Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma
Abstract
:1. Introduction
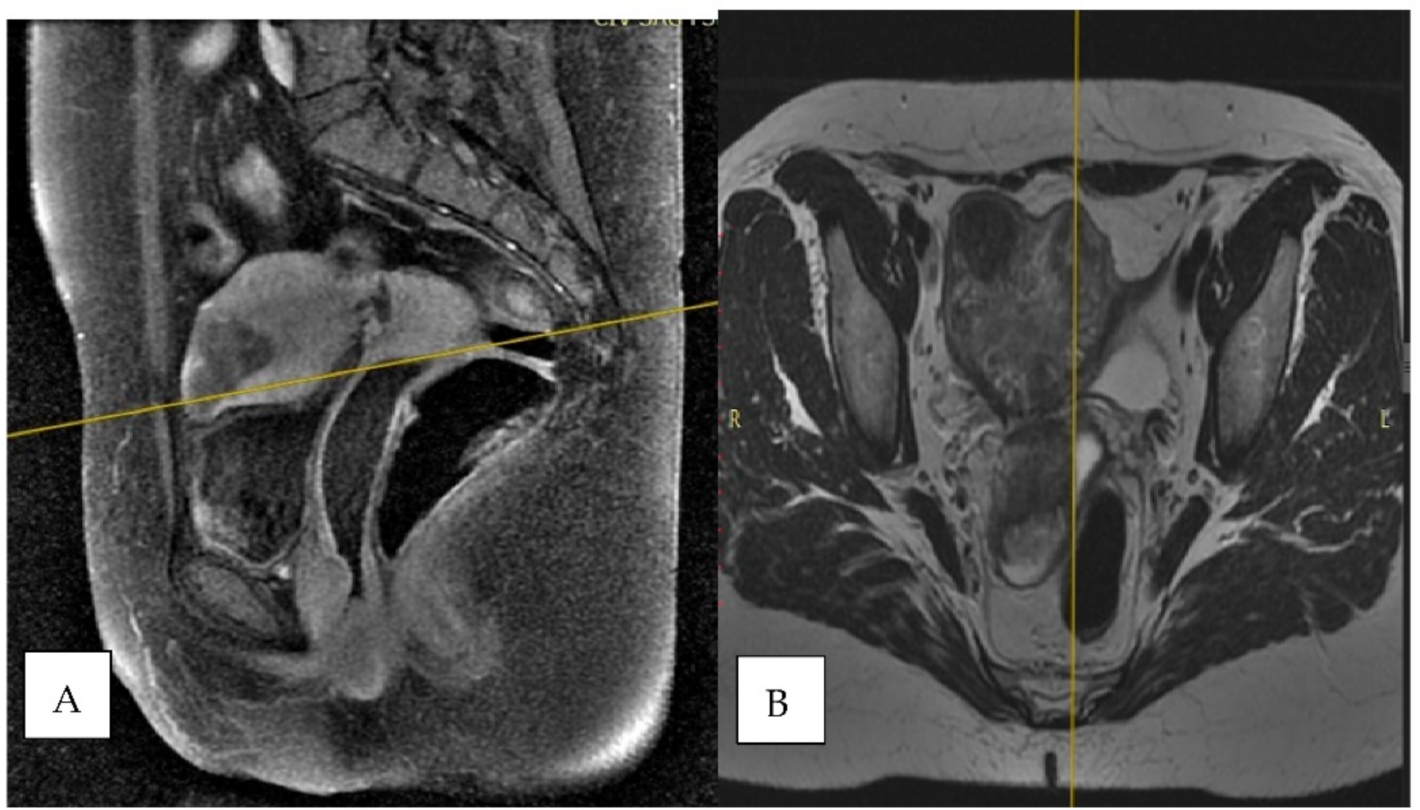
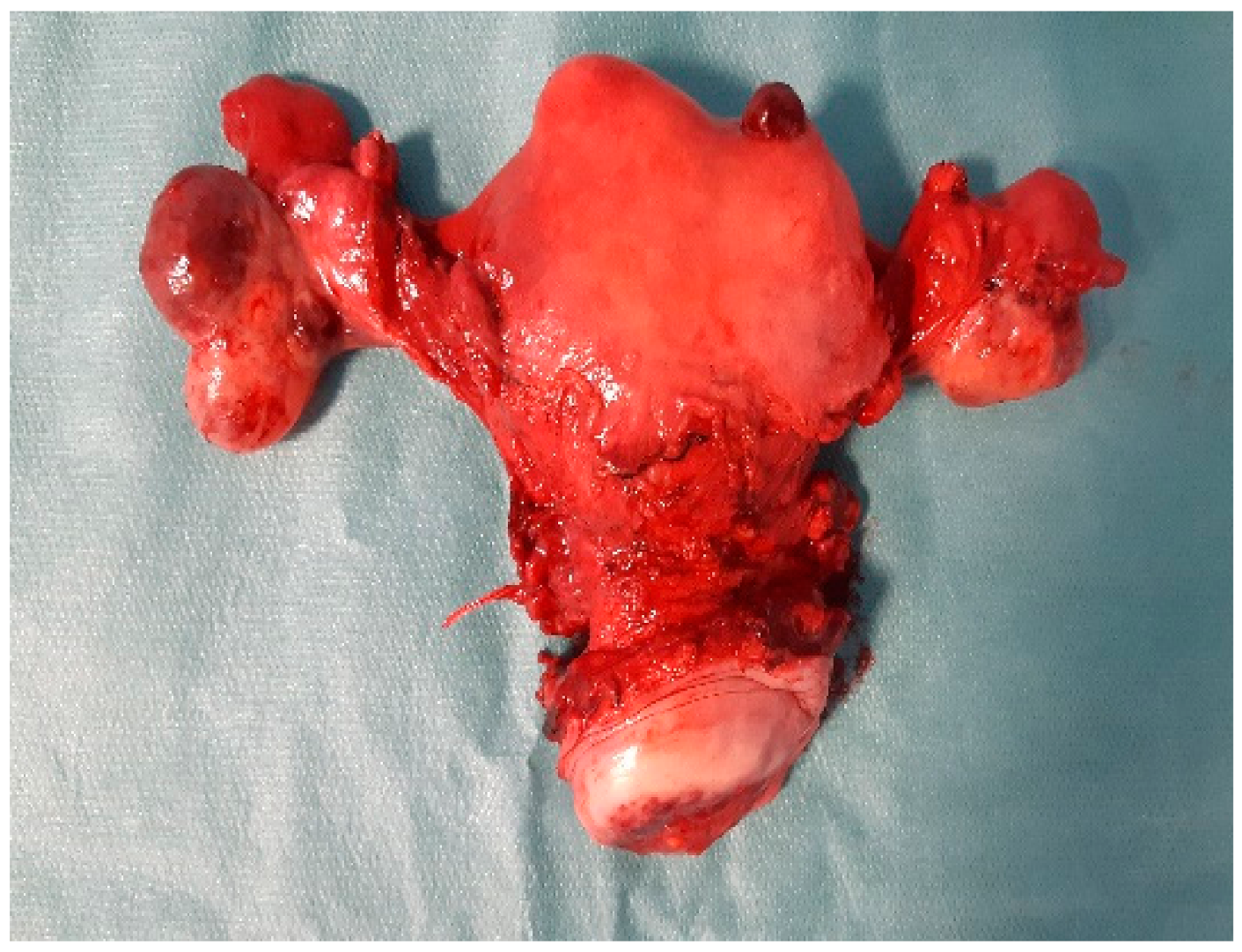
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clement, P.B.; Stall, J.N.; Young, R.H. Atlas of Gynecologic Surgical Pathology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 314–316. [Google Scholar]
- Shih, I.-M.; Kurman, R.J. Epithelioid trophoblastic tumour. A neoplasm distinct from choriocarcinoma and placental site trophoblastic tumour simulating carcinoma. Am. J. Surg. Pathol. 1998, 22, 1393–1403. [Google Scholar] [CrossRef]
- Genest, D.R.B.R.; Fisher, R.A. Gestational trophoblastic disease. In WHO Classification of Tumours Pathology and Genetics of Tumours of the Breast and Female Genital Organs; Tavassoli, F.A., Ed.; IARC Press: Lyon, France, 2003; pp. 250–254. [Google Scholar]
- Lybol, C.; Thomas, C.M.; Bulten, J.; van Dijck, J.A.; Sweep, F.C.; Massuger, L.F. Increase in the incidence of gestational trophoblastic disease in The Netherlands. Gynecol. Oncol. 2011, 121, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Y.; Wan, X.; Qian, C.; Zhou, C.; Chen, X. Epithelioid trophoblastic tumour: A clinico-pathological and immunohistochemical study of seven cases. Med. Oncol. 2011, 28, 294–299. [Google Scholar]
