Gestational Diabetes Mellitus per Different Diagnostic Criteria, Risk Factors, Obstetric Outcomes and Postpartum Glycemia: A Prospective Study in Ghana
Abstract
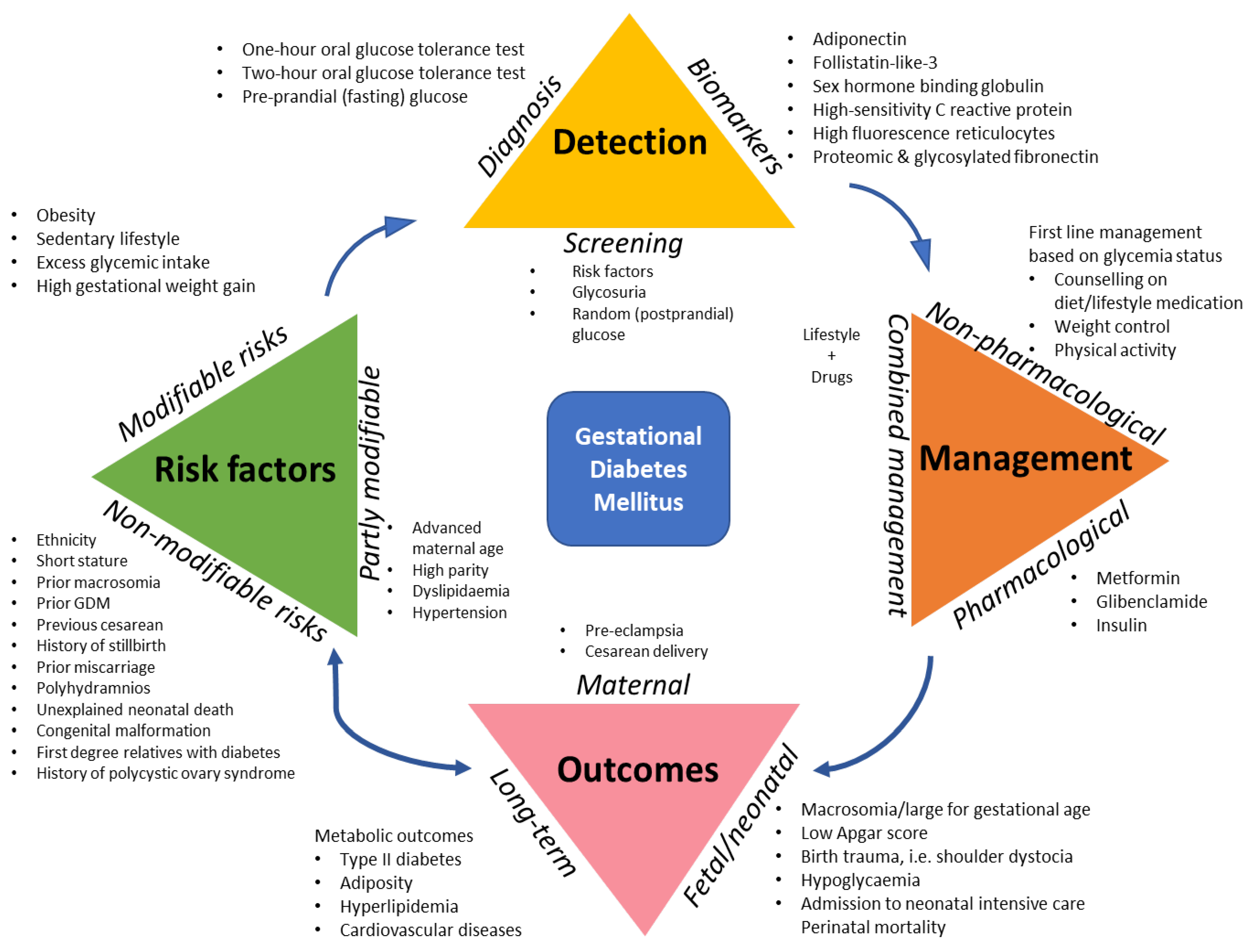
1. Introduction
2. Material and Methods
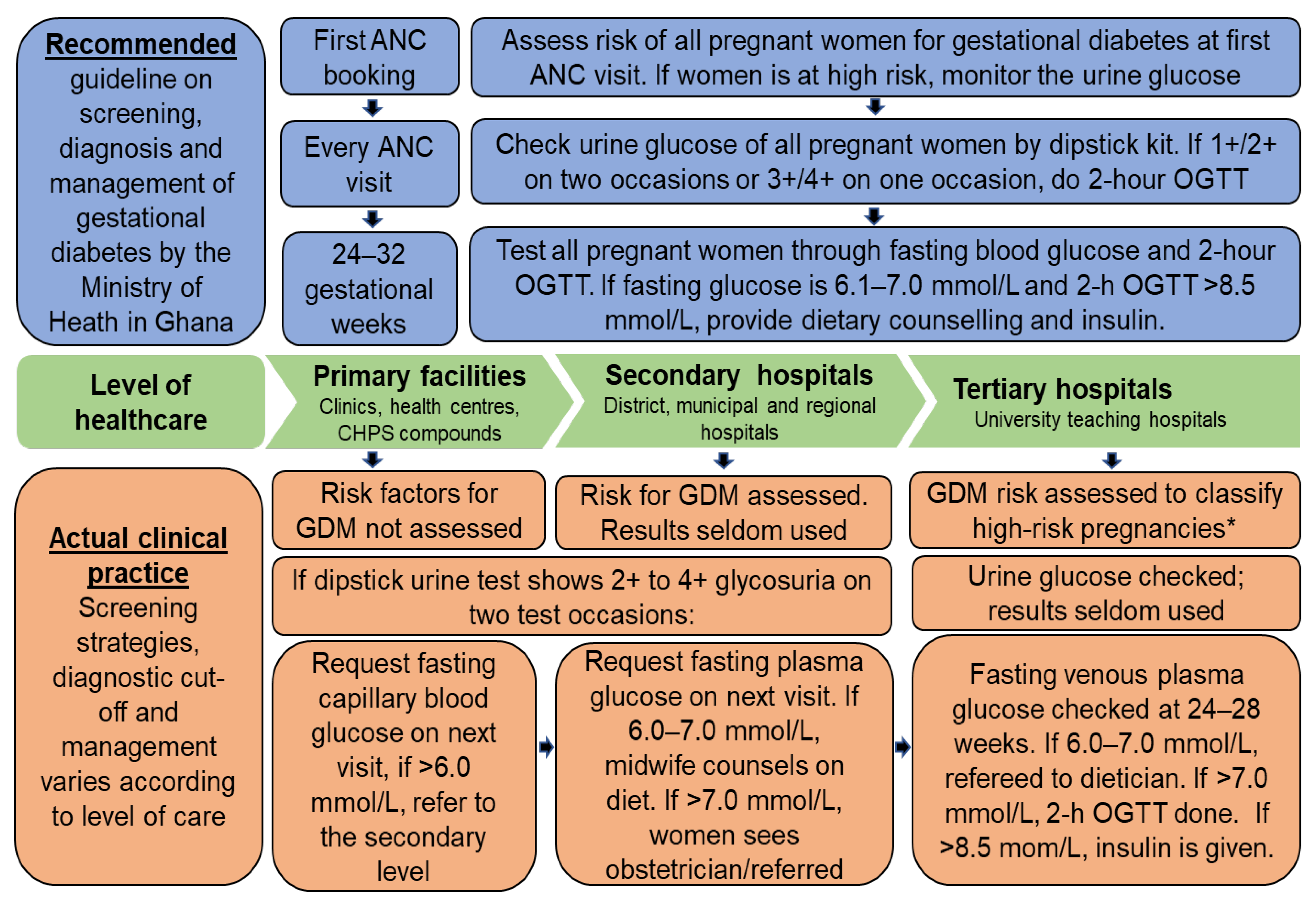
2.1. Study Context and Design
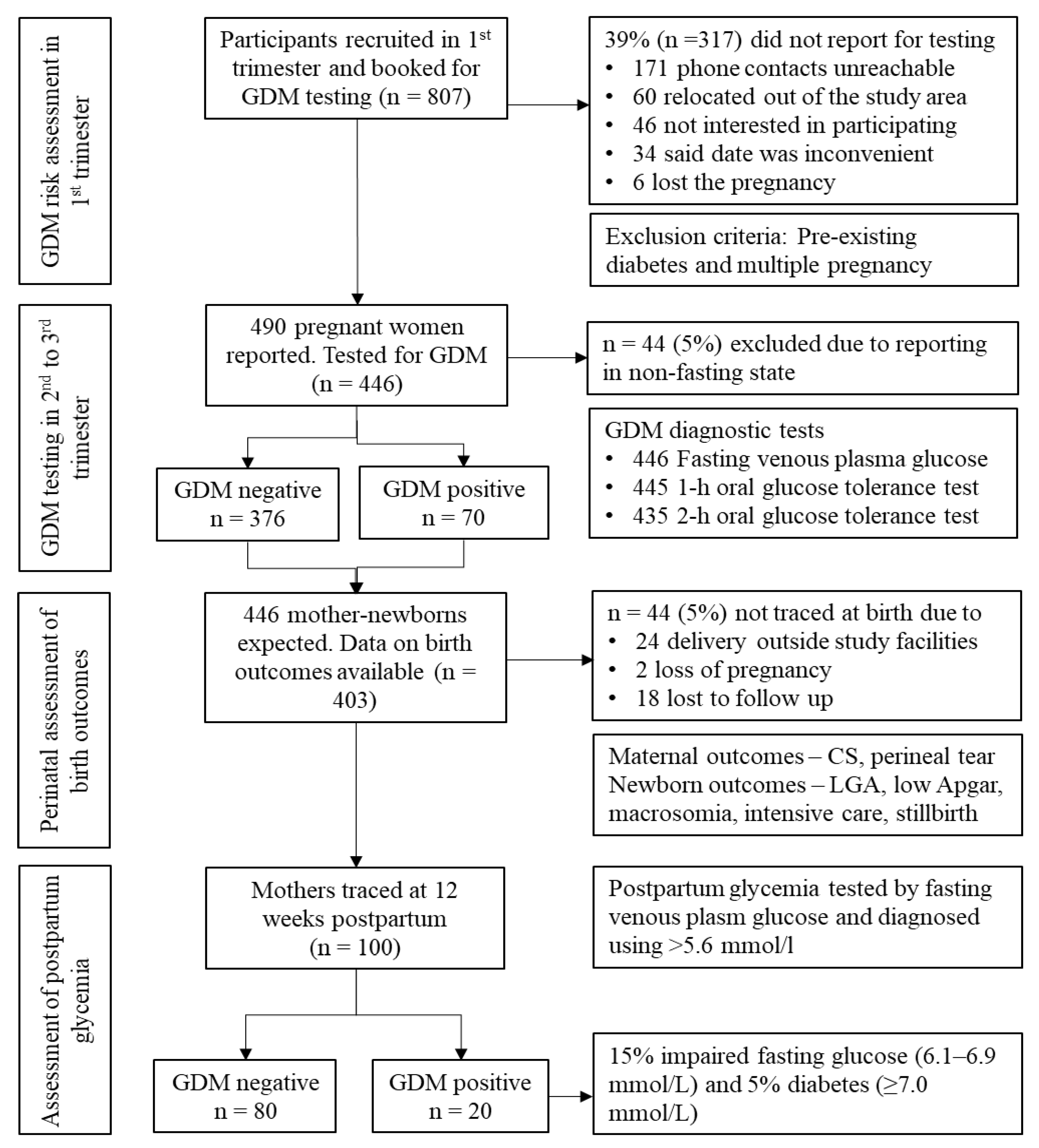
2.2. Participants
2.3. Data Collection
2.3.1. Anthropometric, Health and Dietary Indicators
2.3.2. GDM Testing and Diagnosis
2.3.3. Pregnancy Outcomes
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Prevalence of GDM
3.2. Risk Factors for GDM
3.3. Perinatal Outcomes
3.4. Postpartum Glycemic Status
4. Discussion
4.1. Strengths and Limitations of This Study
4.2. Implications for Clinical and Public Health Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, J.M. On puerperal diabetes. Trans. Obstet. Soc. Lond. 1882, 24, 256. [Google Scholar]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- IADPSG Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Shaw, J.; Karuranga, S. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Mwanri, A.W.; Kinabo, J.; Ramaiya, K. Gestational diabetes mellitus in sub-Saharan Africa: Systematic review and metaregression on prevalence and risk factors. Trop. Med. Int. Health 2015, 20, 983–1002. [Google Scholar] [CrossRef]
- Muche, A.A.; Olayemi, O.O.; Gete, Y.K. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: A systematic review and meta-analysis. Arch. Public Health 2019, 77, 1–20. [Google Scholar] [CrossRef]
- Hod, M.; Kapur, A.; Sacks, D.A. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M. Gestational diabetes and pregnancy outcomes-a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef]
