A Case of Thrombotic Thrombocytopenic Purpura without Pathognomonic Schistocytes
Abstract
1. Introduction
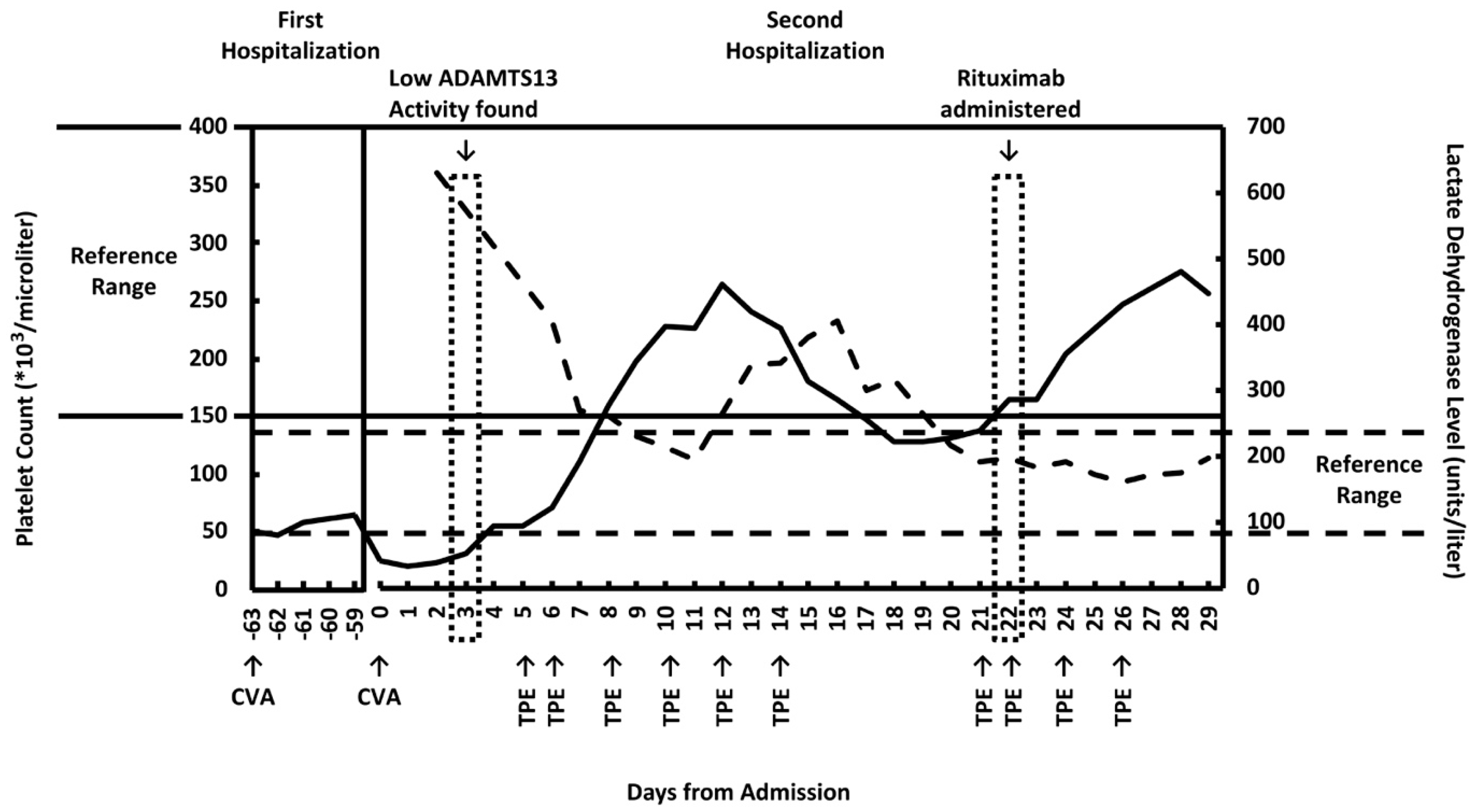
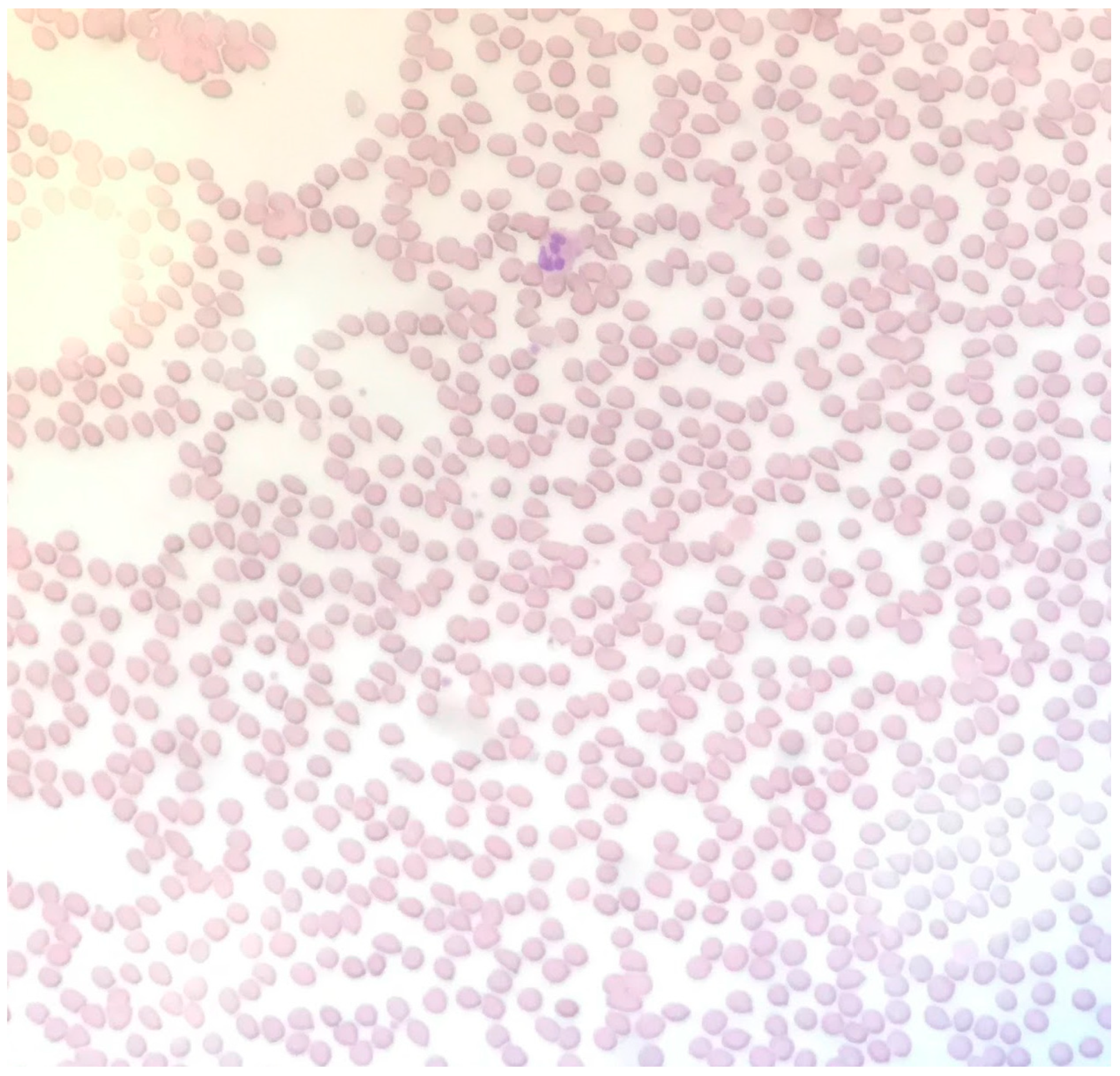
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, H.M. Pathophysiology of thrombotic thrombocytopenic purpura. Int. J. Hematol. 2010, 91, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Pathophysiology of thrombotic thrombocytopenic purpura. Blood 2017, 130, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.E.; Crum, E.D. Atypical Presentations of Thrombotic Thrombocytopenic Purpura. Int. J. Hematol. 2002, 76, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Shulman, K. Rituximab induces remission of cerebral ischemia caused by thrombotic thrombocytopenic purpura. Eur. J. Haematol. 2003, 70, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.A.; Yomtovian, R.; Tsai, H.M.; Silver, B.; Rutherford, C.; Sarode, R. Relapsed Thrombotic Thrombocytopenic Purpura Presenting as an Acute Cerebrovascular Accident. J. Clin. Apher. 2004, 19, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Imanirad, I.; Rajasekhar, A.; Zumberg, M. A Case Series of Atypical Presentations of Thrombotic Thrombocytopenic Purpura. J. Clin. Apher. 2012, 27, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kalish, Y.; Rottenstreich, A.; Rund, D.; Hochberg-Klein, S. Atypical presentations of thrombotic thrombocytopenic purpura: A diagnostic role for ADAMTS13. J. Thromb. Thrombolysis 2016, 42, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Badugu, P.; Idowu, M. Atypical Thrombotic Thrombocytopenic Purpura Presenting as Stroke. Case Rep. Hematol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Chan, K.H.; Kaur, P.; Modi, V.; Maroules, M. Atypical Presentation of Thrombotic Thrombocytopenic Purpura without Hematological Features. Int. J. Hematol. Oncol. Stem Cell Res. 2020, 14, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Lian, E.C. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N. Engl. J. Med. 1998, 339, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M. Chapter 49: Thrombotic Thrombocytopenic Purpura, Hemolytic-Uremic Syndrome, and Related Disorders. In Wintrobe’s Clinical Hematology, 14th ed.; Greer, J.P., Arber, D.A., Glader, B.E., List, A.F., Means, R.M., Rodgers, G.M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 3434–3510. [Google Scholar]
- Tsai, H.M. Chapter 6: Acquired thrombotic thrombocytopenic purpura-a disease due to inhibitors of ADAMTS13. In ADAMTS13-Biology and Disease; Rodgers, G.M., Ed.; Springer: Basel, Switzerland, 2015; pp. 91–128. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Yan, M. A Case of Thrombotic Thrombocytopenic Purpura without Pathognomonic Schistocytes. Clin. Pract. 2021, 11, 223-227. https://doi.org/10.3390/clinpract11020033
Yu K, Yan M. A Case of Thrombotic Thrombocytopenic Purpura without Pathognomonic Schistocytes. Clinics and Practice. 2021; 11(2):223-227. https://doi.org/10.3390/clinpract11020033
Chicago/Turabian StyleYu, Kevin, and Min Yan. 2021. "A Case of Thrombotic Thrombocytopenic Purpura without Pathognomonic Schistocytes" Clinics and Practice 11, no. 2: 223-227. https://doi.org/10.3390/clinpract11020033
APA StyleYu, K., & Yan, M. (2021). A Case of Thrombotic Thrombocytopenic Purpura without Pathognomonic Schistocytes. Clinics and Practice, 11(2), 223-227. https://doi.org/10.3390/clinpract11020033