- Vencken, P.M.; Ewing, P.C.; Zweemer, R.P. Epithelioid trophoblastic tumor: A case report and review of literature. J. Clin. Pathol. 2006, 59, 1307–1308. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.J.; Cheng, C.J.; Chen, W.Y. Transformation of a post-cesarean section placental site nodule into a coexisting epithelioid trophoblastic tumor and placental site trophoblastic tumor: A case report. Diagn. Pathol. 2013, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; An, S.; Jang, S.J.; Kim, H.R. Extrauterine epithelioid trophoblastic tumor of lung in a 35-year-old woman. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 471–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, E.M.; Smith, A.L.; Desouki, M.M.; Olawaiye, A.B. Epithelioid trophoblastic tumor: A case report and review of the literature. Case Rep. Obstet. Gynecol. 2012, 2012, 862472. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, J.; Zhang, Y.; Xiong, H.; Sheng, X. Epithelioid trophoblastic tumor coexisting with choriocarcinoma around an abdominal wall cesarean scar: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 178. [Google Scholar] [CrossRef]
- Almarzooqi, S.; Ahmad Al-Safi, R.; Fahad Al-Jassar, W.; Akhter, S.M.J.; Chiab-Rassou, Y.; Albawardi, A. Epithelioid trophoblastic tumor: Report of two cases in postmenopausal women with literature review and emphasis on cytological findings. Acta Cytol. 2014, 58, 198–210. [Google Scholar] [CrossRef]
- Stănculescu, R.V.; Bauşic, V.; Vlădescu, T.C.; Vasilescu, F.; Brătilă, E. Epithelioid trophoblastic tumor: A case report and literature review. Rom. J. Morphol. Embryol. 2016, 57, 1365–1370. [Google Scholar]
- McGregor, S.M.; Furtado, L.V.; Montag, A.G.; Brooks, R.; Lastra, R.R. Expanding the Clinicopathologic Spectrum of a Rare Malignancy. Int. J. Gynecol. Pathol. 2020, 39, 8–18. [Google Scholar] [CrossRef]
- Lewin, S.N.; Aghajanian, C.; Moreira, A.L.; Soslow, R.A. Extrauterine epithelioid trophoblastic tumours presenting as primary lung carcinomas. Morphologic and immunohistochemical features to resolve a diagnostic dilemma. Am. J. Surg. Pathol. 2009, 33, 1809–1814. [Google Scholar] [CrossRef]
- Hsiue, E.H.-C.; Hsu, C.; Tseng, L.-H.; Lu, T.-P.; Kuo, K.-T. Epithelioid trophoblastic tumour around an abdominal cesarean scar: A pathologic and molecular genetic analysis. Int. J. Gynecol. Pathol. 2017, 36, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.pathologyoutlines.com/topic/placentaETT.html (accessed on 21 May 2021).