- O’Sullivan, E.; Avalos, G.; O’Reilly, M. Atlantic Diabetes in Pregnancy (DIP): The prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 2011, 54, 1670–1675. [Google Scholar] [CrossRef]
- Eades, C.E.; Styles, M.; Leese, G.P. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: An observational follow-up study. BMC Pregnancy Childbirth 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Madsen, L.R.; Skajaa, G.O. Gestational diabetes: A clinical update. World J. Diabetes 2015, 6, 1065. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M. Consensus in gestational diabetes mellitus: Looking for the holy grail. J. Clin. Med. 2018, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Cundy, T.; Ackermann, E.; Ryan, E.A. Gestational diabetes: New criteria may triple the prevalence but effect on outcomes is unclear. BMJ 2014, 348, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M. Overdiagnosis of Gestational Diabetes Mellitus in Pregnant Woman: A Case Report. J. Women’s Health Care 2015, 4, 2167. [Google Scholar] [CrossRef]
- Twohig, H.; Hodges, V.; Mitchell, C. Pre-diabetes: Opportunity or overdiagnosis? Br. J. Gen. Pract. 2018, 62, 172–173. [Google Scholar] [CrossRef]
- Glasziou, P. Sustainable health care and the problem of overdiagnosis. Pathology 2017, 49, S14. [Google Scholar] [CrossRef]
- Moynihan, R. Preventing overdiagnosis–winding back the harms of too much medicine. Port. J. Nephrol. Hypert. 2016, 30, 14–15. [Google Scholar]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Silver, R.M. GDM: More diabetes, more good or more harm? BJOG Int. J. Obstet. Gynaecol. 2019, 127, 123. [Google Scholar] [CrossRef]
