Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia
Abstract
:1. Introduction
2. Methods
Statistical Analysis
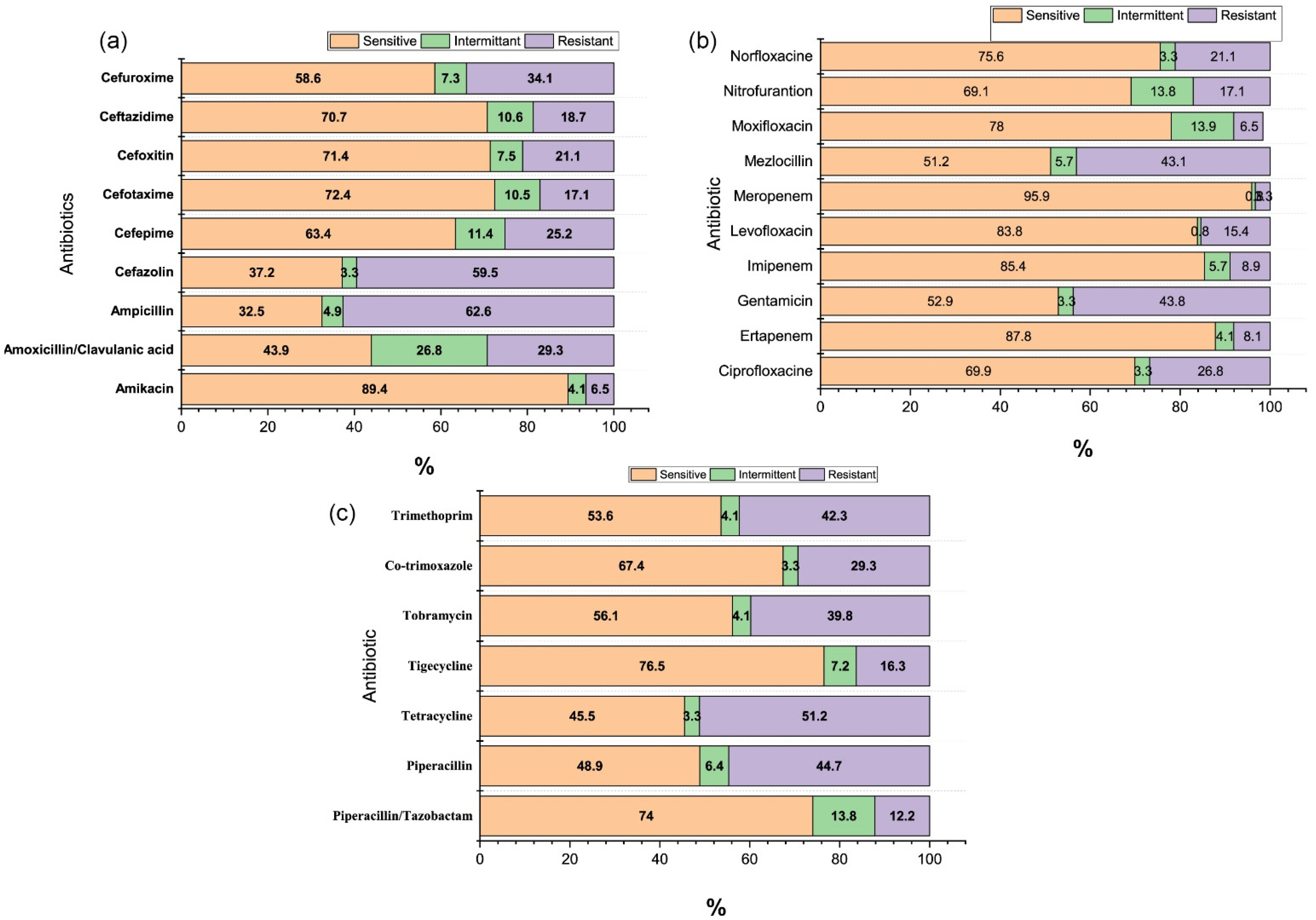
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mohammed, M.A.; Alnour, T.M.; Shakurfo, O.M.; Aburass, M.M. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac. J. Trop. Med. 2016, 9, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J. Antimicrob. Chemother. 1994, 33, 51–62. [Google Scholar] [CrossRef]
- Sewify, M.; Nair, S.; Warsame, S.; Murad, M.; Alhubail, A.; Behbehani, K.; Al-Refaei, F.; Tiss, A. Prevalence of Urinary Tract Infection and Antimicrobial Susceptibility among Diabetic Patients with Controlled and Uncontrolled Glycemia in Kuwait. J. Diabetes Res. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- WHO. Urinary Tract Infections. Available online: https://www.who.int/gpsc/information_centre/cauda-uti_eccmid.pdf (accessed on 13 January 2021).
