Use of Cardiac Contractility Modulation in an Older Patient with Non-Ischemic Dilated Cardiomyopathy: A Case Report
Abstract
:1. Introduction
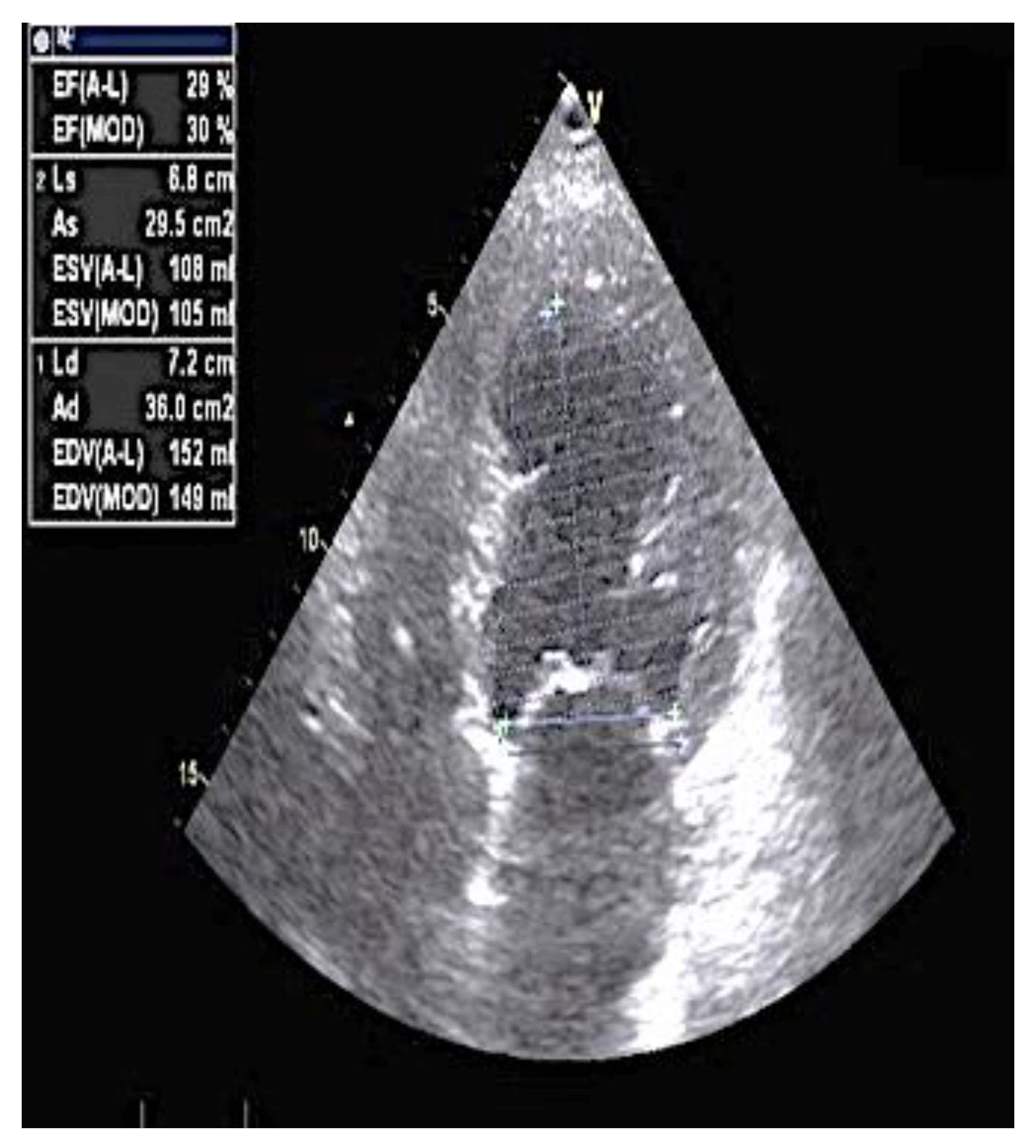
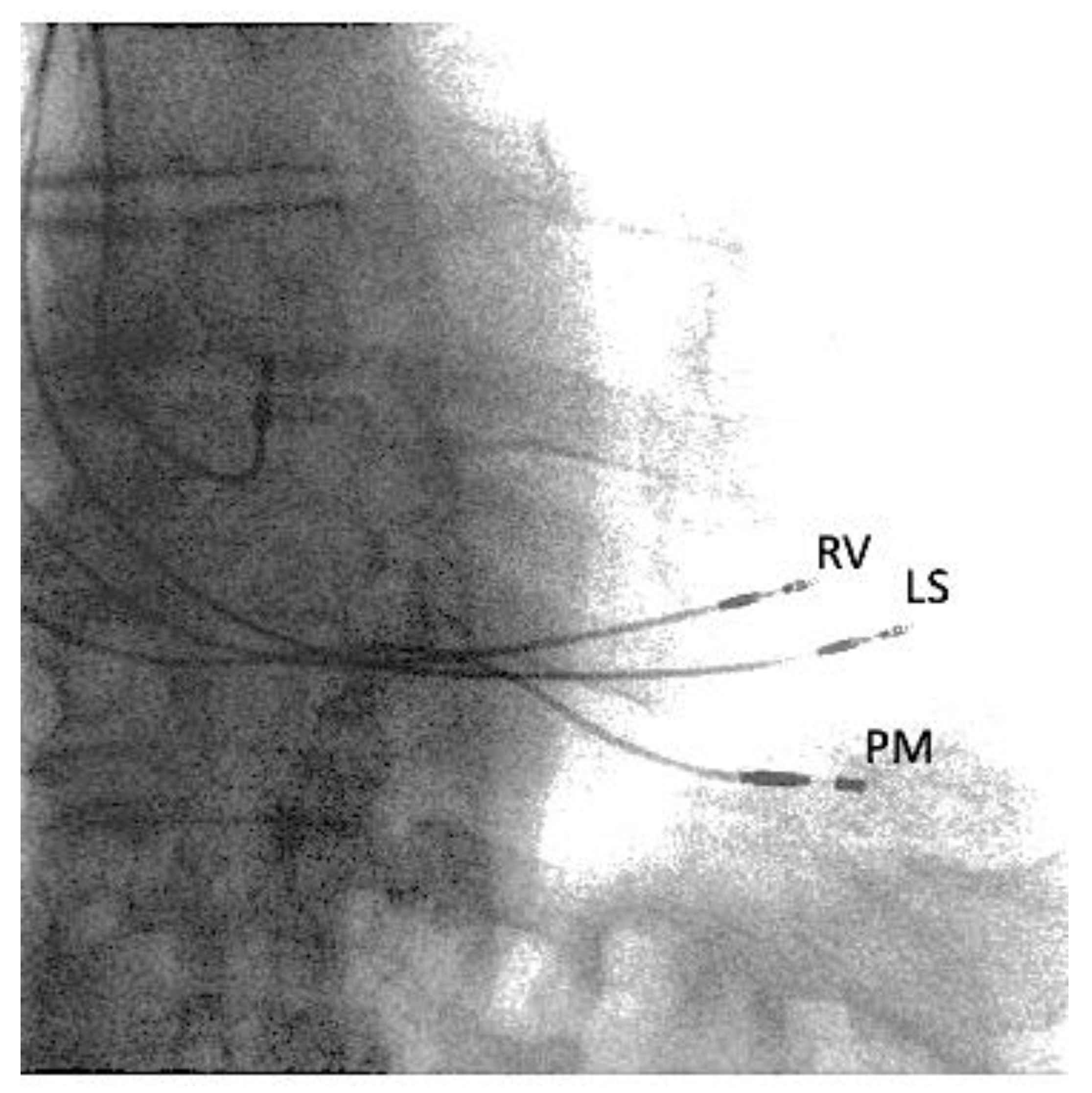
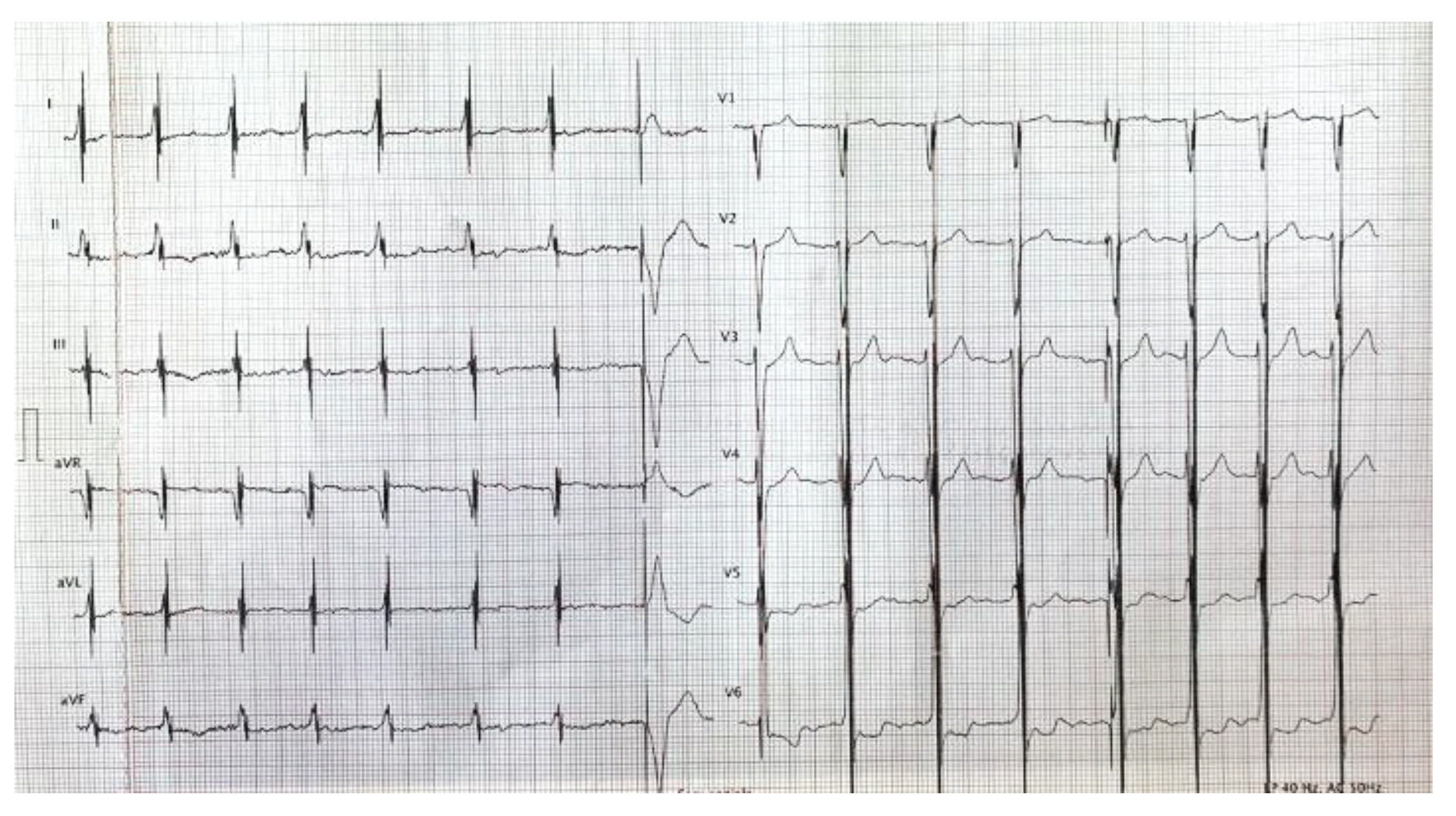
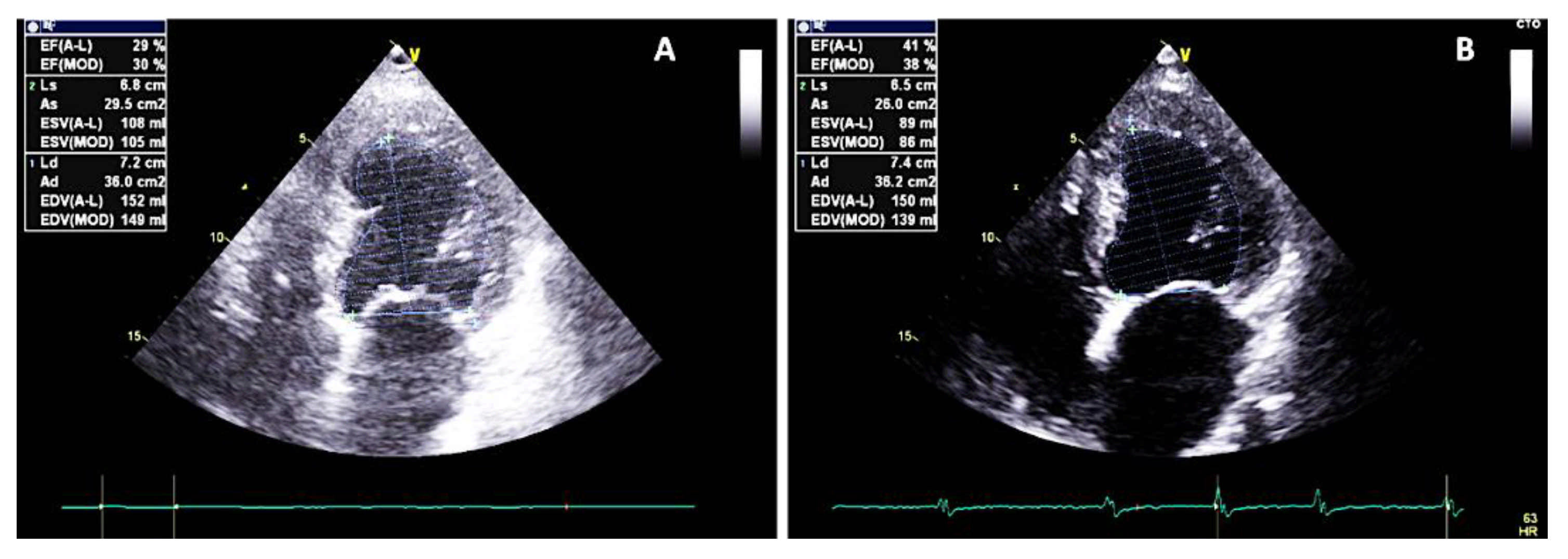
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L.J. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [PubMed]
- Kuschyk, J.; Falk, P.; Demming, T.; Marx, O.; Morley, D.; Rao, I.; Burkhoff, D. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur. J. Heart Fail. 2021, 23, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Kahwash, R.; Abraham, W.T. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Future Cardiol. 2020, 16, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Biffi, M.; Aspromonte, N.; Bongiorni, M.G.; Clemenza, F.; D’Onofrio, A.; De Ferrari, G.M.; Giallauria, F.; Grimaldi, M.; Olivia, F.; Senni, M.; et al. Cardiac contractility modulation in heart failure with reduced ejection fraction: Critical review of evidence and application perspectives. G. Ital. Cardiol. (Rome) 2021, 22, 727–741. [Google Scholar]
