Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results/Discussion
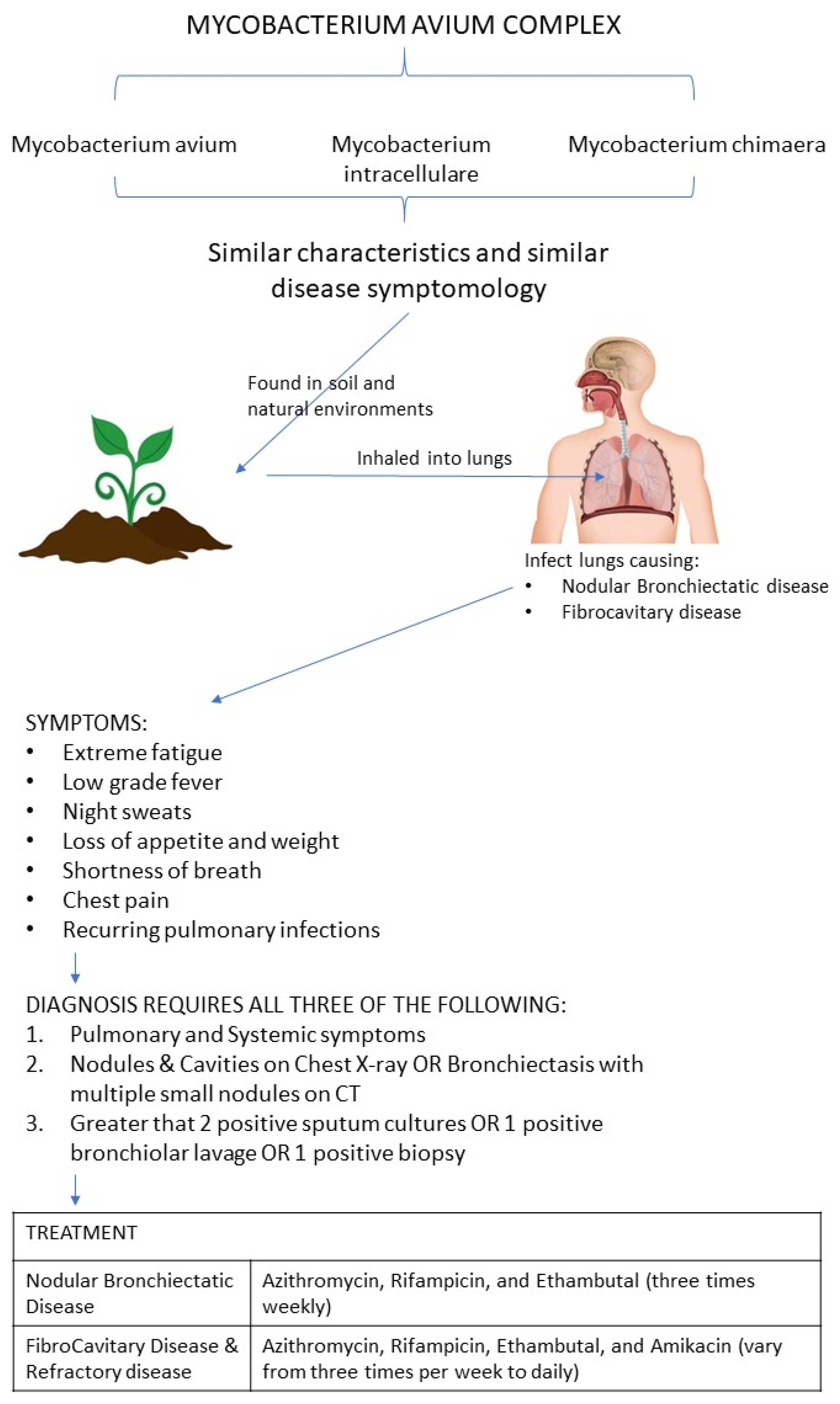
3.1. Pathogenesis of Mycobacterium avium Complex (MAC)
3.2. Prevalence of Mycobacterium avium Complex (MAC)
3.3. MAC Assessment Toola and Clinical Diagnosis of Mycobacterium avium Complex (MAC) Infection
3.4. Treatment of Mycobacterium avium Complex (MAC) Infection
3.5. Pathogenesis of Chronic Obstructive Pulmonary Disease (COPD)
3.6. Immunosuppressant Effects of Steroids in COPD Patients
3.7. Redox Imbalance in COPD Patients
3.8. Glutathione Deficiency in COPD and MAC Infected Patients
3.9. Increased Susceptibility for MAC Infections among COPD Patients
3.10. Immune Alterations Due to MAC
3.11. Parallels between HIV-Aids Patient’s Systemic Immunosuppression and COPD Induced Localized Immunosuppression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matesanz Lopez, C.; Loras Gallego, C.; Cacho Calvo, J.; Thuissard Vasallo, I.J.; Rio Ramirez, M.T. Patients with non-tuberculous mycobacteria in respiratory samples: A 5-year epidemiological study. Rev. Esp. Quimioter. 2021, 34, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Auster, L.; Sutton, M.; Gwin, M.C.; Nitkin, C.; Bonfield, T.L. Optimization of In Vitro Mycobacterium avium and Mycobacterium intracellulare Growth Assays for Therapeutic Development. Microorganisms 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfyffer, G.E.; Inderlied, C.B. Chapter 174—Mycobacteria. Mosby 2010, 1777–1800. [Google Scholar] [CrossRef]
- Meier, E.; Pennington, K.; Gallo de Moraes, A.; Escalante, P. Characteristics of Mycobacterium avium complex (MAC) pulmonary disease in previously treated lung cancer patients. Respir Med. Case Rep. 2017, 22, 70–73. [Google Scholar] [CrossRef]
- Swenson, C.; Zerbe, C.S.; Fennelly, K. Host Variability in NTM Disease: Implications for Research Needs. Front. Microbiol. 2018, 9, 2901. [Google Scholar] [CrossRef]
- Bachofner, J.A.; Ikenberg, K.; Schulthess, B.; Nemeth, J. Disseminated Mycobacterium simiae and Mycobacterium avium infection causing an immune reconstitution inflammatory syndrome in a female patient with HIV infection. BMJ Case Rep. 2021, 14, e241037. [Google Scholar] [CrossRef]
- Shu, C.C.; Wang, J.Y.; Wu, M.F.; Wu, C.T.; Lai, H.C.; Lee, L.N.; Chiang, B.L.; Yu, C.J. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci. Rep. 2017, 7, 42004. [Google Scholar] [CrossRef]
- Schuurbiers, M.M.F.; Bruno, M.; Zweijpfenning, S.M.H.; Magis-Escurra, C.; Boeree, M.; Netea, M.G.; van Ingen, J.; van de Veerdonk, F.; Hoefsloot, W. Immune defects in patients with pulmonary Mycobacterium abscessus disease without cystic fibrosis. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef]
- Dronamraju, V.; Singh, N.; Poon, J.; Shah, S.; Gorga, J.; Ojeda-Martinez, H.; McFarlane, S. Assessment of Bronchiectasis in HIV Patients among an Urban Population. Case Rep. Pulmonol. 2020, 2020, 8903809. [Google Scholar] [CrossRef]