- Dibua, U.M.; Onyemerela, I.S.; Nweze, E.I. Frequency, urinalysis and susceptibility profile of pathogens causing urinary tract infections in Enugu State, southeast Nigeria. Rev. Do Inst. Med. Trop. São Paulo 2014, 56, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.G.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Sarojamma, V.; Ramakrishna, V. Prevalence of ESBL-Producing Klebsiella pneumoniae Isolates in Tertiary Care Hospital. ISRN Microbiol. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A.; Kumar, A.K. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med. J. 2004, 25, 570–574. [Google Scholar]
- Mathai, D.; Jones, R.; Pfaller, M. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: A report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn. Microbiol. Infect. Dis. 2001, 40, 129–136. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Jones, M.E.; Thornsberry, C.; Critchley, I.; Kelly, L.J.; Sahm, D.F. Prevalence of antimicrobial resistance among urinary tract pathogens isolated from female outpatients across the US in 1999. Int. J. Antimicrob. Agents 2001, 18, 121–127. [Google Scholar] [CrossRef]
- Khan, A.U.; Zaman, M.S. Multiple drug resistance pattern in urinary tract infection patients in Aligarh. Biomed. Res. 2006, 17, 179–181. [Google Scholar]
- Kader, A.A.; Angamuthu, K. Extended-spectrum beta-lactamases in urinary isolates of Escherichia coli, Klebsiella pneumoniae and other gram-negative bacteria in a hospital in Eastern Province, Saudi Arabia. Saudi Med. J. 2005, 26, 956–959. [Google Scholar]
- Rawat, D.; Nair, D. Extended-spectrum ß-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2010, 2, 263–274. [Google Scholar] [CrossRef]
- Yadav, K.K.; Adhikari, N.; Khadka, R.; Pant, A.D.; Shah, B. Multidrug resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: A cross-sectional study in National Kidney Center, Nepal. Antimicrob. Resist. Infect. Control. 2015, 4, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharavi, M.J.; Zarei, J.; Roshani-Asl, P.; Yazdanyar, Z.; Sharif, M.; Rashidi, N. Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pana, Z.D.; Zaoutis, T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Research 2018, 7, 1347. [Google Scholar] [CrossRef] [PubMed]
- Norafika, N.A.; Arbianti, N.; Prihatiningsih, S.; Indriani, D.W. A retrospective cross-sectional study of urinary tract infections and prevalence of antibiotic resistant pathogens in patients with diabetes mellitus from a public hospital in Surabaya, Indonesia. Germs 2020, 10, 157–166. [Google Scholar] [CrossRef]
- Munoz-Dávila, M.J.; Roig, M.; Yagüe, G.; Blázquez, A.; Salvador, C.; Segovia, M. Comparative evaluation of Vitek 2 identification and susceptibility testing of urinary tract pathogens directly and isolated from chromogenic media. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 773–780. [Google Scholar] [CrossRef]
- Wilson, M.L.; Gaido, L. Laboratory Diagnosis of Urinary Tract Infections in Adult Patients. Clin. Infect. Dis. 2004, 38, 1150–1158. [Google Scholar] [CrossRef] [Green Version]
- Hay, A.D.; Birnie, K.; Busby, J.; Delaney, B.; Downing, H.; Dudley, J.; Durbaba, S.; Fletcher, M.; Harman, K.; Hollingworth, W.; et al. The Diagnosis of Urinary Tract infection in Young children (DUTY): A diagnostic prospective observational study to derive and validate a clinical algorithm for the diagnosis of urinary tract infection in children presenting to primary care with an acute illness. Health Technol. Assess. 2016, 20, 1–294. [Google Scholar]
- Al-Zahrani, J.; Al Dossari, K.; Gabr, A.H.; Ahmed, A.-F.; Al Shahrani, S.A.; Al-Ghamdi, S. Antimicrobial resistance patterns of Uropathogens isolated from adult women with acute uncomplicated cystitis. BMC Microbiol. 2019, 19, 237. [Google Scholar] [CrossRef]
- Ling, T.K.W.; Tam, P.C.; Liu, Z.K.; Cheng, A.F.B. Evaluation of VITEK 2 Rapid Identification and Susceptibility Testing System against Gram-Negative Clinical Isolates. J. Clin. Microbiol. 2001, 39, 2964–2966. [Google Scholar] [CrossRef] [Green Version]