- Oppong, S.A.; Ntumy, M.Y.; Amoakoh-Coleman, M. Gestational diabetes mellitus among women attending prenatal care at Korle-Bu Teaching Hospital, Accra, Ghana. Int. J. Gynecol. Obstet. 2015, 131, 246–250. [Google Scholar] [CrossRef]
- Ministry of Health Ghana. Standard Treatment Guidelines. In Accra Ghana Ministry of Health and Ghana National Drugs Programme (GNDP), 6th ed.; Ministry of Health: Accra, Ghana, 2017. [Google Scholar]
- Beyuo, T.; Obed, S.A.; Adjepong-Yamoah, K.K. Metformin versus Insulin in the Management of Pre-Gestational Diabetes Mellitus in Pregnancy and Gestational Diabetes Mellitus at the Korle Bu Teaching Hospital: A Randomized Clinical Trial. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Ghana Health Service. Family Health Division Annual Report 2016; Ghana Health Service: Accra, Ghana, 2017; pp. 1–68. [Google Scholar]
- Agbozo, F.; Amardi-Mfoafo, J.; Dwase, H. Nutrition knowledge, dietary patterns and anthropometric indices of older persons in four peri-urban communities in Ga West municipality, Ghana. Afr. Health Sci. 2018, 18, 743–755. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy. Management of Diabetes and Its Complications from Preconception to the Postnatal Period. NICE Guideline 3 Methods, Evidence and Recommendations; National Institute for Health and Care Excellence and National Collaborating Centre for Women’s and Children’s Health: London, UK, 2015; p. 681.
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 2018, 42 (Suppl. 1), S1–S325. [Google Scholar]
- Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin No. 190, gestational diabetes mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization and International Diabetes Federation; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Macaulay, S.; Ngobeni, M.; Dunger, D.B. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res. Clin. Pract. 2018, 139, 278–287. [Google Scholar] [CrossRef]
- Olagbuji, B.N.; Atiba, A.S.; Olofinbiyi, B.A. Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan African population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 27–32. [Google Scholar] [CrossRef]
- Njete, H.; John, B.; Mlay, P. Prevalence, predictors and challenges of gestational diabetes mellitus screening among pregnant women in northern Tanzania. Trop. Med. Int. Health 2018, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Rheeder, P. Screening for gestational diabetes mellitus in a South African population: Prevalence, comparison of diagnostic criteria and the role of risk factors. SAMJ South Afr. Med. J. 2017, 107, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Utz, B.; Assarag, B.; Smekens, T. Detection and initial management of gestational diabetes through primary health care services in Morocco: An effectiveness-implementation trial. PLoS ONE 2018, 13, e0209322. [Google Scholar] [CrossRef] [PubMed]
- Feighan, C.; Devine, H.; Daniel, U. The emotional journey of gestational diabetes. Lancet Diabetes Endocrinol. 2017, 5, 924. [Google Scholar] [CrossRef][Green Version]
- Government of India. Diagnosis and Management of Gestational Diabetes Mellitus. Technical and Operational Guidelines: Ministry of Health and Family Welfare; Maternal Health Division: New Delhi, India, 2018.
