Matching Imaging and Remodulation Effects: Benefits of Cardiac Contractility Modulation Shown by Global Longitudinal Strain: A Case Report
Abstract
:1. Introduction
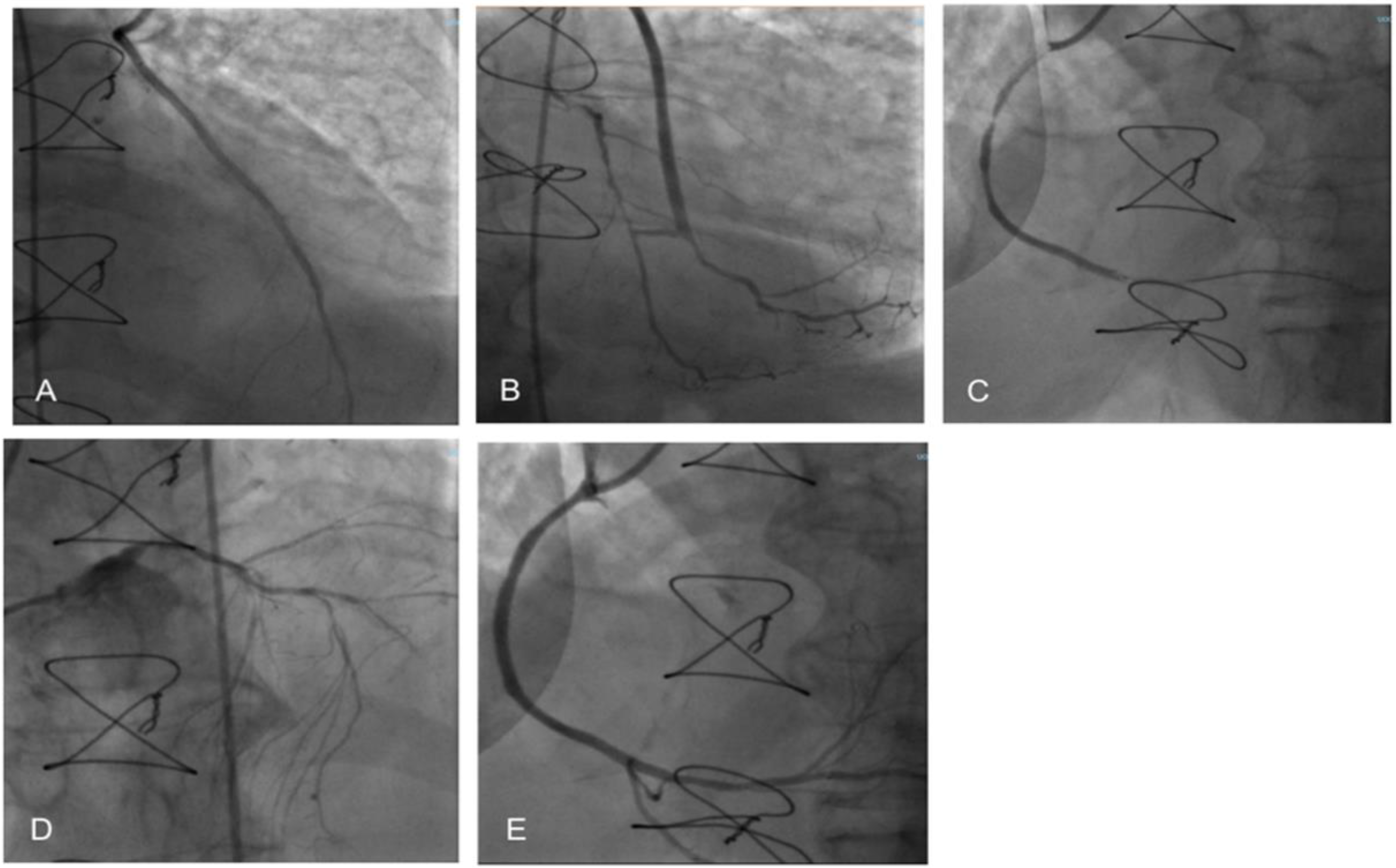
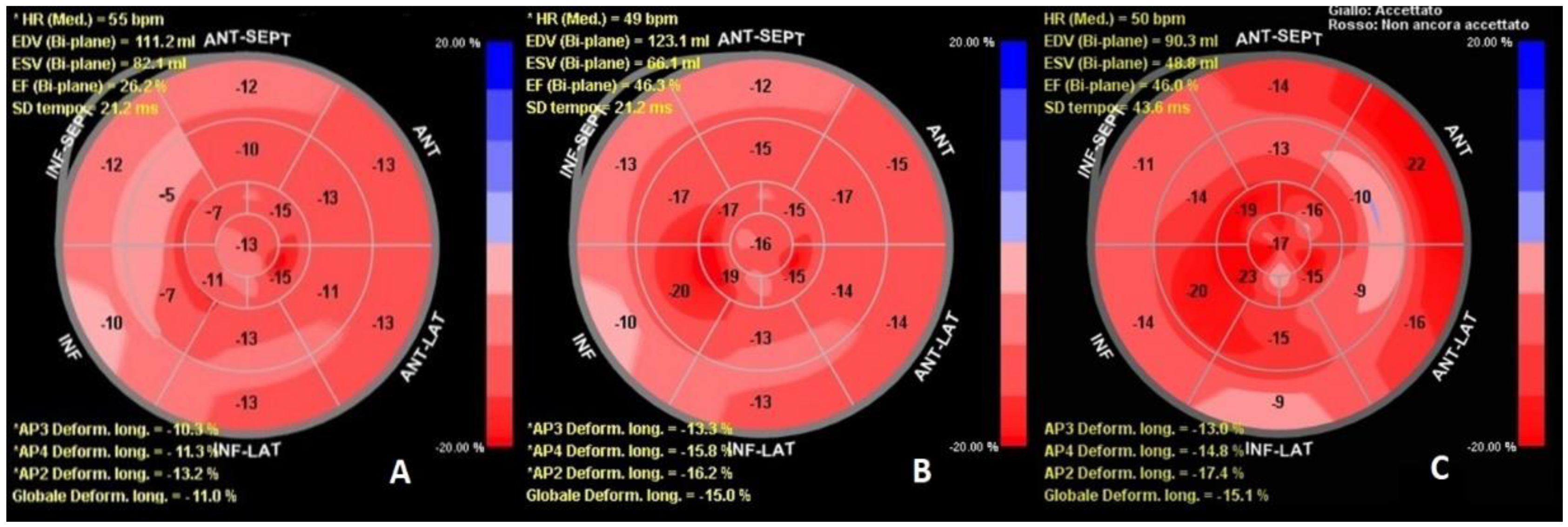
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices, and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Kahwash, R.; Burkhoff, D.; Abraham, W.T. Cardiac contractility modulation in patients with advanced heart failure. Expert Rev. Cardiovasc. Ther. 2013, 11, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Vigorito, C.; Piepoli, M.F.; Coats, A.J.S. Effects of cardiac contractility modulation by non-excitatory electrical stimulation on exercise capacity and quality of life: An individual patient’s data meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014, 175, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zacà, V.; Ballo, P.; D’Andrea, A.; Muraru, D.; Losi, M.; et al. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–78. [Google Scholar] [CrossRef]
- Cicchitti, V.; Radico, F.; Bianco, F.; Gallina, S.; Tonti, G.; De Caterina, R. Heart failure due to right ventricular apical pacing: The importance of flow patterns. Ep Eur. 2016, 18, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Mando, R.; Goel, A.; Habash, F.; Saad, M.; Ayoub, K.; Vallurupalli, S.; Maskoun, W. Outcomes of Cardiac Contractility Modulation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Cardiovasc. Ther. 2019, 2019, 9769724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Borggrefe, M.; Neuser, H.; Ohlow, M.