- Brunckhorst, C.B.; Shemer, I.; Mika, Y.; Ben-Haim, S.A.; Burkhoff, D. Cardiac contractility modulation by non-excitatory currents: Studies in isolated cardiac muscle. Eur. J. Heart Fail. 2006, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Rastogi, S.; Minden, H.H.; Meyhöfer, J.; Burkhoff, D.; Sabbah, H.N. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J. Am. Coll. Cardiol. 2008, 51, 1784–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohri, S.; He, K.L.; Dickstein, M.; Mika, Y.; Shimizu, J.; Shemer, I.; Yi, G.-H.; Wang, J.; Ben-Haim, S.; Burkhoff, D. Cardiac contractility modulation by electric currents applied during the refractory period. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1642–H1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borggrefe, M.; Mann, D.L. Cardiac Contractility Modulation in 2018. 11 December. Circulation 2018, 138, 2738–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giallauria, F.; Cuomo, G.; Parlato, A.; Raval, N.Y.; Kuschyk, J.; Coats, A.J.S. A comprehensive individual patient data meta-analysis of the effects of cardiac contractility modulation on functional capacity and heart failure-related quality of life. ESC Heart Fail. 2020, 7, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Limongelli, G.; Ammendola, E.; Verrengia, M.; Gravino, R.; Pacileo, G. Risk Stratification of Sudden Cardiac Death in Patients with Heart Failure: An update. J. Clin. Med. 2018, 7, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.; Elliott, P.M. Risk Stratification for Sudden Cardiac Death in Non-Ischaemic Dilated Cardiomyopathy. Curr. Cardiol. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manganelli, G.; Fiorentino, A.; Ceravolo, G.; Minichiello, S.; Bianchino, G.; Bellizzi, G.; Pacileo, G.; Masarone, D. Use of Cardiac Contractility Modulation in an Older Patient with Non-Ischemic Dilated Cardiomyopathy: A Case Report. Clin. Pract. 2021, 11, 835-840. https://doi.org/10.3390/clinpract11040098