- Ghana Statistical Service. 2010 Population and Housing Census, Regional Analytical Report, Volta Region; Ghana Statistical Service: Accra, Ghana, 2013; pp. 1–153.
- Mathes, T.; Jaschinski, T.; Pieper, D. Adherence influencing factors—A systematic review of systematic reviews. Arch. Public Health 2014, 72, 37. [Google Scholar] [CrossRef] [PubMed]
- Galbete, C.; Nicolaou, M.; Meeks, K.A. Food consumption, nutrient intake, and dietary patterns in Ghanaian migrants in Europe and their compatriots in Ghana. Food Nutr. Res. 2017, 61, 1341809. [Google Scholar] [CrossRef] [PubMed]
- Agbozo, F.; Atitto, P.; Jahn, A. Nutrient composition and dietary diversity of on-site lunch meals, and anthropometry of beneficiary children in private and public primary schools in Ghana. Nutr. Health 2018, 24, 241–249. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Criteria | Fasting Plasma Glucose (n = 446) | 2-h OGTT (n = 435) | FPG and/or 2-h OGTT (n = 446) | ||
|---|---|---|---|---|---|
| Cut-Off mmol/L | % | Cut-Off mmol/L | % | % | |
| IADPSG a/WHO/FIGO/ADA | 5.1 | 23.8 | 8.5 | 9.0 | 26.5 |
| 1999 WHO | 7.0 | 2.7 | 7.8 | 14.3 | 14.9 |
| NICE b | 5.6 | 10.8 | 7.8 b | 14.3 | 20.3 |
| CDA c | 5.3 | 16.9 | 9.0 | 5.1 | 18.9 |
| ACOG/Carpenter and Coustan d | 5.3 | 16.9 | 8.6 | 7.8 | 20.0 |
| ACOG/NDDG d | 5.8 | 8.3 | 9.2 | 4.4 | 10.6 |
| Ghana protocol | 6.1 | 5.8 | 8.5 | 9.0 | 11.9 |
| Variables | Polychotomous Sub-Groups | GDM (n = 70) n (%) | No GDM (n = 376) n (%) | p-Value * |
|---|---|---|---|---|
| Maternal age (years) | <20 | 4 (5.5) | 31 (7.5) | 0.489 |
| 20–29 | 32 (43.8) | 212 (51.5) | ||
| 30–39 | 32 (43.9) | 158 (38.0) | ||
| ≥40 | 5 (6.8) | 13 (3.1) | ||
| Parity (no. of children) | None | 11 (21.6) | 89 (35.5) | 0.173 |
| 1 child | 15 (29.4) | 70 (27.9) | ||
| 2 children | 12 (23.5) | 52 (20.7) | ||
| ≥3 children | 13 (25.5) | 40 (16.0) | ||
| Woman’s education | None/primary | 11 (21.6) | 30 (12.0) | 0.196 |
| Secondary/vocational | 33 (64.7) | 182 (73.1) | ||
| Tertiary | 7 (13.7) | 37 (14.9) | ||
| Partner’s education | None/primary * | 8 (16.7) | 16 (6.7) | 0.032 |
| Secondary/vocational * | 24 (50.0) | 158 (65.8) | ||
| Tertiary | 16 (33.3) | 66 (27.5) | ||
| Level of care | Health centre * | 22 (30.1) | 59 (14.1) | 0.001 |
| District hospital | 47 (64.4) | 299 (71.7) | ||
| Teaching hospital | 4 (5.5) | 59 (14.1) | ||
| Body mass index | Underweight | 4 (7.8) | 21 (8.4) | 0.061 |
| Normal weight * | 24 (47.1) | 158 (63.2) | ||
| Overweight * | 19 (37.3) | 50 (20.0) | ||
| Obese | 4 (7.8) | 21 (8.4) | ||
| Caloric intake a | Low | 36 (54.5) | 198 (57.9) | 0.022 |
