Changes in Maternal Heart Rate Variability in Response to the Administration of Routine Obstetric Medication in Hospitalized Patients: Study Protocol for a Cohort Study (MAMA-Heart Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim of the Study
2.2. Clinical Setting
2.3. Clinical Data Acquisition
2.4. Routinely Administered Medications in Obstetric Care Settings
2.5. Study Design
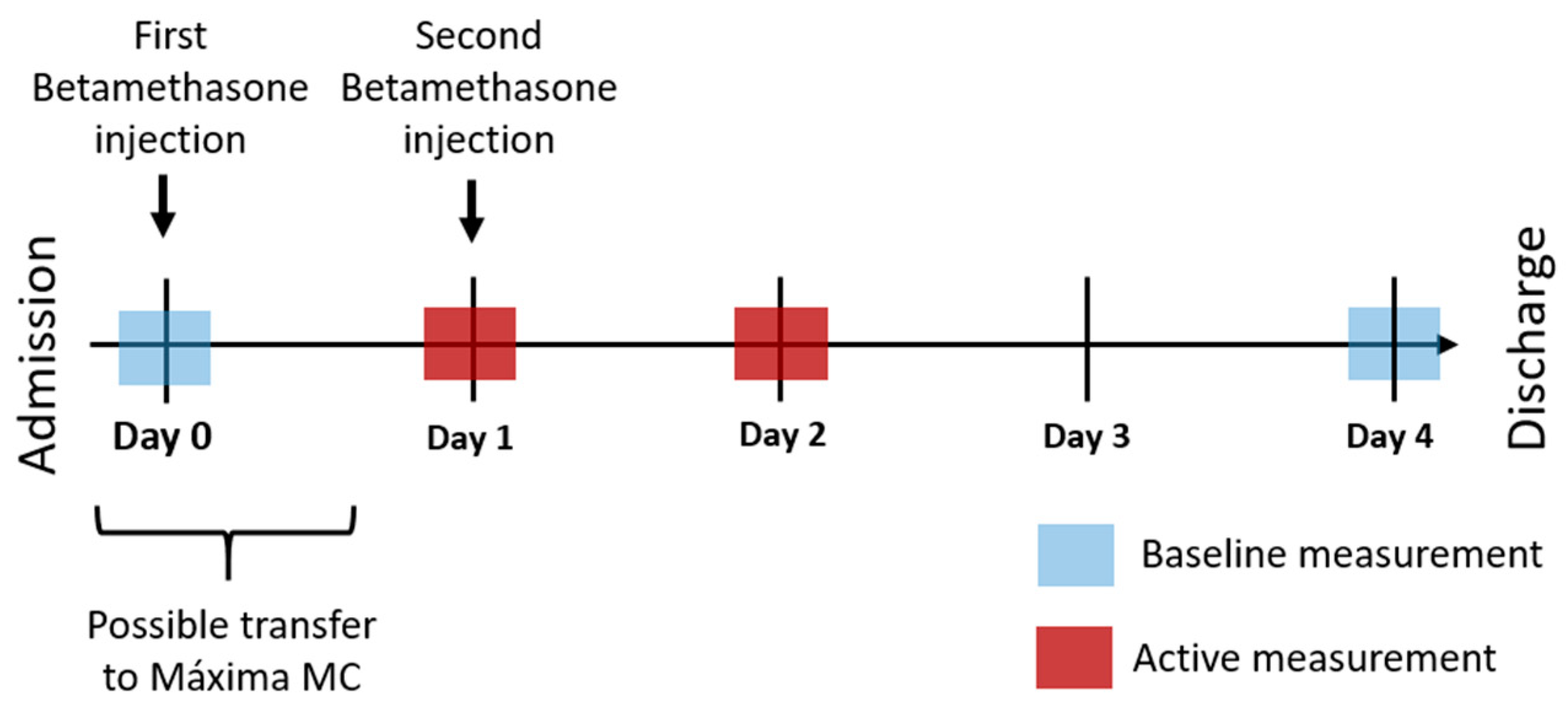
2.5.1. Primary Phase
2.5.2. Secondary Phase
2.6. Primary and Secondary Analyses
2.6.1. Primary Phase
2.6.2. Secondary Phase
2.7. Study Parameters
- Maternal condition:
- o
- Patient characteristics, including age, BMI, and ethnicity.