- Pennington, K.M.; Vu, A.; Challener, D.; Rivera, C.G.; Shweta, F.N.U.; Zeuli, J.D.; Temesgen, Z. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J. Clin. Tuberc. Other Mycobact. Dis 2021, 24, 100244. [Google Scholar] [CrossRef]
- Prato, B.D.; Altieri, A.M.; Carlucci, B.; Mori, P.A.; Parrella, R.; Stainer, A.; Giacomi, F.; Pesci, A.; Faverio, P.; On Behalf of Gruppo di Studio AIPO “Patologie Infettive Respiratorie e Tubercolosi”. Non-tuberculous mycobacterial pulmonary disease: An Italian National survey. Sarcoidosis Vasc. Diffuse Lung Dis. 2018, 35, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.D.; McAdam, K.P. The re-emergence of tuberculosis. Annu. Rev. Public Health 1994, 15, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Vande Weygaerde, Y.; Cardinaels, N.; Bomans, P.; Chin, T.; Boelens, J.; André, E.; Van Braeckel, E.; Lorent, N. Clinical relevance of pulmonary non-tuberculous mycobacterial isolates in three reference centres in Belgium: A multicentre retrospective analysis. BMC Infect. Dis. 2019, 19, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringshausen, F.C.; Wagner, D.; de Roux, A.; Diel, R.; Hohmann, D.; Hickstein, L.; Welte, T.; Rademacher, J. Prevalence of Nontuberculous Mycobacterial Pulmonary Disease, Germany, 2009–2014. Emerg. Infect. Dis. 2016, 22, 1102–1105. [Google Scholar] [CrossRef]
- Ringshausen, F.C.; Apel, R.M.; Bange, F.C.; de Roux, A.; Pletz, M.W.; Rademacher, J.; Suhling, H.; Wagner, D.; Welte, T. Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005–2011. BMC Infect. Dis. 2013, 13, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Lee, E.H.; Jung, I.; Park, G.; Kang, Y.A. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium avium complex pulmonary disease: A systematic review and meta-analysis. Respir Res. 2019, 20, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, H.; Kinjo, T.; Nei, Y.; Yamashiro, S.; Fujita, J.; Kishaba, T. Causative species of nontuberculous mycobacterial lung disease and comparative investigation on clinical features of Mycobacterium abscessus complex disease: A retrospective analysis for two major hospitals in a subtropical region of Japan. PLoS ONE 2017, 12, e0186826. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, M.D.S.; Nunes, L.S.; David, S.M.M.; Dias, C.F.; Barth, A.L.; Unis, G. Nontuberculous mycobacterial lung disease in a high tuberculosis incidence setting in Brazil. J. Bras. Pneumol. 2018, 44, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Tabusadani, M.; Yamane, K.; Kakuta, T.; Kuroyama, Y.; Mori, K.; Ono, K.; Omatsu, S.; Kawahara, K.; Toyoda, Y.; et al. Is the Leicester Cough Questionnaire useful for nontuberculous mycobacterial lung disease? Respir. Investig 2021, 59, 120–125. [Google Scholar] [CrossRef]
