Liver Cirrhosis and Hepatocellular Carcinoma Diagnosed from Chylothorax: A Case Report
Abstract
:1. Introduction
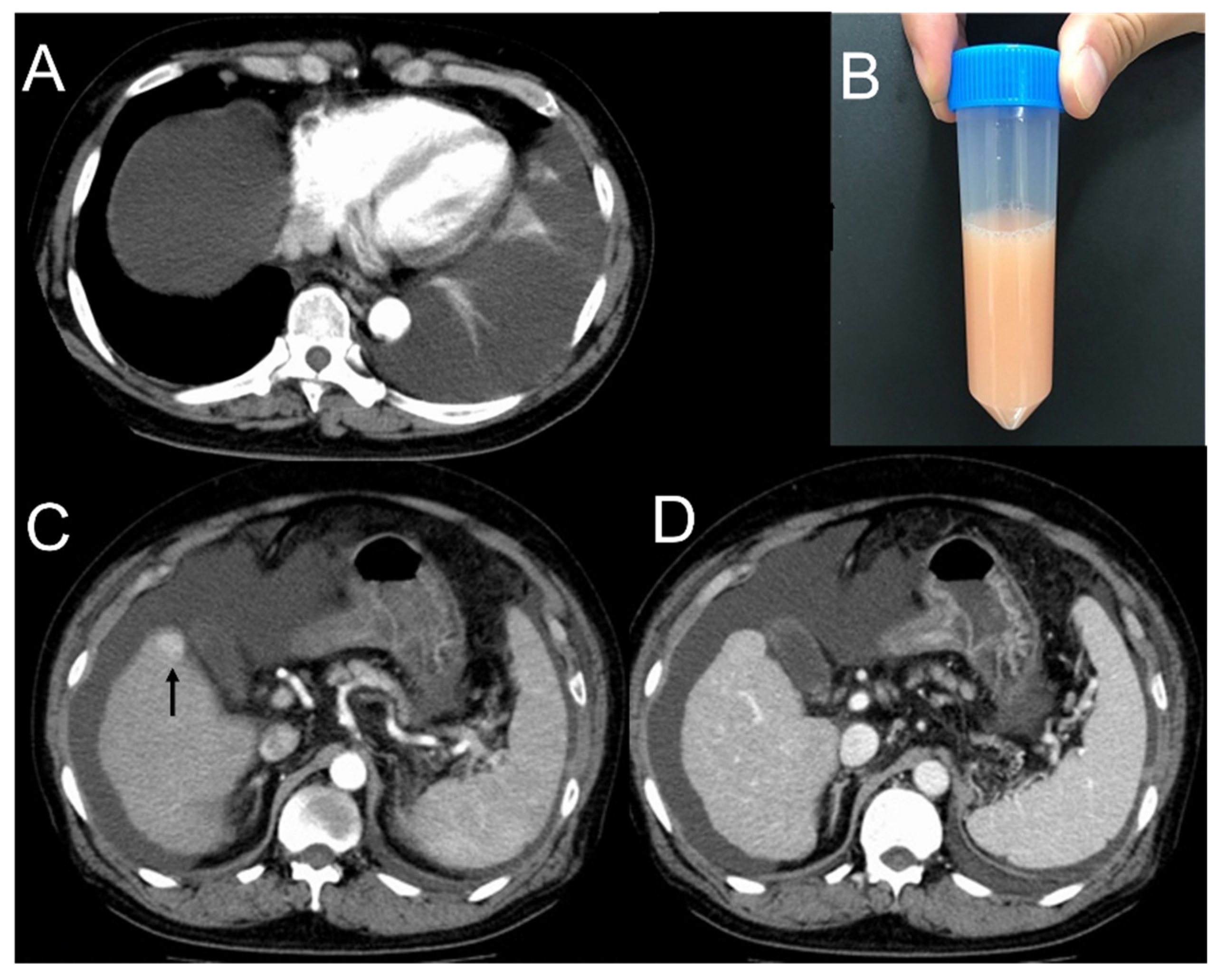
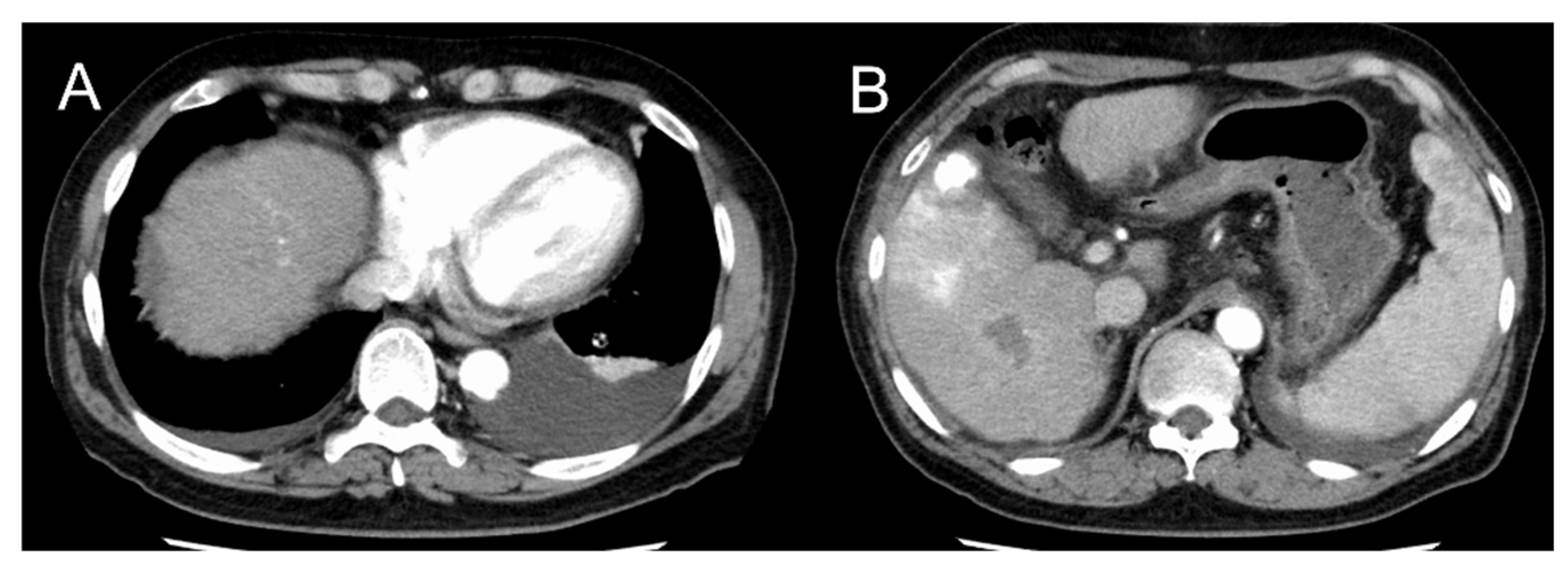
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Romero, S. Nontraumatic chylothorax. Curr. Opin. Pulm. Med. 2000, 6, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Doerr, C.H.; Allen, M.S.; Nichols, F.C., 3rd; Ryu, J.H. Etiology of chylothorax in 203 patients. Mayo Clin. Proc. 2005, 80, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, S.; Janning, M.; Daukeva, L.; Bokemeyer, C.; Fiedler, W. Chylothorax in a patient with Hodgkin’s lymphoma: A case report and review of the literature. Tumori J. 2013, 99, e96–e99. [Google Scholar] [CrossRef]
- Tsauo, J.; Shin, J.H.; Han, K.; Yoon, H.-K.; Ko, G.-Y.; Ko, H.-K.; Gwon, D.-I. Transjugular Intrahepatic Portosystemic Shunt for the Treatment of Chylothorax and Chylous Ascites in Cirrhosis: A Case Report and Systematic Review of the Literature. J. Vasc. Interv. Radiol. 2016, 27, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Badillo, R.; Rockey, D.C. Hepatic hydrothorax: Clinical features, management, and outcomes in 77 patients and review of the literature. Medicine 2014, 93, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guzman, E.; Culver, D.A.; Stoller, J.K. Transudative chylothorax: Report of two cases and review of the literature. Lung 2005, 183, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, T.B.; Ferrara, S.L.; Miller, F.J.; Roberts, A.C.; Hassanein, T. Transjugular intrahepatic portosystemic shunt creation as treatment for refractory chylous ascites and chylothorax in a patient with cirrhosis. J. Vasc. Interv. Radiol. 2004, 15 Pt 1, 85–89. [Google Scholar] [CrossRef]
- Higuchi, Y.; Tomizawa, M.; Kakegawa, S.; Suzuki, K.; Ishihara, S.; Kobayashi, H. A case of chylothorax associated with liver cirrhosis successfully treated by β-blocker. AJRS 2014, 3, 70–74. (In Japanese) [Google Scholar]
