Delayed Onset Minimal Change Disease as a Manifestation of Lupus Podocytopathy
Abstract
1. Introduction
2. Case Report
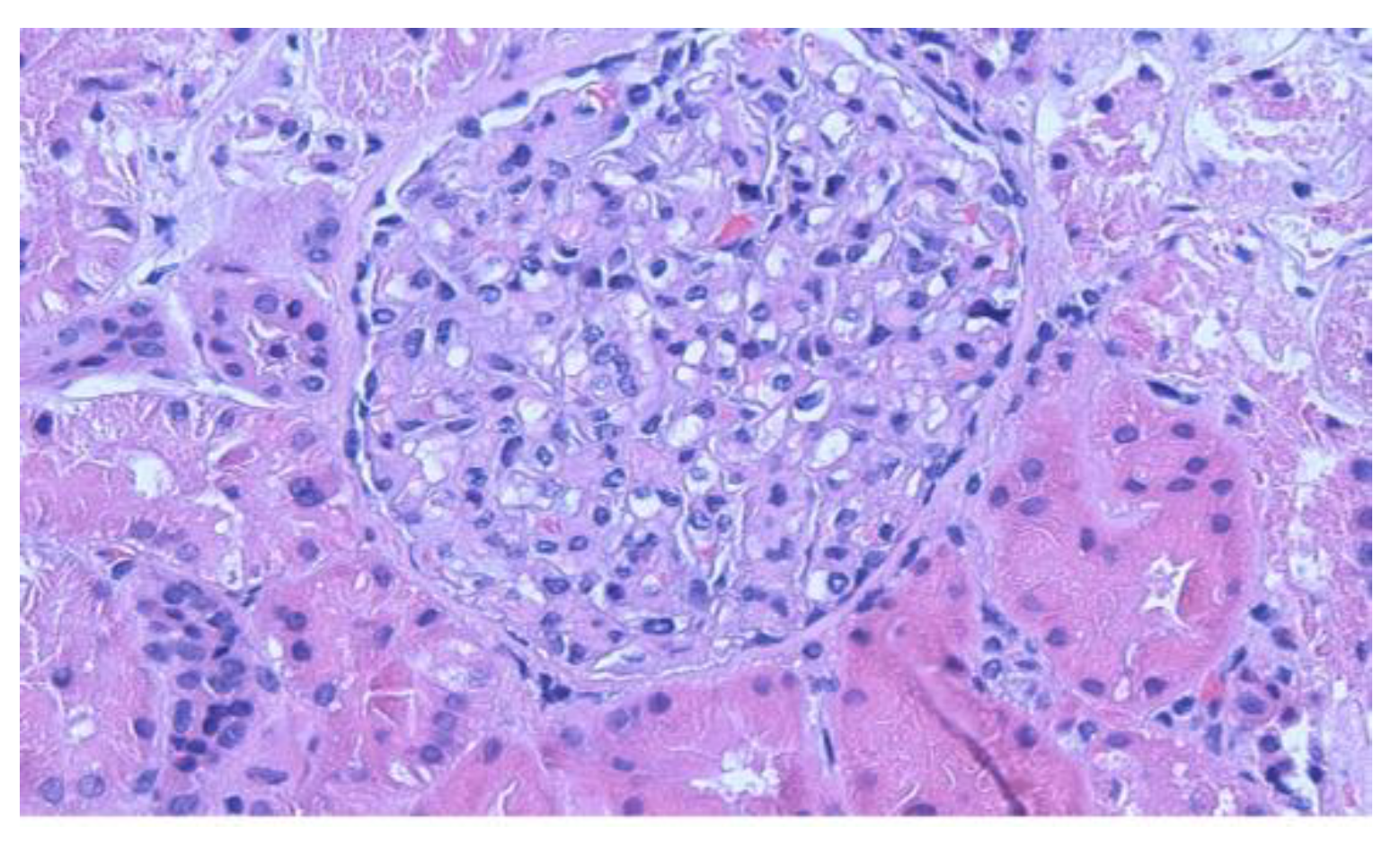
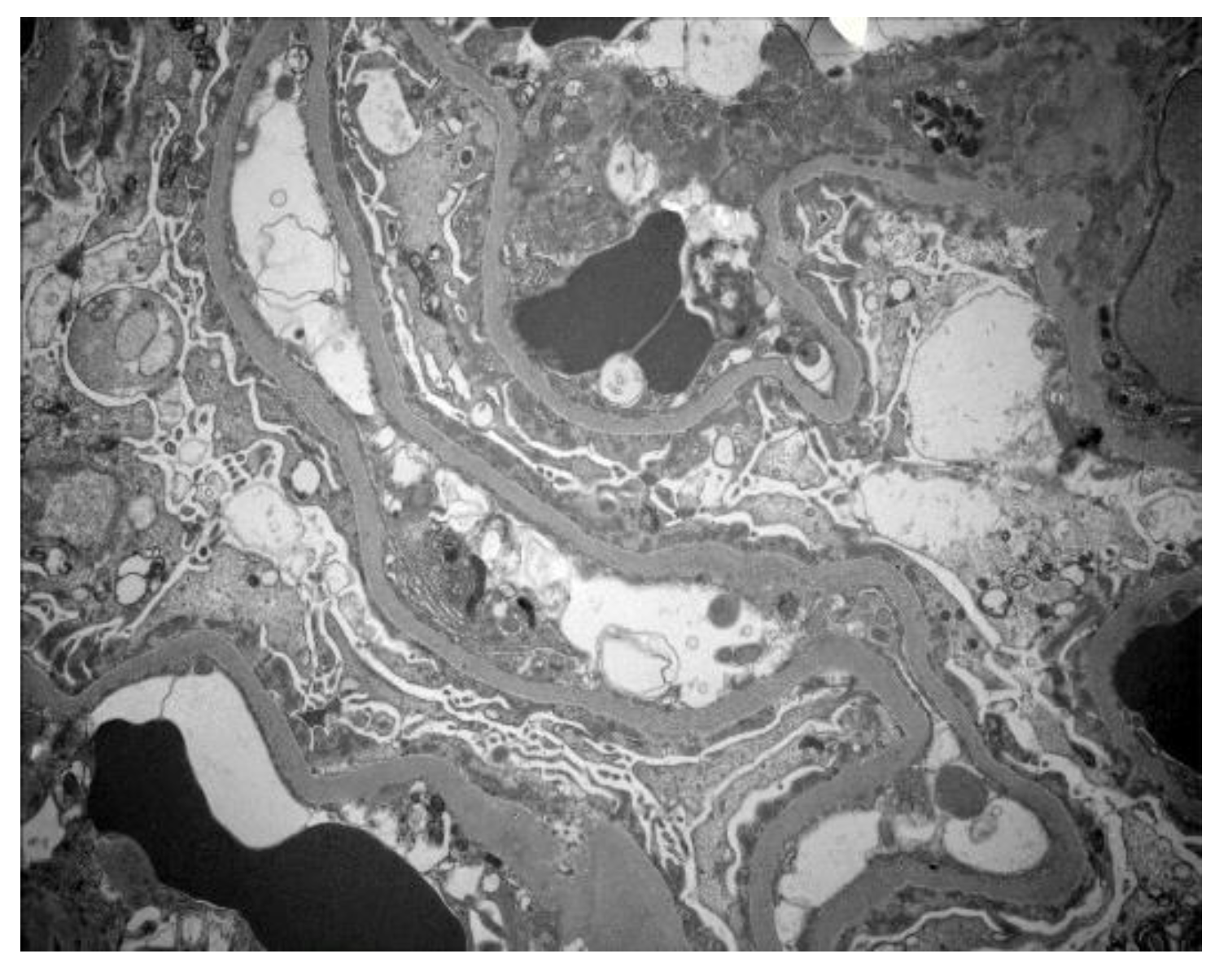
2.1. Results
2.2. Management
2.3. Outcome
3. Discussion
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tiffin, N.; Adeyemo, A.; Okpechi, I. A diverse array of genetic factors contributes to the pathogenesis of systemic lupus erythe-matosus. Orphanet J. Rare Dis. 2013, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Bertsias, G.; Cervera, R.; Boumpas, D.T. Systemic lupus erythematosus: Pathogenesis and clinical features. In EULAR Textbook on Rheumatic Diseases; BMJ: Geneva, Switzerland, 2012; pp. 476–505. [Google Scholar]
- Sadun, R.E.; Ardoin, S.P.; Schanberg, L.E. Systemic lupus erythematosus. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., Stanton, B.F., Geme, J.W., Schor, N.F., Berhrman, R.E., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 1176–1180. [Google Scholar]
- Niaudet, P.; Salomon, R. Systemic lupus erythematosus. In Pediatric Nephrology, 6th ed.; Avner, E.D., Harmon, W.E., Niaudet, P., Eds.; Springer: Heidelburg, Germany, 2009; pp. 1127–1154. [Google Scholar]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Dube, G.K.; Markowitz, G.S.; Radhakrishnan Appel, G.B.; D’Agati, V.D. Minimal change disease in systemic lupus erythematosus. Clin. Nephrol. 2002, 57, 120–126. [Google Scholar] [CrossRef]
- Hertig, A.; Droz, A.; Lesavre, P.; Grünfeld, J.P. SLE and idiopathic nephrotic syndrome: Coincidence or not? Am. J. Kidney Dis. 2002, 40, 1179–1184. [Google Scholar] [CrossRef]
- Horino, T.; Takao, T.; Morita, T.; Ito, H.; Hashimoto, K. Minimal change nephrotic syndrome associated with systemic lupus ery-thematosus. Nephrol. Dial Transplant. 2006, 21, 230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hickman, P.L.; Nolph, K.D.; Jacobs, R.; Luger, A.M.; Walker, S.E. Idiopathic focal segmental glomerulosclerosis in a patient with sys-temic lupus erythematosus: An unusual combination. Am. J. Kidney Dis. 1994, 23, 582–586. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Wang, S.; Chen, H.; Liu, Z.; Zeng, C.; Zhang, H.; Liu, Z. Clinical–Morphological Features and Outcomes of Lupus Podocytopathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Markowitz, G.S. Lupus Podocytopathy: A Distinct Entity. Clin. J. Am. Soc. Nephrol. 2016, 11, 547–548. [Google Scholar] [CrossRef]