| Moderate | 12 (18.2) | 81 (23.7) | ||
| High | 18 (27.3) | 63 (18.4) |
| Risk Categories | Dichotomized Exposure Variables | Cochran-Mantel-Haenszel Test | Unconditional Binary Logistic Regression | |||||
|---|---|---|---|---|---|---|---|---|
| GDM a (n = 70) n (%) | No GDM (n = 376) n (%) | p-Value | Crude Model | Adjusted Model | ||||
| uOR | 95% CI | aOR | 95% CI | |||||
| Socio-demographic data | Age >35 years | 16 (23.9) | 46 (12.0) | 0.019 | 2.29 | 1.21–4.36 | 4.06 | 0.58–8.73 |
| Unmarried | 13 (28.3) | 65 (27.3) | 0.859 | 1.05 | 0.52–2.12 | - | - | |
| Rural residency | 14 (28.6) | 71 (29.1) | 0.941 | 0.98 | 0.49–1.92 | - | - | |
| Low education: woman | 11 (21.6) | 30 (12.0) | 0.077 | 2.01 | 0.93–4.33 | - | - | |
| Low education: partner | 8 (16.7) | 16 (6.7) | 0.039 | 2.80 | 1.12–6.97 | - | - | |
| Primary-level facility | 16 (31.4) | 38 (15.1) | 0.009 | 2.56 | 1.29–5.08 | - | - | |
| Anthropometric indicators | Overweight/obese | 13 (20.0) | 39 (10.7) | 0.041 | 2.08 | 1.04–4.16 | 2.13 | 1.13–4.03 |
| Weight >90 kg b | 4 (6.1) | 11 (3.0) | 0.182 | 2.08 | 0.64–6.75 | - | - | |
| Height <150 cm b | 7 (13.7) | 26 (10.4) | 0.466 | 1.37 | 0.56–3.35 | - | - | |
| High weight gain c | 12 (24.0) | 51 (20.6) | 0.574 | 1.21 | 0.59–2.49 | - | - | |
| MUAC >30 cm d | 22 (34.9) | 80 (21.3) | 0.024 | 1.99 | 1.12–3.52 | 2.97 | 1.31–5.58 | |
| Obstetric history | Parity >3 children | 8 (12.9) | 22 (6.2) | 0.066 | 2.25 | 0.95–5.31 | 2.42 | 0.39–4.75 |
| Gravida >5 pregnancies | 5 (7.9) | 19 (5.0) | 0.365 | 1.63 | 0.58–4.53 | - | - | |
| Prior macrosomia >4 kg | 1 (16.7) | 8 (18.6) | 0.909 | 2.87 | 0.09–8.56 | - | - | |
| Prior neonatal death | 5 (10.2) | 21 (8.0) | 0.576 | 1.32 | 0.47–3.67 | 4.06 | 0.88–18.87 | |
| Prior cesarean section | 10 (20.0) | 43 (16.3) | 0.539 | 1.28 | 0.59–2.75 | 1.15 | 0.33–4.03 | |
| History of abortions | 18 (50.0) | 62 (32.0) | 0.040 | 2.13 | 1.04–4.37 | 4.01 | 1.09–14.76 | |
| Multiple pregnancies | 2 (4.0) | 7 (2.7) | 0.439 | 1.51 | 0.31–7.49 | - | - | |
| Medical conditions | Diabetes in family | 5 (7.5) | 24 (6.3) | 0.787 | 1.20 | 0.44–3.26 | 1.50 | 0.31–7.31 |
| Family hypertension | 7 (13.7) | 22 (8.8) | 0.296 | 1.65 | 0.67–4.11 | 1.21 | 0.34–4.36 | |
| Glycosuria e | 4 (5.5) | 11 (2.6) | 0.171 | 2.14 | 0.66–6.91 | 3.65 | 0.76–17.42 | |
| Hypertension | 9 (17.6) | 47 (18.7) | 0.989 | 1.93 | 0.42–2.04 | - | - | |
| Preeclampsia | 6 (9.1) | 6 (1.6) | 0.004 | 6.23 | 1.15–19.96 | 3.98 | 0.50–31.42 | |
| Antepartum depression | 13 (32.5) | 60 (26.2) | 0.442 | 1.36 | 0.66–2.80 | - | - | |