- o
- Pregnancy characteristics, including gestational age, results of prenatal screening, and complications in pregnancy.
- o
- Obstetric history, including gravidity, parity, and previous pregnancy or labor complications.
- o
- Family history, including genetic or congenital diseases or a history of hypertension or preeclampsia.
- o
- Medical condition, including preexisting diseases (i.e., cardiovascular disease, pre-existing hypertension, autoimmune disorders, neurologic disorders).
- o
- Routine measurements, including blood pressure, laboratory test results, physical examination results, ultrasound results.
- Fetal/neonatal condition, including fetal growth, congenital diseases, birth weight, APGAR score, CTG measurements, and umbilical cord blood gases.
- Labor and delivery, including mode of delivery and clinical notes.
- Administration of medications, including timing, dosage, and reasons for administration.
2.8. Subject Inclusion and Exclusion Criteria
2.9. Sample Size
2.10. Statistical Analysis
2.11. Data Handling and Storage
2.12. Ethics and Dissemination
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802. [Google Scholar] [CrossRef]
- Fu, Q. Hemodynamic and electrocardiographic aspects of uncomplicated singleton pregnancy. Adv. Exp. Med. Biol. 2018, 1065, 413–431. [Google Scholar]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- May, L. Cardiac physiology of pregnancy. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1325–1344. ISBN 978-0-470-65071-4. [Google Scholar]
- Khlybova, S.V.; Tsirkin, V.I.; Dvoryanskii, S.A.; Makarova, I.A.; Trukhin, A.N. Heart rate variability in normal and complicated pregnancies. Hum. Physiol. 2008, 34, 625–632. [Google Scholar] [CrossRef]
- Frey, H.A.; Klebanoff, M.A. The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef]
- Umesawa, M.; Kobashi, G. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens. Res. Off. J. JPN. Soc. Hypertens. 2017, 40, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; McClure, E.M.; Goudar, S.S.; Patel, A.; Esamai, F.; Garces, A.; Chomba, E.; Althabe, F.; Moore, J.; Kodkany, B.; et al. A prospective study of maternal, fetal and neonatal deaths in low- and middle-income countries. Bull. World Health Organ. 2014, 92, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Look, P.F.V. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, B.; Zheng, F. Low-dose aspirin at ≤16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: A systematic review and meta-analysis. Exp. Med. 2018, 15, 4361–4369. [Google Scholar]
- Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 127: Management of preterm labor. Obstet. Gynecol. 2012, 119, 1308–1317. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 202: Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019, 133, e1. [Google Scholar]
- Reyes, L.M.; Usselman, C.W.; Davenport, M.H.; Steinback, C.D. Sympathetic nervous system regulation in human normotensive and hypertensive pregnancies. Hypertension 2018, 71, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Lee, C.T.; Hains, S.M.J.; Kisilevsky, B.S. Maternal heart rate variability and fetal behavior in hypertensive and normotensive pregnancies. Biol. Res. Nurs. 2008, 10, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Julius, S.; Majahalme, S. The changing face of sympathetic overactivity in hypertension. Ann. Med. 2000, 32, 365–370. [Google Scholar] [CrossRef]
- Yousif, D.; Bellos, I.; Penzlin, A.I.; Hijazi, M.M.; Illigens, B.M.-W.; Pinter, A.; Siepmann, T. Autonomic dysfunction in preeclampsia: A systematic review. Front. Neurol. 2019, 10, 816. [Google Scholar] [CrossRef]