- Oliva-Damaso, N.; Payan, J.; Oliva-Damaso, E.; Pereda, T.; Bomback, A.S. Lupus Podocytopathy: An Overview. Adv. Chronic Kidney Dis. 2019, 26, 369–375. [Google Scholar] [CrossRef]
- Galindo-Izquierdo, M.; Rodriguez-Almaraz, E.; Pego-Reigosa, J.M.; López-Longo, F.J.; Calvo-Alén, J.; Olivé, A.; Fernández-Nebro, A.; Martinez-Taboada, V.; Vela-Casasempere, P.; Freire, M. Characterization of patients with lupus nephritis included in a large cohort from the Spanish Society of Rheumatology registry of patients with systemic lupus erythematosus (RE-LESSER). Medicine 2016, 95, e2891. [Google Scholar] [CrossRef]
- Narváez, J.; Ricse, M.; Gomà, M.; Mitjavila, F.; Fulladosa, X.; Capdevila, O.; Torras, J.; Juanola, X.; Pujol-Farriols, R.; Nolla, J.M. The value of repeat biopsy in lupus nephritis flares. Medicine 2017, 96, e7099. [Google Scholar] [CrossRef]
- Al Arfaj, A.S.; Khalil, N.; Al Saleh, S. Lupus nephritis among 624 cases of systemic lupus erythematosus in Riyadh, Saudi Ara-bia. Rheumatol. Int. 2009, 29, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Croca, S.C.; Rodrigues, T.; Isenberg, D. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology 2011, 50, 1424–1430. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Lim, S.S.; E Patzer, R.; McClellan, W.M.; Kramer, M.; Klein, M.; Pastan, S.; Gordon, C.; Helmick, C.G.; Drenkard, C. Incidence of End-Stage Renal Disease Among Newly Diagnosed Systemic Lupus Erythematosus Patients: The Georgia Lupus Registry. Arthritis Care Res. 2016, 68, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, G.; Nakamoto, M.; Yasunaga, C.; Takeda, K.; Matsuo, K.; Urabe, M.; Goya, T.; Sakemi, T. Systemic lupus erythematosus in a patient with remitting minimal change ne-phrotic syndrome. Clin. Nephrol. 1997, 48, 327–330. [Google Scholar]