-A.; Röger, S.; Goette, A.; Remppis, B.A.; Kuck, K.-H.; Najarian, K.B.; Gutterman, D.D.; et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2019, 21, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, A.; Bonacchi, G.; La Fazia, V.M.; Stifano, G.; Sergi, D. Matching Imaging and Remodulation Effects: Benefits of Cardiac Contractility Modulation Shown by Global Longitudinal Strain: A Case Report. Clin. Pract. 2022, 12, 113-117. https://doi.org/10.3390/clinpract12010015
Matteucci A, Bonacchi G, La Fazia VM, Stifano G, Sergi D. Matching Imaging and Remodulation Effects: Benefits of Cardiac Contractility Modulation Shown by Global Longitudinal Strain: A Case Report. Clinics and Practice. 2022; 12(1):113-117. https://doi.org/10.3390/clinpract12010015
Chicago/Turabian StyleMatteucci, Andrea, Giacomo Bonacchi, Vincenzo M. La Fazia, Giuseppe Stifano, and Domenico Sergi. 2022. "Matching Imaging and Remodulation Effects: Benefits of Cardiac Contractility Modulation Shown by Global Longitudinal Strain: A Case Report" Clinics and Practice 12, no. 1: 113-117. https://doi.org/10.3390/clinpract12010015
APA StyleMatteucci, A., Bonacchi, G., La Fazia, V. M., Stifano, G., & Sergi, D. (2022). Matching Imaging and Remodulation Effects: Benefits of Cardiac Contractility Modulation Shown by Global Longitudinal Strain: A Case Report. Clinics and Practice, 12(1), 113-117. https://doi.org/10.3390/clinpract12010015