- Ling, T.K.W.; Liu, Z.K.; Cheng, A.F.B. Evaluation of the VITEK 2 System for Rapid Direct Identification and Susceptibility Testing of Gram-Negative Bacilli from Positive Blood Cultures. J. Clin. Microbiol. 2003, 41, 4705–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Al-Juaid, N.F.; Alenzi, F.Q.; Mattar, E.H.; Bakheet, O.E.-S. Prevalence, antibiotic susceptibility pattern and production of extended-spectrum beta-lactamases amongst clinical isolates of Klebsiella pneumoniae at Armed Forces Hospital in Saudi Arabia. J. Coll. Physicians Surg. Pak. 2009, 19, 264–265. [Google Scholar] [PubMed]
- Balkhi, B.; Mansy, W.; Alghadeer, S.; Alnuaim, A.; AlShehri, A.; Somily, A. Antimicrobial susceptibility of microorganisms causing Urinary Tract Infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Belete, M.A.; Saravanan, M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020, 13, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.B.; Soni, S.T.; Bhagyalaxmi, A.; Patel, N.M. Causative agents of urinary tract infections and their antimicrobial susceptibility patterns at a referral center in Western India: An audit to help clinicians prevent antibiotic misuse. J. Fam. Med. Prim. Care 2019, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Jobin, K.; Kurts, C. Urinary tract infection: Recent insight into the evolutionary arms race between uropathogenic Escherichia coli and our immune system. Nephrol. Dial. Transplant. 2017, 32, 1977–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Wutayd, O.; Al Nafeesah, A.; Adam, I.; Babikir, I. The antibiotic susceptibility patterns of uropathogens isolated in Qassim, Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Shariq, A.; AlSalloom, A.A.; Babikir, I.H.; Alhomoud, B.N. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 2019, 13, 48–55. [Google Scholar]
- Aabed, K.; Moubayed, N.; Alzahrani, S. Antimicrobial resistance patterns among different Escherichia coli isolates in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 3776–3782. [Google Scholar] [CrossRef]
- Bandy, A.; Tantry, B. ESBL Activity, MDR, and Carbapenem Resistance among Predominant Enterobacterales Isolated in 2019. Antibiot. 2021, 10, 744. [Google Scholar] [CrossRef]
- Alamri, A.; Hassan, B.; Hamid, M.E. Susceptibility of hospital-acquired uropathogens to first-line antimicrobial agents at a tertiary health-care hospital, Saudi Arabia. Urol. Ann. 2021, 13, 166–170. [Google Scholar] [CrossRef]
- McIsaac, W.J.; Mazzulli, T.; Moineddin, R.; Raboud, J.; Ross, S. Uropathogen Antibiotic Resistance in Adult Women Presenting to Family Physicians with Acute Uncomplicated Cystitis. Can. J. Infect. Dis. Med Microbiol. 2004, 15, 266–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age Group | % Frequency (n = 123) |
|---|---|
| 18–30 | 48.8 (60) |
| 31–40 | 35.8 (44) |
| 41–50 | 8.9 (11) |
| Above 50 | 6.5 (8) |
| Microorganism (%) | Total n = 123 | Age Group | |||||
|---|---|---|---|---|---|---|---|
| 18–25 | 26–30 | 31–35 | 36–40 | 41–45 | Above 45 | ||
| Acinetobacter baumannii | 4 (3.3) | 2 (50) | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Citrobacter amalonaticus | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enterobacter aerogenes | 1 (0.8) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Enterobacter amnigenus | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Empedobacter brevis | 1 (0.8) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Enterobacter cloacae | 4 (3.3) | 0 (0) | 0 ((0) | 0 (0) | 1 (25%) | 1 (25) | 2 (50) |
| Escherichia coli | 72 (58.5) | 14 (19.4) | 18 (25) | 16 (22.2) | 11 (15.3) | 6 (8.3) | 7 (9.7) |
| Klebsiella oxytoca | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Klebsiella pneumoniae | 10 (8.1) | 2 (20) | 5 (50) | 2 (20) | 1 (10) | 0 (0) | 0 (0) |
| Kocuria kristinae | 2 (1.6) | 1 (50) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Proteus mirabilis | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Providencia rettgeri | 1 (0.8) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Providencia stuartii | 3 (2.4) | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pseudomonas aeruginosa | 2 (1.6) | 1 (50) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