- Villanueva, C.; Albillos, A.; Genescà, J.; Garcia-Pagan, J.C.; Calleja-Panero, J.L.; Aracil, C.; Bañares, R.; Morillas, R.M.; Poca, M.; Peñas, B.; et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2019, 393, 1597–1608. [Google Scholar] [CrossRef]
- Khaliq, M.F.; Noorani, M.M.; Chowdhry, M.; Mohamed, H.; Koirala, A. Transjugular Intrahepatic Portosystemic Shunt (TIPS) in Refractory Transudative Chylothorax due to Liver Cirrhosis. Case Rep. Med. 2020, 2020, 2581040. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xie, F.; Lu, J.; Ni, Q.; Shi, C.; Tang, C.-X.; Yang, J. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, S.; Martín, C.; Hernandez, L.; Verdu, J.; Trigo, C.; Perez-Mateo, M.; Alemany, L. Chylothorax in cirrhosis of the liver: Analysis of its frequency and clinical characteristics. Chest 1998, 114, 154–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Hematology | Biochemistry | Plural Effusion | ||||||
|---|---|---|---|---|---|---|---|---|
| White blood cells | 2700 | /μL | Total protein | 5.8 | g/dL | Color | Milky white | |
| Neutrophils | 70.3 | % | Albumin | 2.9 | g/dL | pH | 8.5 | |
| Lymphocytes | 13.3 | % | Total bilirubin | 1.1 | mg/dL | White blood cells | 500 | /μL |
| Monocytes | 12.3 | % | Direct bilirubin | 0.1 | mg/dL | Neutrophils | 5.0 | % |
| Eosinophils | 3.7 | % | AST | 38 | U/L | Lymphocytes | 60.0 | % |
| Basophils | 0.4 | % | ALT | 33 | U/L | Monocytes | 33.0 | % |
| Red blood cells | 346 × 104 | /μL | LDH | 213 | U/L | Eosinophils | 2.0 | % |
| Hemoglobin | 8.7 | g/dL | ALP | 121 | U/L | Hematocrit | 0.1 | % |
| Hematocrit | 28.2 | % | γ-GTP | 42 | U/L | Total protein | 1.6 | g/dL |
| Platelets | 30.4 × 104 | /μL | CK | 153 | U/L | LDH | 66 | U/L |
| BUN | 11 | mg/dL | Glucose | 189 | mg/dL | |||
| Coagulation | Creatinine | 0.74 | mg/dL | Total cholesterol | 30 | mg/dL | ||
| PT% | 64 | % | Sodium | 139 | mEq/L | Triglyceride | 227 | mg/dL |