- Nelson, P.G.; Nelson, K.B. Innervation of the placenta and uterus: Competition between cytotrophoblasts and nerves? Placenta 2013, 34, 463–466. [Google Scholar] [CrossRef]
- Moors, S.; Staaks, K.J.J.; Westerhuis, M.E.M.H.; Dekker, L.R.C.; Verdurmen, K.M.J.; Oei, S.G.; van Laar, J.O.E.H. Heart rate variability in hypertensive pregnancy disorders: A systematic review. Pregnancy Hypertens. 2020, 20, 56–68. [Google Scholar] [CrossRef]
- Musa, S. Sympathetic activity in preeclampsia, a study of heart rate variability. J. Hypertens. 2018, 36, e5. [Google Scholar] [CrossRef]
- Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Massaro, S.; Pecchia, L. Heart Rate Variability (HRV) analysis: A methodology for organizational neuroscience. Organ. Res. Methods 2019, 22, 354–393. [Google Scholar] [CrossRef]
- Chaswal, M.; Kapoor, R.; Batra, A.; Verma, S.; Yadav, B.S. Heart rate variability and cardiovascular reflex tests for assessment of autonomic functions in preeclampsia. Int. J. Hypertens. 2018, 2018, 8163824. [Google Scholar] [CrossRef]
- Weber, T.M.; Lackner, H.K.; Roessler, A.; Papousek, I.; Kolovetsiou-Kreiner, V.; Lucovnik, M.; Schmid-Zalaudek, K.; Lang, U.; Moertl, M.G. Heart rate variability and baroreceptor reflex sensitivity in early- versus late-onset preeclampsia. PLoS ONE 2017, 12, e0186521. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, N.; Saarelainen, H.; Karkkainen, H.; Valtonen, P.; Lyyra-Laitinen, T.; Laitinen, T.; Vanninen, E.; Heinonen, S. Cardiovascular autonomic responses to head-up tilt in gestational hypertension and normal pregnancy. Blood Press. 2011, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin No. 188: Prelabor rupture of membranes. Obstet. Gynecol. 2018, 131, e1–e14. [Google Scholar]
- Tejera, E.; Areias, M.J.; Rodrigues, A.I.; Nieto-Villar, J.M.; Rebelo, I. Blood pressure and heart rate variability complexity analysis in pregnant women with hypertension. Hypertens. Pregnancy 2012, 31, 91–106. [Google Scholar] [CrossRef]
- Faber, R.; Baumert, M.; Stepan, H.; Wessel, N.; Voss, A.; Walther, T. Baroreflex sensitivity, heart rate, and blood pressure variability in hypertensive pregnancy disorders. J. Hum. Hypertens. 2004, 18, 707–712. [Google Scholar] [CrossRef][Green Version]
- Casati, D.; Stampalija, T.; Ferrazzi, E.; Alberti, A.M.; Scebba, I.; Paganelli, A.; Di Martino, D.; Muggiasca, M.L.; Bauer, A. Maternal cardiac deceleration capacity: A novel insight into maternal autonomic function in pregnancies complicated by hypertensive disorders and intrauterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 6–11. [Google Scholar] [CrossRef]
- Seeck, A.; Baumert, M.; Fischer, C.; Khandoker, A.; Faber, R.; Voss, A. Advanced Poincaré plot analysis differentiates between hypertensive pregnancy disorders. Physiol. Meas. 2011, 32, 1611–1622. [Google Scholar] [CrossRef]
- Koenen, S.V.; Mulder, E.J.H.; Wijnberger, L.D.; Visser, G.H.A. Transient loss of the diurnal rhythms of fetal movements, heart rate, and its variation after maternal betamethasone administration. Pediatr. Res. 2005, 57, 662–666. [Google Scholar] [CrossRef][Green Version]
- Weissman, A.; Tobia, R.S.; Burke, Y.Z.; Maxymovski, O.; Drugan, A. The effects of oxytocin and atosiban on the modulation of heart rate in pregnant women. J. Matern. Fetal Neonatal Med. 2017, 30, 329–333. [Google Scholar] [CrossRef]