| Pseudomonas oryzihabitans | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Salmonella sp | 2 (1.6) | 1 (50) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
| Serratia marcescens | 2 (1.6) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sphingomonas paucimobilis | 5 (4.1) | 2 (40) | 0 (0) | 1 (20) | 0 (0) | 1 (20) | 1 (20) |
| Staphylococcus epidermidis | 5 (4.1) | 1 (20) | 0 (0) | 3 (60) | 0 (0) | 1 (20) | 0 (0) |
| Staphylococcus haemolyticus | 2 (1.6) | 1 (50) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Staphylococcus hominis | 1 (0.8) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Streptococcus agalactiae | 1 (0.8) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Microorganism (%) | Amikacin | Amox + Clav | Ampicillin | Cefazolin | Cefepime | Cefotaxime | Cefoxitin | Ceftazidime | Cefuroxime |
|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 1 (25) | 3 (75) | 3 (75) | 3 (75) | 0 (0) | 0 (0) | 2 (50) | 1 (25) | 2 (50) |
| Citrobacter amalonaticus | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Enterobacter aerogenes | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Enterobacter amnigenus | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Empedobacter brevis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enterobacter cloacae | 0 (0) | 2 (50) | 3 (75) | 3 (75) | 1(25) | 1 (25) | 2 (50) | 2 (50) | 4 (100) |
| Escherichia coli | 0 (0) | 9 (12.5) | 41 (59.6) | 25 (34.7) | 16 (22.2) | 4 (5.6) | 5 (6.9) | 4 (5.6) | 17 (23.6) |
| Klebsiella oxytoca | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) |
| Klebsiella pneumoniae | 0 (0) | 3 (30) | 9 (90) | 4 (40) | 0 (0) | 1 (10) | 2 (20) | 2 (20) | 2 (20) |
| Kocuria kristinae | 0 (0) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 2 (100) | 1 (50) | 1 (50) | 2 (100) |
| Proteus mirabilis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Providencia rettgeri | 0 (0.0) | 1 (100) | 1 (100) | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (100) | 1 (100) |
| Providencia stuartii | 1 (33.3) | 3 (100) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) | 0 (0) | 2 (66,7) |
| Pseudomonas aeruginosa | 0 (0) | 2 (100) | 2(100) | 2 (100) | 2 (100) | 2(100) | 2(100) | 2(100) | 2 (100) |
| Pseudomonas oryzihabitans | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) |
| Salmonellasp | 1 (50) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 1 (50) | 1 (50) |
| Serratia marcescens | 1 (50) | 1 (50%) | 2 (100) | 2 (100) | 1 (50) | 1 (50) | 0 (0) | 2 (100) | 2 (100) |
| Sphingomonas paucimobilis | 1 (20) | 3 (60%) | 3 (60) | 1 (20) | 1 (20) | 1 (20) | 1 (20) | 2 (40) | 1 (20) |
| Staphylococcus epidermidis | 1 (20) | 2 (40%) | 2 (40) | 2 (40) | 1 (20) | 1 (20) | 1 (20) | 2 (40) | 1 (20) |
| Staphylococcus haemolyticus | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| Staphylococcus hominis | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Streptococcus agalactiae | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Microorganism (%) | Ciprofloxacin | Ertapenem | Gentamicin | Imipenem | Levofloxacin | Meropenem | Mezlocillin | Moxifloxacin | Nitrofurantoin |
|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 3 (75) | 2 (50) | 3 (75) | 1 (25) | 2 (50) | 2 (50) | 2 (50) | 0 (0) | 2 (50) |
| Citrobacter amalonaticus | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Enterobacter aerogenes | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Enterobacter amnigenus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Empedobacter brevis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0(0) | 0 (0) | 0 (0) |
| Enterobacter cloacae | 3 (75) | 1 (25) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 4 (100) | 1 (25) | 2 (50) |
| Escherichia coli | 5 (6.9) | 0 (0) | 13 (18.1) | 2 (2.8) | 3 (4.2) | 1 (1.4) | 35 (48.6) | 3 (4.2) | 0 (0) |
| Klebsiella oxytoca | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Klebsiella pneumoniae | 4 (40) | 0 (0) | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 7 (70) | 0 (0) | 1 (10) |