- Mirkovic, J.; Garcia, E.; Sholl, L.M.; Lindeman, N.I.; MacConaill, L.E.; Dong, F.; Quade, B.J.; Horowitz, N.S.; Crum, C.P.; Howitt, B. Targeted genomic profiling and PD-L1 expresion in epithelioid trophoblastic tumours and placental site trophoblastic tumours. Abstract. Mod. Pathol. 2017, 30, 302A. [Google Scholar]
- Veras, E.; Kurman, R.J.; Wang, T.-L.; Shih, I.-M. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int. J. Gynecol. Pathol. 2017, 36, 146–153. [Google Scholar] [CrossRef] [Green Version]
- WHO. Classification of Tumours Editorial Board. In Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4, pp. 323–326. [Google Scholar]
- Frijstein, M.M.; Lok, C.A.R.; van Trommel, N.E.; Ten Kate-Booij, M.J.; Massuger, L.F.A.G.; van Werkhoven, E.; Kaur, B.; Tidy, J.A.; Sarwar, N.; Golfier, F.; et al. Management and prognostic factors of epithelioid trophoblastic tumours: Results from the International Society for the Study of Trophoblastic Diseases database. Gynecol. Oncol. 2019, 152, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Froeling, F.E.; Ramaswami, M.; Papanastasopoulos, R.; Kaur, B.; Sebire, N.J.; Short, D.; Fisher, R.A.; Sarwar, N.; Wells, M.; Singh, K.; et al. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br. J. Cancer 2019, 120, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Xiang, Y.; Guo, L.; Ren, T.; Feng, F.; Wan, X.; Xiao, Y. Analysis of clinicopathologic prognostic factors in 9 patients with epithelioid trophoblastic tumour. Int. J. Gynecol. Cancer 2011, 21, 1124–1130. [Google Scholar] [CrossRef]
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Placental site trophoblastic tumour and epithelioid trophoblastic tumour: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol. Oncol. 2019, 153, 684–693. [Google Scholar] [CrossRef]
- Davis, M.R.; Howitt, B.E.; Quade, B.J.; Crum, C.P.; Horowitz, N.S.; Goldstein, D.P.; Berkowitz, R.S. Epithelioid trophoblastic tumour: A single institution case series at the New England Trophoblastic Disease Center. Gynecol. Oncol. 2015, 137, 456–461. [Google Scholar] [CrossRef]
- Taylor, F.H.B. Pharmacotherapy of placental site and epithelioid trophoblastic tumours. Expert Opin. Orphan Drugs 2015, 3, 75–78. [Google Scholar] [CrossRef]
- Yang, J.; Zong, L.; Wang, J.; Feng, F.; Xiang, Y. Epithelioid Trophoblastic Tumours: Treatments, Outcomes, and Potential Therapeutic Targets. J. Cancer 2019, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]