- Moysés-Neto, M.; Costa, R.S.; Rodrigues, F.F.; Neto, O.M.V.; Reis, M.A.; Louzada-Junior, P.; Romão, E.A.; Dantas, M. Minimal change disease: A variant of lupus nephritis. NDT Plus 2011, 4, 20–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.F.; Chen, Y.H.; Chen, D.Q.; Liu, Z.Z.; Xu, F.; Zeng, C.H.; Hu, W.X. Mesangial proliferative lupus nephritis with podocytopathy: A special entity of lupus nephritis. Lupus 2018, 27, 303–311. [Google Scholar] [CrossRef]
- Almaani, S.; Parikh, S.V. Membranous Lupus Nephritis: A Clinical Review. Adv. Chronic Kidney Dis. 2019, 26, 393–403. [Google Scholar] [CrossRef]
- Abdelnabi, H.H. Is podocytopathy another image of renal affection in p-SLE? Pediatr. Rheumatol. Online J. 2021, 19, 57. [Google Scholar] [CrossRef]
- Yap, H.K.; Cheung, W.; Murugasu, B.; Sim, S.K.; Seah, C.C.; Jordan, S.C. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: Evidence for increased IL-13 mRNA expression in relapse. J. Am. Soc. Nephrol. 1999, 10, 529–537. [Google Scholar] [CrossRef]
- Kraft, S.W.; Schwartz, M.M.; Korbet, S.M.; Lewis, E.J. Glomerular podocytopathy in patients with systemic lupus erythematosus. J. Am. Soc. Nephrol. 2005, 16, 175–179. [Google Scholar] [CrossRef]
- Heymann, F.; Meyer-Schwesinger, C.; Hamilton-Williams, E.; Hammerich, L.; Panzer, U.; Kaden, S.; Quaggin, S.E.; Floege, J.; Gröne, H.-J.; Kurts, C. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Investig. 2009, 119, 1286–1297. [Google Scholar] [CrossRef]
- Ishimoto, T.; Shimada, M.; Araya, C.E.; Huskey, J.; Garin, E.H.; Johnson, R.J. Minimal change disease: A CD80 podocytopathy? Semin. Nephrol. 2011, 31, 320–325. [Google Scholar] [CrossRef]
- Garin, E.H.; Boggs, K.P. Synergy of monocytes and lymphocytes from idiopathic minimal lesion nephrotic patients in relapse in the production of the supernatant factor that increases rat glomerular basement membrane sulfate uptake. Int. J. Pediatr. Nephrol. 1987, 8, 187–192. [Google Scholar]
- Kerjaschki, D. Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Investig. 2001, 108, 1583. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.G.; Gillespie, K.M.; Mathieson, P.W. Effects of type 2 cytokines on glomerular epithelial cells. Exp. Nephrol. 2001, 9, 275–283. [Google Scholar] [CrossRef]