| Kocuria kristinae | 1 (50) | 1 (50) | 2 (100) | 1 (50) | 2 (100) | 0 (0) | 1 (50) | 1 (50) | 1 (50) |
| Proteus mirabilis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (100) |
| Providencia rettgeri | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Providencia stuartii | 3 (100) | 1 (33.3) | 3 (100) | 0 (0) | 2 (66.6) | 1 (33.3) | 0 (0) | 0 (0) | 3 (100) |
| Pseudomonas aeruginosa | 2(100) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| Pseudomonas oryzihabitans | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Salmonella sp | 2 (100) | 1 (50) | 2 (100) | 1 (50) | 2 (100) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Serratia marcescens | 0 (0) | 0 (0) | 1 (50) | 2 (100) | 1 (50) | 0 (0) | 0 (0) | 1 (50) | 0 (0) |
| Sphingomonas paucimobilis | 2 (40) | 1 (20) | 2 (40) | 1 (20) | 1 (20) | 0 (0) | 0 (0) | 1 (20) | 2 (40) |
| Staphylococcus epidermidis | 2 (40) | 0 (0) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20) |
| Staphylococcus haemolyticus | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| Staphylococcus hominis | 0 (0) | 0 (0) | 0 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Streptococcus agalactiae | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Microorganism (%) | Norfloxacin | Piperacillin-Tazobactam | Piperacillin | Tetracycline | Tigecycline | Tobramycin | Co-trimoxazole | Trimethoprim |
|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 2 (50) | 3 (75) | 2 (50) | 2 (50) | 2 (50) | 2 (50) | 3 (75) | 3 (75) |
| Citrobacter amalonaticus | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) |
| Enterobacter aerogenes | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) |
| Enterobacter amnigenus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0 | 1 (100) | 0 (0) |
| Empedobacter brevis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) |
| Enterobacter cloacae | 2 (50) | 0 (0) | 3 (75) | 4 (100) | 0 (0) | 2 (50) | 3 (75) | 2 (50) |
| Escherichia coli | 4 (5.6) | 7 (9.7)) | 33 (45.8) | 35 (48.6) | 5 (6.9) | 19 (26.4) | 38 (52.8) | 32 (44.4) |
| Klebsiella oxytoca | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) |
| Klebsiella pneumoniae | 3 (30) | 1 (10) | 7 (70) | 4 (40) | 2 (20) | 5 (50) | 5 (50) | 4 (40) |
| Kocuria kristinae | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 1 (50) | 2 (100) | 1 (50) | 0 (0) |
| Proteus mirabilis | 0 (0) | 0 (0) | 0 (0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Providencia rettgeri | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| Providencia stuartii | 3 (100) | 2 (66.7) | 1 (33.3) | 3 (100) | 1 (33.3) | 2 (66.7) | 3 (100) | 1 (33.3) |
| Pseudomonas aeruginosa | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 2 (100) | 2 (100) | 0 (0) |
| Pseudomonas oryzihabitans | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| Salmonella sp | 1 (50) | 0 (0) | 0 (0) | 2 (100) | 1 (50) | 2 (100) | 2 (100) | 0 (0) |
| Serratia marcescens | 0 (0) | 1 (50) | 2 (100) | 1 (50) | 1 (50) | 2 (100) | 2 (100) | 2 (100) |
| Sphingomonas paucimobilis | 1 (20) | 0 (0) | 1 (20) | 2 (40) | 1 (20) | 2 (40) | 2 (40) | 1 (20) |
| Staphylococcus epidermidis | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 4 (80) | 5 (100) | 1 (20) |
| Staphylococcus haemolyticus | 2 (100) | 0 (0) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| Staphylococcus hominis | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Streptococcus agalactiae | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmary, M.Y. Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clin. Pract. 2021, 11, 650-658. https://doi.org/10.3390/clinpract11030080
Alasmary MY. Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clinics and Practice. 2021; 11(3):650-658. https://doi.org/10.3390/clinpract11030080
Chicago/Turabian StyleAlasmary, Mohammed Yahia. 2021. "Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia" Clinics and Practice 11, no. 3: 650-658. https://doi.org/10.3390/clinpract11030080
APA StyleAlasmary, M. Y. (2021). Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clinics and Practice, 11(3), 650-658. https://doi.org/10.3390/clinpract11030080





