Fast-Onset Diffuse Interstitial Lung Disease in Anti-MDA5 Antibodies-Associated Amyopathic Dermatomyositis
Abstract
1. Introduction
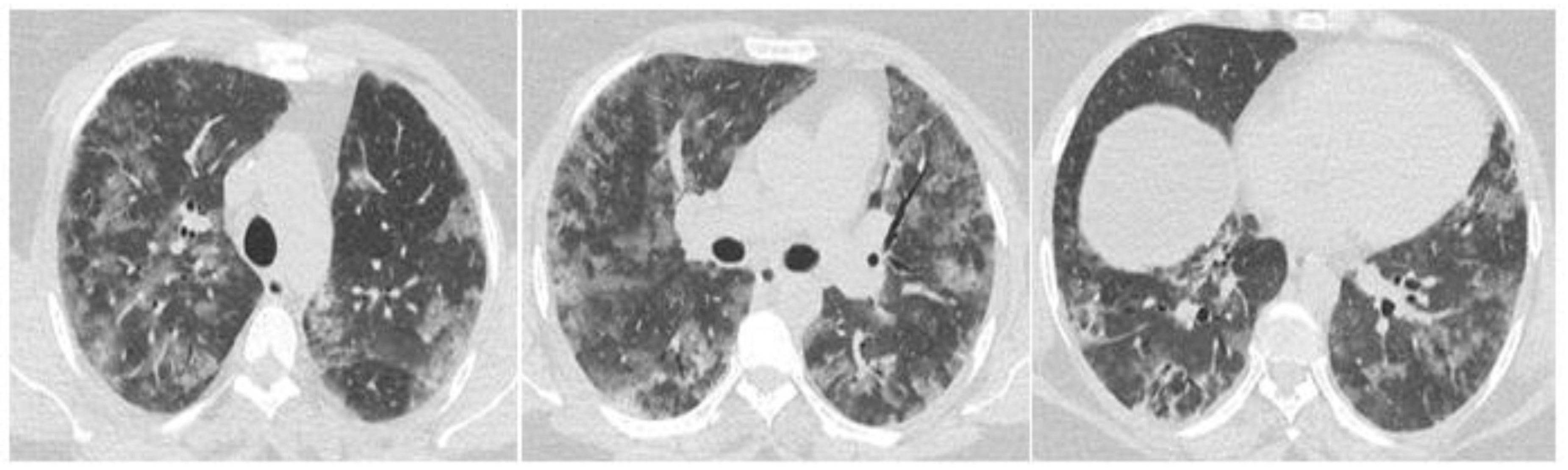
2. Case Report
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betaimi, L.; Taos, H.; Djanette, H.; Nabila, S.; Samia, L.; Rafika, B.; Abdelkrim, B. Une myocardite compliquant une myopathie inflammatoire type dermatomyosite à anti-MDA5. Rev. Neurol. 2018, 174, S38. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Nast, A.; Worm, M.; Dengler, R.; Dörner, T.; Ganter, H.; Hohlfeld, R.; Melms, A.; Melzer, N.; Rösler, K.; et al. Guidelines on dermatomyositis—Excerpt from the interdisciplinary S2k guidelines on myositis syndromes by the German Society of Neurology. J. Dtsch. Dermatol. Ges. 2016, 14, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Ceribelli, A.; Fredi, M.; Taraborelli, M.; Cavazzana, I.; Tincani, A.; Selmi, C.; Chan, J.Y.; Chan, E.K.; Satoh, M.; Franceschini, F. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin. Exp. Rheumatol. 2014, 32, 891–897. [Google Scholar] [PubMed]
- Yamaguchi, K.; Yamaguchi, A.; Kashiwagi, C.; Sawada, Y.; Taguchi, K.; Umetsu, K.; Oshima, K.; Uchida, M.; Suzuki, M.; Kono, S.; et al. Differential clinical features of patients with clinically amyopathic dermatomyositis who have circulating anti-MDA5 autoantibodies with or without myositis-associated auto antibodies. Respir Med. 2018, 140, 1–5. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Ip, R.W.; Wong, V.T.; Yip, R.M. Analysis of anti-melanoma differentiation-associated gene 5 antibody in Hong Kong Chinese patients with idiopathic inflammatory myopathies: Diagnostic utility and clinical correlations. Int. J. Rheum. Dis. 2018, 21, 1076–1081. [Google Scholar] [CrossRef]
- Kurtzman, D.J.B.; Vleugels, R.A. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: A concise review with an emphasis on distinctive clinical features. J. Am. Acad. Dermatol. 2018, 78, 776–785. [Google Scholar] [CrossRef]
- Girard, C.; Vincent, T.; Bessis, D. Dermatomyositis and acute interstitial lung disease associated with MDA-5 antibodies: An atypical case. Ann. Dermatol. Venereol. 2013, 140, 628–634. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kuwana, M.; Hoshino, K.; Hasegawa, M.; Kaji, K.; Matsushita, T.; Komura, K.; Nakamura, M.; Kodera, M.; Suga, N.; et al. Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: A multicenter cross-sectional study. Arch. Dermatol. 2011, 147, 391–398. [Google Scholar]
- Muro, Y.; Sugiura, K.; Hoshino, K.; Akiyama, M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology 2012, 51, 800–804. [Google Scholar] [CrossRef]
- Kotani, T.; Makino, S.; Takeuchi, T.; Kagitani, M.; Shoda, T.; Hata, A.; Tabushi, Y.; Hanafusa, T. Early intervention with corticosteroids and cyclosporin A and 2 h post dose blood concentration monitoring improves the prognosis of acute/subeacuteinterstitiel pneumonia in dermatomyositis. J. Rhumatol. 2008, 35, 254–259. [Google Scholar]
- Abe, Y.; Matsushitab, M.; Tada, K.; Yamaji, K.; Takasaki, Y.; Tamura, N. Clinical characteristics and change in the antibody titres of patients with anti MDA5 antibody-positive inflammatory myositis. Rhumatology 2017, 56, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Kora, T.; Fujikawa, K.; Horai, Y.; Okada, A.; Kawashiri, S.Y.; Iwamoto, N.; Suzuki, T.; Nakashima, Y.; Tamai, M.; Arima, K.; et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibodytesting for predictingthe prognosis of japanese patients with DM. Rhumatology 2012, 51, 1278–1284. [Google Scholar]