- Weatherall, M.; Marsh, S.; Shirtcliffe, P.; Williams, M.; Travers, J.; Beasley, R. Quality of life measured by the St George’s Respiratory Questionnaire and spirometry. Eur. Respir. J. 2009, 33, 1025–1030. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.X.; Shao, C.; Li, S.; Xu, K.; Huang, H.; Xu, Z.J. The clinical analysis of chronic obstructive pulmonary disease patients complicated with nontuberculous mycobacterial pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2019, 42, 826–831. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Bottger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Fiogbe, A.A.; Liistro, G.; Hoton, D.; Pieters, T. Mycobacterium avium tumoral infection mimicking a lung adenocarcinoma: A potential diagnostic pitfall. Rev. Pneumol. Clin. 2016, 72, 147–151. [Google Scholar] [CrossRef]
- Wyrostkiewicz, D.; Szturmowicz, M.; Bartoszuk, I.; Siemion-Szczesniak, I.; Jakubowska, L.; Augustynowicz-Kopec, E.; Kus, J. Nontuberculous mycobacterial lung disease in a patient with COPD and bronchiectasis, with radiological signs of lung tumor. Adv. Respir Med. 2018, 86, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Rajdev, K.; Pervaiz, S.; Hasan Siddiqui, A.; Azam, M.; Chalhoub, M. Pulmonary Cavitary Disease Secondary to Mycobacterium xenopi Complicated by Respiratory Failure. Cureus 2018, 10, e3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brode, S.K.; Ling, S.C.; Chapman, K.R. Alpha-1 antitrypsin deficiency: A commonly overlooked cause of lung disease. CMAJ 2012, 184, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Taraseviciene-Stewart, L.; Voelkel, N.F. Molecular pathogenesis of emphysema. J. Clin. Investig. 2008, 118, 394–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, K.R.; Dharwal, V.; Patel, V.K.; Galvao, I.; Wadhwa, R.; Malyla, V.; Shen, S.S.; Budden, K.F.; Hansbro, N.G.; Vaughan, A.; et al. Role of Lung Microbiome in Innate Immune Response Associated With Chronic Lung Diseases. Front. Med. 2020, 7, 554. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Wong Fok Lung, T.; Prince, A. Pulmonary Pathogens Adapt to Immune Signaling Metabolites in the Airway. Front. Immunol 2020, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Falk, J.A.; Minai, O.A.; Mosenifar, Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Liu, V.X.; Winthrop, K.L.; Lu, Y.; Sharifi, H.; Nasiri, H.U.; Ruoss, S.J. Association between Inhaled Corticosteroid Use and Pulmonary Nontuberculous Mycobacterial Infection. Ann. Am. Thorac. Soc. 2018, 15, 1169–1176. [Google Scholar] [CrossRef]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef]
- Scheinman, R.I.; Cogswell, P.C.; Lofquist, A.K.; Baldwin, A.S., Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 1995, 270, 283–286. [Google Scholar] [CrossRef] [Green Version]
- Ayroldi, E.; Riccardi, C. Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB J. 2009, 23, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Reynaud, Q.; Bricca, R.; Cavalli, Z.; Nove-Josserand, R.; Durupt, S.; Reix, P.; Burgel, P.R.; Durieu, I. Risk factors for nontuberculous mycobacterial isolation in patients with cystic fibrosis: A meta-analysis. Pediatr. Pulmonol. 2020, 55, 2653–2661. [Google Scholar] [CrossRef]