- Mérida, E.; Praga, M. NSAIDs and Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2019, 14, 1280–1282. [Google Scholar] [CrossRef]
- Maas, R.J.; Deegens, J.K.; Wetzels, J.F. Permeability factors in idiopathic nephrotic syndrome: Historical perspectives and lessons for the future. Nephrol. Dial. Transplant. 2014, 29, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Sellier-Leclerc, A.L.; Duval, A.; Riveron, S.; Macher, M.-A.; Deschenes, G.; Loirat, C.; Verpont, M.-C.; Peuchmaur, M.; Ronco, P.; Monteiro, R.C.; et al. A humanized mouse model of idiopathic nephrotic syndrome suggests a patho-genic role for immature cells. J. Am. Soc. Nephrol. 2007, 18, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, L.; Bruneau, S.; Naulet, J.; Renaudin, K.; Buzelin, F.; Usal, C.; Smit, H.; Condamine, T.; Soulillou, J.-P.; Dantal, J. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J. Am. Soc. Nephrol. 2009, 20, 57–67. [Google Scholar] [CrossRef]
- Yang, T.; Nast, C.C.; Vo, A.; Jordan, S.C. Rapid remission of steroid and mycophenolate mofetil (mmf)-resistant minimal change nephrotic syndrome after rituximab therapy. Nephrol. Dial. Transplant. 2008, 23, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, M.; Wu, H.; Lu, Q. The pathological role of B cells in systemic lupus erythematosus: From basic research to clinical. Autoimmunity 2020, 53, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Moutsatsou, P. Estrogen Receptor Signaling and Its Relationship to Cytokines in Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 317452. [Google Scholar] [CrossRef]
- Yung, R.L. Mechanisms of lupus: The role of estrogens. Clin. Exp. Rheumatol. 1999, 17, 271–275. [Google Scholar]
- Lu, Q.; Wu, A.; Tesmer, L.; Ray, N.; Yousif, N.; Richardson, B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J. Immunol. 2007, 179, 6352–6358. [Google Scholar] [CrossRef]
- Liu, K.; Kurien, B.T.; Zimmerman, S.L.; Kaufman, K.M.; Taft, D.H.; Kottyan, L.C.; Lazaro, S.; Weaver, C.A.; Ice, J.A.; Adler, A.J.; et al. X chromosome dose and sex bias in autoimmune diseases: Increased prevalence of 47,XXX in systemic lupus erythematosus and sjögren’s syndrome. Arthritis Rheumatol. 2016, 68, 1290–1300. [Google Scholar] [CrossRef]