- Verdurmen, K.M.J.; Hulsenboom, A.D.J.; van Laar, J.O.E.H.; Oei, S.G. Effect of tocolytic drugs on fetal heart rate variability: A systematic review. J. Matern. Fetal Neonatal Med. 2017, 30, 2387–2394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noben, L.; Verdurmen, K.M.J.; Warmerdam, G.J.J.; Vullings, R.; Oei, S.G.; van Laar, J.O.E.H. The fetal electrocardiogram to detect the effects of betamethasone on fetal heart rate variability. Early Hum. Dev. 2019, 130, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nensi, A.; Silva, D.A.D.; von Dadelszen, P.; Sawchuck, D.; Synnes, A.R.; Crane, J.; Magee, L.A. MAG-CP Collaborative Group (Appendix 1). Effect of magnesium sulphate on fetal heart rate parameters: A systematic review. J. Obstet. Gynaecol. Can. 2014, 36, 1055–1064. [Google Scholar] [CrossRef]
- Verdurmen, K.M.; Renckens, J.; van Laar, J.O.; Oei, S.G. The influence of corticosteroids on fetal heart rate variability: A systematic review of the literature. Obstet. Gynecol. Surv. 2013, 68, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Verdurmen, K.M.J.; Warmerdam, G.J.J.; Lempersz, C.; Hulsenboom, A.D.J.; Renckens, J.; Dieleman, J.P.; Vullings, R.; van Laar, J.O.E.H.; Oei, S.G. The influence of betamethasone on fetal heart rate variability, obtained by non-invasive fetal electrocardiogram recordings. Early Hum. Dev. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Eerikäinen, L.M.; Bonomi, A.G.; Schipper, F.; Dekker, L.R.C.; Vullings, R.; de Morree, H.M.; Aarts, R.M. Comparison between electrocardiogram- and photoplethysmogram-derived features for atrial fibrillation detection in free-living conditions. Physiol. Meas. 2018, 39, 084001. [Google Scholar] [CrossRef]
- Radha, M.; de Groot, K.; Rajani, N.; Wong, C.C.P.; Kobold, N.; Vos, V.; Fonseca, P.; Mastellos, N.; Wark, P.A.; Velthoven, N.; et al. Estimating blood pressure trends and the nocturnal dip from photoplethysmography. Physiol. Meas. 2019, 40, 025006. [Google Scholar] [CrossRef]
- Sartor, F.; Gelissen, J.; van Dinther, R.; Roovers, D.; Papini, G.B.; Coppola, G. Wrist-worn optical and chest strap heart rate comparison in a heterogeneous sample of healthy individuals and in coronary artery disease patients. BMC Sports Sci. Med. Rehabil. 2018, 10, 10. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.W.; Newnham, J.P.; Challis, J.G.; Jobe, A.H.; Stock, S.J. The clinical use of corticosteroids in pregnancy. Hum. Reprod. Update 2015, 22, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Shanks, A.L.; Grasch, J.L.; Quinney, S.K.; Haas, D.M. Controversies in antenatal corticosteroids. Semin. Fetal Neonatal Med. 2019, 24, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.I.; Najib, N.M. Pharmacokinetics of betamethasone after single-dose intramuscular administration of betamethasone phosphate and betamethasone acetate to healthy subjects. Clin. Ther. 2012, 34, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ballard, L.; Ballard, A. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am. J. Obstet. Gynecol. 1995, 173, 254–262. [Google Scholar] [CrossRef]
- Moss, T.J.M.; Doherty, D.A.; Nitsos, I.; Harding, R.; Newnham, J.P. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am. J. Obstet. Gynecol. 2003, 189, 1751–1757. [Google Scholar] [CrossRef]
- He, C.; Fan, H.; Tan, J.; Zou, J.; Zhu, Y.; Yang, K.; Hu, Q. Pharmacokinetics of betamethasone and betamethasone 17-monopropionate in Chinese healthy volunteers after intramuscular injection of betamethasone phosphate/betamethasone dipropionate. Arzneim. Forsch. 2011, 61, 417–420. [Google Scholar] [CrossRef]
- Jobe, A.H.; Milad, M.A.; Peppard, T.; Jusko, W.J. Pharmacokinetics and pharmacodynamics of intramuscular and oral betamethasone and dexamethasone in reproductive age women in India. Clin. Transl. Sci. 2020, 13, 391–399. [Google Scholar] [CrossRef]
- MSD. Celestone Chronodose, Suspensie Voor Injectie 5.7 mg/mL; Merck Sharp & Dohme B.V.: Haarlen, The Netherlands, 2018. [Google Scholar]