| Dyslipidemia f | 8 (15.7) | 63 (25.3) | 0.153 | 0.55 | 0.25–1.23 | 0.91 | 0.16–5.11 | |
| Malaria infection | 5 (12.5) | 14 (6.0) | 0.170 | 2.25 | 0.76–6.62 | - | - | |
| HIV positive | 2 (5.1) | 2 (0.9) | 0.082 | 5.84 | 0.79–42.74 | - | - | |
| Nutritional status | Anaemia (Hb < 11 g/dL) | 24 (60.0) | 130 (55.6) | 0.365 | 1.20 | 0.61–2.37 | - | - |
| High caloric intake g | 18 (28.6) | 56 (18.5) | 0.080 | 1.76 | 1.95–3.28 | 2.91 | 1.05–8.07 | |
| Maternal and Newborn Outcomes | Fasting Plasma Glucose Values | 2-h OGTT Values | ||||
|---|---|---|---|---|---|---|
| Coef.crude | 95% CI | p-Value | Coef.crude | 95% CI | p-Value | |
| Cesarean section * | 0.185 | −0.087, 0.457 | 0.183 | 0.330 | −0.140, 0.801 | 0.168 |
| Episiotomy * | −0.235 | −0.601, 0.130 | 0.207 | −0.490 | −1.121, 0.140 | 0.127 |
| Perineal tear * | 0.204 | −0.168, 0.575 | 0.281 | 0.143 | −0.506, 0.793 | 0.664 |
| Preeclampsia * | 0.087 | −0.193, 0.368 | 0.541 | 0.149 | −0.339, 0.637 | 0.548 |
| Prolong labour | 0.028 | −0.098, 0.155 | 0.660 | 0.077 | −0.026, 0.122 | 0.200 |
| Est. blood loss | 0.196 | 0.087, −0.306 | 0.001 | 0.290 | 0.010–0.482 | 0.003 |
| Hemoglobin | 0.024 | −0.065, 0.114 | 0.592 | 0.043 | −0.105, 0.193 | 0.563 |
| Gestational age | 0.056 | −0.004, 0.116 | 0.067 | 0.034 | −0.072, 0.140 | 0.529 |
| Birth weight | 0.251 | 0.008, 0.494 | 0.043 | 0.562 | 0.141, 0.983 | 0.009 |
| Birth length | 0.001 | −0.034, 0.036 | 0.969 | 0.003 | −0.059, 0.065 | 0.923 |
| Head circumference | 0.056 | −0.001, 0.114 | 0.056 | 0.043 | −0.059, 0.147 | 0.405 |
| Apgar at 5 min | −0.036 | −0.119, 0.064 | 0.558 | −0.064 | −0.236, 0.072 | 0.296 |
| Ponderal index a | 0.159 | −0.030, 0.349 | 0.100 | 0.273 | −0.060, 0.607 | 0.108 |
| Newborn glucose | 0.058 | −0.156, 0.273 | 0.583 | 0.029 | −0.420, 0.478 | 0.897 |
| Resuscitation * | 0.172 | −0.081, 0.426 | 0.181 | 0.272 | −0.142, 0.687 | 0.197 |
| Intensive care * | −0.286 | −0.881, 0.307 | 0.343 | −0.734 | −1.757, 0.288 | 0.158 |
| Birth asphyxia * | 0.850 | −0.461, 2.163 | 0.203 | 0.457 | −1.792, 2.706 | 0.690 |
| Perinatal death b,* | 0.719 | −0.353, 1.792 | 0.188 | 0.645 | −1.193, 2.484 | 0.490 |
| Maternal and Newborn Outcomes | FPG ≥ 5.1 mmol/L a | 2-h OGTT ≥ 8.5 mmol/L a | FPG ≥ 6.1 mmol/L b | FPG ≥ 5.6 mmol/L and/or 2-h OGTT ≥ 8.5 mmol/L c | FPG ≥ 5.6 mmol/L and/or 2-h OGTT ≥ 8.5 mmol/L d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| uRR | 95% CI (p-Value) | uRR | 95% CI (p-Value) | uRR | 95% CI (p-Value) | uRR | 95% CI (p-Value) | aRR | 95% CI (p-Value) | |
| Cesarean section | 1.84 | 0.98–3.46 (0.057) | 1.44 | 0.57–3.62 (0.434) | 1.15 | 0.36–3.69 (0.806) | 1.70 | 0.84–3.44 (0.138) | 1.88 | 0.96–3.67 (0.063) |