| PT-INR | 1.25 | Potassium | 3.6 | mEq/L | ADA | 9 | U/L | |
| APTT | 28.9 | sec | Chloride | 107 | mEq/L | |||
| Fibrinogen | 225 | mg/dL | Calcium | 7.8 | mg/dL | Cytology | (−) | |
| D-dimer | 13.3 | μg/mL | Glucose | 198 | mg/dL | |||
| HbA1c | 5.9 | % | Culture | |||||
| Tumor marker | Total cholesterol | 99 | mg/dL | Routine | (−) | |||
| AFP | 69.7 | ng/mL | LDL-cholesterol | 70 | mg/dL | Mycobacterium | (−) | |
| CEA | 1.5 | ng/mL | HDL-cholesterol | 26 | mg/dL | |||
| CA19-9 | 36.9 | U/mL | Triglyceride | 56 | mg/dL | |||
| Soluble IL-2 receptor | 842 | U/mL | BNP | 34.4 | pg/mL | |||
| CRP | 1.46 | mg/dL | ||||||
| Virus marker | ANA | 40 | titer | |||||
| HBV antigen | (−) | |||||||
| HCV antibody | (−) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Hachisu, Y.; Shibasaki, M.; Ezawa, K.; Iwashita, H.; Jingu, A.; Arai, H.; Horie, T.; Takise, A. Liver Cirrhosis and Hepatocellular Carcinoma Diagnosed from Chylothorax: A Case Report. Clin. Pract. 2021, 11, 582-586. https://doi.org/10.3390/clinpract11030073
Ito K, Hachisu Y, Shibasaki M, Ezawa K, Iwashita H, Jingu A, Arai H, Horie T, Takise A. Liver Cirrhosis and Hepatocellular Carcinoma Diagnosed from Chylothorax: A Case Report. Clinics and Practice. 2021; 11(3):582-586. https://doi.org/10.3390/clinpract11030073
Chicago/Turabian StyleIto, Kenta, Yoshimasa Hachisu, Mitsuhiko Shibasaki, Kazuma Ezawa, Hiroshi Iwashita, Asuka Jingu, Hirotaka Arai, Takeo Horie, and Atsushi Takise. 2021. "Liver Cirrhosis and Hepatocellular Carcinoma Diagnosed from Chylothorax: A Case Report" Clinics and Practice 11, no. 3: 582-586. https://doi.org/10.3390/clinpract11030073
APA StyleIto, K., Hachisu, Y., Shibasaki, M., Ezawa, K., Iwashita, H., Jingu, A., Arai, H., Horie, T., & Takise, A. (2021). Liver Cirrhosis and Hepatocellular Carcinoma Diagnosed from Chylothorax: A Case Report. Clinics and Practice, 11(3), 582-586. https://doi.org/10.3390/clinpract11030073