Manganelli G, Fiorentino A, Ceravolo G, Minichiello S, Bianchino G, Bellizzi G, Pacileo G, Masarone D. Use of Cardiac Contractility Modulation in an Older Patient with Non-Ischemic Dilated Cardiomyopathy: A Case Report. Clinics and Practice. 2021; 11(4):835-840. https://doi.org/10.3390/clinpract11040098
Chicago/Turabian StyleManganelli, Gianvito, Antonio Fiorentino, Gianluca Ceravolo, Stefana Minichiello, Giuseppe Bianchino, Gennaro Bellizzi, Giuseppe Pacileo, and Daniele Masarone. 2021. "Use of Cardiac Contractility Modulation in an Older Patient with Non-Ischemic Dilated Cardiomyopathy: A Case Report" Clinics and Practice 11, no. 4: 835-840. https://doi.org/10.3390/clinpract11040098
APA StyleManganelli, G., Fiorentino, A., Ceravolo, G., Minichiello, S., Bianchino, G., Bellizzi, G., Pacileo, G., & Masarone, D. (2021). Use of Cardiac Contractility Modulation in an Older Patient with Non-Ischemic Dilated Cardiomyopathy: A Case Report. Clinics and Practice, 11(4), 835-840. https://doi.org/10.3390/clinpract11040098






