://doi.org/10.3390/clinpract12030037