| Perineal tear | 1.82 | 0.77–4.31 (0.171) | 1.30 | 0.348–4.87 (0.694) | 2.11 | 0.53–8.35 (0.287) | 1.90 | 0.72–4.97 (0.189) | 2.90 | 1.08–5.56 (0.043) |
| PPH e | 1.26 | 0.23- 6.68 (0.786) | 3.72 | 0.69–19.80 (0.123) | 2.47 | 0.28–21.78 (0.414) | 1.82 | 0.35–9.41 (0.473) | 4.65 | 0.31–9.58 (0.265) |
| Preterm | 0.90 | 0.28–2.87 (0.860) | 0.44 | 0.05–3.54 (0.448) | 1.07 | 0.12–8.87 (0.950) | 0.92 | 0.25–3.38 (0.912) | 0.73 | 0.20–2.61 (0.856) |
| LGA f | 1.72 | 0.71–4.19 (0.226) | 3.36 | 1.14–9.85 (0.027) | 3.56 | 0.89–14.28 (0.072) | 1.63 | 0.60–4.38 (0.331) | 2.66 | 0.86–5.04 (0.254) |
| Resuscitated | 1.07 | 0.57–2.01 (0.821) | 0.66 | 0.23–1.86 (0.437) | 0.88 | 0.27–2.88 (0.842) | 1.28 | 0.63–2.62 (0.489) | 2.90 | 0.93–9.01 (0.065) |
| Birth asphyxia g | 1.67 | 0.21–2.06 (0.490) | 1.96 | 1.21–4.39 (0.963) | 3.19 | 1.79–12.86 (0.042) | 1.61 | 0.32–8.13 (0.495) | 3.24 | 1.01–10.44 (0.039) |
| Macrosomia (≥4 kg) | 1.50 | 0.36–6.20 (0.569) | 2.80 | 0.55–14.29 (0.213) | 2.05 | 0.24–17.55 (0.509) | 1.37 | 0.27–6.85 (0.695) | - | - |
| NICU | 0.31 | 0.03–2.53 (0.278) | 0.90 | 0.36–2.24 (0.822) | 1.80 | 0.21–15.15 (0.589) | 0.51 | 0.06–4.14 (0.530) | - | - |
| Perinatal death | 1.48 | 0.13–16.63 (0.748) | 2.38 | 0.21–26.82 (0.482) | 7.96 | 0.68–92.62 (0.097) | 2.38 | 0.21–26.82 (0.482) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbozo, F.; Abubakari, A.; Zotor, F.; Jahn, A. Gestational Diabetes Mellitus per Different Diagnostic Criteria, Risk Factors, Obstetric Outcomes and Postpartum Glycemia: A Prospective Study in Ghana. Clin. Pract. 2021, 11, 257-271. https://doi.org/10.3390/clinpract11020039
Agbozo F, Abubakari A, Zotor F, Jahn A. Gestational Diabetes Mellitus per Different Diagnostic Criteria, Risk Factors, Obstetric Outcomes and Postpartum Glycemia: A Prospective Study in Ghana. Clinics and Practice. 2021; 11(2):257-271. https://doi.org/10.3390/clinpract11020039
Chicago/Turabian StyleAgbozo, Faith, Abdulai Abubakari, Francis Zotor, and Albrecht Jahn. 2021. "Gestational Diabetes Mellitus per Different Diagnostic Criteria, Risk Factors, Obstetric Outcomes and Postpartum Glycemia: A Prospective Study in Ghana" Clinics and Practice 11, no. 2: 257-271. https://doi.org/10.3390/clinpract11020039
APA StyleAgbozo, F., Abubakari, A., Zotor, F., & Jahn, A. (2021). Gestational Diabetes Mellitus per Different Diagnostic Criteria, Risk Factors, Obstetric Outcomes and Postpartum Glycemia: A Prospective Study in Ghana. Clinics and Practice, 11(2), 257-271. https://doi.org/10.3390/clinpract11020039