| Case No. | Publication | Age | Recent Gestation History | Interval to Antecedent Pregnancy | Maximum Serum β-hCG | Symptom | Management |
|---|---|---|---|---|---|---|---|
| 1 | Vencken et al. [6] | 43 | Abortion | 13 years | <2 | Amenorrhoea | surgery |
| 2 | Chen et al. [7] | 42 | Term | 1 year | 1 week after surgery | Vaginal Bleeding | surgery |
| 3 | Kim et al. [8] | 35 | Term | - | N | Abdominal pain | surgery |
| 4 | Scott et al. [9] | 44 | Term | 4 years | N | Abdominal pain | surgery |
| 5 | Yang et al. [10] | 39 | Term | 9 years | N | Abdominal mass | surgery and adjuvant chemotherapy |
| 6 | Almarzooqi et al. [11] | 47 | – | – | N | Vaginal Bleeding | surgery |
| 7 | Stănculescu et al. [12] | 35 | Abortion | 10 years | N | Vaginal Bleeding | surgery |
| 8 | McGregor et al. [13] | 68 | Term | 30 years | N | Vaginal Bleeding | surgery |
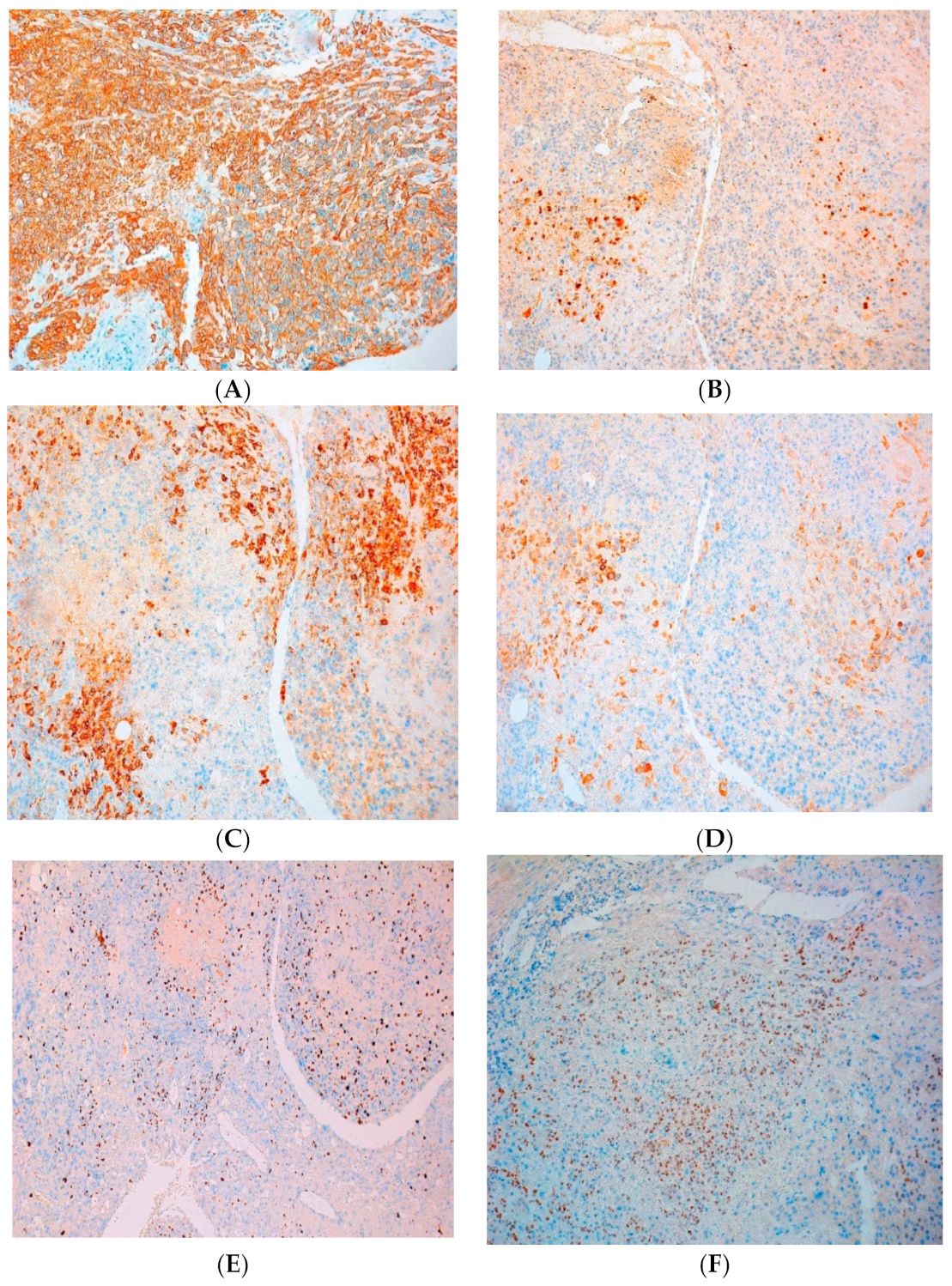
| Immunohistochemical Stain | PSTT | ETT | Results for Our Case |
|---|---|---|---|
| CD10 | positive | positive | intense and diffuse positive in tumour cells |
| p63 | negative | positive | positive, zonal extended in tumour cells |
| CKAE1/AE3 | positive | positive | diffusely positive in tumour cells |
| EMA | positive | positive | positive in focus in tumour cells |
| Ki67 | 5–10% | >10% | positive in 50% of tumor cells |
| Cyclin D1 | negative | positive | positive in focus in tumor cells |
| Inhibin alpha | negative | positive | focal positive in tumor cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniţei, M.-G.; Lazăr, D.-E.; Pleşca, R.A.; Terinte, C.; Iulian, R.; Viorel, S. Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma. Clin. Pract. 2021, 11, 631-639. https://doi.org/10.3390/clinpract11030078
Aniţei M-G, Lazăr D-E, Pleşca RA, Terinte C, Iulian R, Viorel S. Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma. Clinics and Practice. 2021; 11(3):631-639. https://doi.org/10.3390/clinpract11030078
Chicago/Turabian StyleAniţei, Maria-Gabriela, Diana-Elena Lazăr, Raluca Alina Pleşca, Cristina Terinte, Radu Iulian, and Scripcariu Viorel. 2021. "Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma" Clinics and Practice 11, no. 3: 631-639. https://doi.org/10.3390/clinpract11030078
APA StyleAniţei, M.-G., Lazăr, D.-E., Pleşca, R. A., Terinte, C., Iulian, R., & Viorel, S. (2021). Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma. Clinics and Practice, 11(3), 631-639. https://doi.org/10.3390/clinpract11030078