- Koya, M.; Pichler, R.; Jefferson, J.A. Minimal-change disease secondary to etanercept. Clin. Kidney J. 2012, 5, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Piga, M.; Chessa, E.; Ibba, V.; Mura, V.; Floris, A.; Cauli, A.; Mathieu, A. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: Systematic literature review and analysis of a monocentric cohort. Autoimmun. Rev. 2014, 13, 873–879. [Google Scholar] [CrossRef]
- Raveh, D.; Shemesh, O.; Ashkenazi, Y.J.; Winkler, R.; Barak, V. Tumor necrosis factor-alpha blocking agent as a treatment for ne-phrotic syndrome. Pediatr. Nephrol. 2004, 19, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okamura, M.; Nakajima, S.; Hayakawa, K.; Huang, T.; Yao, J.; Kitamura, M. Suppression of nephrin expression by TNF-alpha via interfering with the cAMP-retinoic acid receptor pathway. Am. J. Physiol. Renal Physiol. 2010, 298, F1436–F1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hu, W. Lupus podocytopathy: A distinct entity of lupus nephritis. J. Nephrol. 2018, 31, 629–634. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, R.; Zeng, X.; Acharya, R.; Upadhyay, K. Delayed Onset Minimal Change Disease as a Manifestation of Lupus Podocytopathy. Clin. Pract. 2021, 11, 747-754. https://doi.org/10.3390/clinpract11040089
Aly R, Zeng X, Acharya R, Upadhyay K. Delayed Onset Minimal Change Disease as a Manifestation of Lupus Podocytopathy. Clinics and Practice. 2021; 11(4):747-754. https://doi.org/10.3390/clinpract11040089
Chicago/Turabian StyleAly, Rasha, Xu Zeng, Ratna Acharya, and Kiran Upadhyay. 2021. "Delayed Onset Minimal Change Disease as a Manifestation of Lupus Podocytopathy" Clinics and Practice 11, no. 4: 747-754. https://doi.org/10.3390/clinpract11040089
APA StyleAly, R., Zeng, X., Acharya, R., & Upadhyay, K. (2021). Delayed Onset Minimal Change Disease as a Manifestation of Lupus Podocytopathy. Clinics and Practice, 11(4), 747-754. https://doi.org/10.3390/clinpract11040089