- Muro, Y.; Sugiura, K.; Hoshino, K.; Akiyama, M.; Tamakoshi, K. Epidemiologic study of clinically amyopathic dermatomyositis and anti-melanoma differentiation-associated gene 5 antibodies in central Japan. Arthritis Res. Ther. 2011, 13, R214. [Google Scholar] [CrossRef]
- Cao, H.; Pan, M.; Kang, Y.; Xia, Q.; Li, X.; Zhao, X.; Shi, R.; Lou, J.; Zhou, M.; Kuwana, M.; et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res. 2012, 64, 1602–1610. [Google Scholar] [CrossRef]
- Boutry, N.; Larde, A.; Demondion, X.; Flipo, R.M.; van Holsbeeck, M.; Cotten, A. Polyarthrite débutante: Apport de l’échographie des articulations métacarpophalangienne. J. Radiol. 2003, 84, 659–665. [Google Scholar]
- Nagata, K.; Tomii, K.; Nanjo, S.; Kubota, M.; Tachikawa, R.; Nishio, M. Four cases of interstitiel pneumonia associated with amyopathic dermatomyositis characterized by the anti-CADM 140 antibody. Nihon Koyuki Gakkai Zasshi 2011, 49, 30–36. [Google Scholar]
- Ikeda, S.; Arita, M.; Morita, M.; Ikeo, S.; Ito, A.; Tokioka, F.; Noyama, M.; Misaki, K.; Notohara, K.; Ishida, T. Interstitial lung disease in clinically amyopathic dermatomyositis with and without anti-MDA-5 antibody: To lump or split? BMC Pulm. Med. 2015, 15, 159. [Google Scholar] [CrossRef]
- Kohsaka, H.; Mimori, T.; Kanda, T.; Shimizu, J.; Sunada, Y.; Fujimoto, M.; Kawaguchi, Y.; Jinnin, M.; Muro, Y.; Ishihara, S.; et al. Treatment consensus for management of polymyositis and dermatomyositis among rheumatologists, neurologists and dermatologists. Neurol. Clin. Neurosci. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Kemada, H.; Nagasawa, H.; Ogawa, H.; Sekiguchi, N.; Takei, H.; Tokuhira, M.; Amaro, K.; Takeuchi, T. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitiel pneumonia in patients with dermatomyositis. J. Rheumatol. 2005, 32, 1719–1726. [Google Scholar]
- So, H.; Wong, V.T.L.; Lao, V.W.N.; Pang, H.T.; Yip, R.M.L. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin. Rheumatol. 2018, 37, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Gono, T.; Kawaguchi, Y.; Hara, M.; Masuda, I.; Katsumata, Y.; Shinozaki, M.; Ota, Y.; Ozeki, E.; Yamanaka, H. Increased ferritin predicts development and severity of acute interstitiel lung disease as a complication of dermatomyositis. Rhumatology 2010, 49, 1354–1360. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aissaoui, H.; Alsibai, K.D.; Khayath, N. Fast-Onset Diffuse Interstitial Lung Disease in Anti-MDA5 Antibodies-Associated Amyopathic Dermatomyositis. Clin. Pract. 2021, 11, 235-240. https://doi.org/10.3390/clinpract11020035
Aissaoui H, Alsibai KD, Khayath N. Fast-Onset Diffuse Interstitial Lung Disease in Anti-MDA5 Antibodies-Associated Amyopathic Dermatomyositis. Clinics and Practice. 2021; 11(2):235-240. https://doi.org/10.3390/clinpract11020035
Chicago/Turabian StyleAissaoui, Houari, Kinan Drak Alsibai, and Naji Khayath. 2021. "Fast-Onset Diffuse Interstitial Lung Disease in Anti-MDA5 Antibodies-Associated Amyopathic Dermatomyositis" Clinics and Practice 11, no. 2: 235-240. https://doi.org/10.3390/clinpract11020035
APA StyleAissaoui, H., Alsibai, K. D., & Khayath, N. (2021). Fast-Onset Diffuse Interstitial Lung Disease in Anti-MDA5 Antibodies-Associated Amyopathic Dermatomyositis. Clinics and Practice, 11(2), 235-240. https://doi.org/10.3390/clinpract11020035