- Fernandez Fernandez, E.; De Santi, C.; De Rose, V.; Greene, C.M. CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2018, 12, 483–492. [Google Scholar] [CrossRef]
- Robinson, B.; Munjal, S.; D’Agostino, J.; Venketaraman, V. Liposomal-Glutathione as a Potential Therapeutic Agent to Control HIV-1 Infection and Tuberculosis. Eur. Med. J. 2018, 3, 62–69. [Google Scholar]
- King, E.M.; Weaver, V.K.; Kestler, M.H. Treatment Dilemmas in Disseminated Nontuberculous Mycobacterial Infections With Interferon-gamma Autoantibodies. Open Forum Infect. Dis. 2021, 8, ofab253. [Google Scholar] [CrossRef] [PubMed]
- Dotta, L.; Vairo, D.; Giacomelli, M.; Moratto, D.; Tamassia, N.; Vermi, W.; Lonardi, S.; Casanova, J.L.; Bustamante, J.; Giliani, S.; et al. Transient Decrease of Circulating and Tissular Dendritic Cells in Patients With Mycobacterial Disease and with Partial Dominant IFNgammaR1 Deficiency. Front. Immunol. 2020, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.M.; Chadwick, M.V.; Nicholson, A.G.; Wilson, R.; Thornton, D.J.; Kirkham, S.; Sheehan, J.K. Interaction between mycobacteria and mucus on a human respiratory tissue organ culture model with an air interface. Exp. Lung Res. 2004, 30, 17–29. [Google Scholar] [CrossRef]
- Balavoine, C.; Andrejak, C.; Marchand-Adam, S.; Blanc, F.X. Relationships between COPD and nontuberculous mycobacteria pulmonary infections. Rev. Mal. Respir. 2017, 34, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Secott, T.E.; Lin, T.L.; Wu, C.C. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 2002, 70, 2670–2675. [Google Scholar] [CrossRef] [Green Version]
- Hojo, M.; Iikura, M.; Hirano, S.; Sugiyama, H.; Kobayashi, N.; Kudo, K. Increased risk of nontuberculous mycobacterial infection in asthmatic patients using long-term inhaled corticosteroid therapy. Respirology 2012, 17, 185–190. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Chong, S.; Jung, J.W.; Park, I.W.; Choi, B.W.; Lim, C.; Lee, C.U.; Kim, Y.S.; Choi, H.W.; Choi, J.C. Natural course of the nodular bronchiectatic form of Mycobacterium avium complex lung disease: Long-term radiologic change without treatment. PLoS ONE 2017, 12, e0185774. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munjal, S.; Munjal, S.; Gao, J.; Venketaraman, V. Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients. Clin. Pract. 2021, 11, 619-630. https://doi.org/10.3390/clinpract11030077
Munjal S, Munjal S, Gao J, Venketaraman V. Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients. Clinics and Practice. 2021; 11(3):619-630. https://doi.org/10.3390/clinpract11030077
Chicago/Turabian StyleMunjal, Shafaa, Shalok Munjal, Jingya Gao, and Vishwanath Venketaraman. 2021. "Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients" Clinics and Practice 11, no. 3: 619-630. https://doi.org/10.3390/clinpract11030077
APA StyleMunjal, S., Munjal, S., Gao, J., & Venketaraman, V. (2021). Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients. Clinics and Practice, 11(3), 619-630. https://doi.org/10.3390/clinpract11030077