- Castaldo, R.; Montesinos, L.; Melillo, P.; Massaro, S.; Pecchia, L. To what extent can we shorten hrv analysis in wearable sensing? A case study on mental stress detection. In Proceedings of the EMBEC & NBC 2017; Eskola, H., Väisänen, O., Viik, J., Hyttinen, J., Eds.; Springer: Singapore, 2018; Volume 65, pp. 643–646. [Google Scholar]
- Pinheiro, N.; Couceiro, R.; Henriques, J.; Muehlsteff, J.; Quintal, I.; Gonçalves, L.; Carvalho, P. Can PPG Be Used for HRV Analysis? In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2945–2949. [Google Scholar]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. CCR 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Voss, A.; Malberg, H.; Schumann, A.; Wessel, N.; Walther, T.; Stepan, H.; Faber, R. Baroreflex sensitivity, heart rate, and blood pressure variability in normal pregnancy. Am. J. Hypertens. 2000, 13, 1218–1225. [Google Scholar] [CrossRef]
- Alam, T.; Choudhary, A.; Sendil, D. Maternal heart rate variability during different trimesters of pregnancy. Natl. J. Physiol. Pharm. Pharm. 2018, 8, 1475. [Google Scholar] [CrossRef]
- Quintana, D.S. Statistical considerations for reporting and planning heart rate variability case-control studies: Reporting heart rate variability studies. Psychophysiology 2017, 54, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shen, J.; Zhou, G.; Shen, L.; Zhou, S.; Li, X. Effects of magnesium sulfate on heart rate, blood pressure variability and baroreflex sensitivity in preeclamptic rats treated with L-NAME. Hypertens. Pregnancy 2013, 32, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Cottin, F.; Malcurat, V.; Zorgati, H.; Prieur, F.; Labsy, Z.; Do, M.C.; Gagey, O.; Collomp, K. Effect of oral glucocorticoid intake on autonomic cardiovascular control. SpringerPlus 2015, 4, 622. [Google Scholar] [CrossRef] [PubMed]
- Koenen, S.V.; Mecenas, C.A.; Smith, G.S.; Jenkins, S.; Nathanielsz, P.W. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0.7 of gestation. Am. J. Obstet. Gynecol. 2002, 186, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Brotman, D.J.; Girod, J.P.; Garcia, M.J.; Patel, J.V.; Gupta, M.; Posch, A.; Saunders, S.; Lip, G.Y.H.; Worley, S.; Reddy, S. Effects of short-term glucocorticoids on cardiovascular biomarkers. J. Clin. Endocrinol. Metab. 2005, 90, 3202–3208. [Google Scholar] [CrossRef]
- Verdurmen, K.; Van, L.J.; Oei, S. Corticosteroids and fetal heart rate variability. J. Matern. Fetal Neonatal Med. 2014, 27, 361. [Google Scholar]
- Grym, K.; Niela-Vilén, H.; Ekholm, E.; Hamari, L.; Azimi, I.; Rahmani, A.; Liljeberg, P.; Löyttyniemi, E.; Axelin, A. Feasibility of smart wristbands for continuous monitoring during pregnancy and one month after birth. BMC Pregnancy Childbirth 2019, 19, 34. [Google Scholar] [CrossRef]
- Choi, A.; Shin, H. Photoplethysmography sampling frequency: Pilot assessment of how low can we go to analyze pulse rate variability with reliability? Physiol. Meas. 2017, 38, 586–600. [Google Scholar] [CrossRef]
- Samways, J.W.; Vause, S.; Kontopantelis, E.; Eddleston, J.; Ingleby, S.; Roberts, A.; Clarke, B. Maternal heart rate during the first 48 h postpartum: A retrospective cross sectional study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 41–47. [Google Scholar] [CrossRef][Green Version]
- Mahendru, A.A.; Everett, T.R.; Wilkinson, I.B.; Lees, C.C.; McEniery, C.M. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J. Hypertens. 2014, 32, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Jevitt, C.M.; Sahebzamani, F.; Beckstead, J.W.; Keefe, D.L. Breastfeeding status and maternal cardiovascular variables across the postpartum. J. Womens Health 2013, 22, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bester, M.; Moors, S.; Joshi, R.; Nichting, T.J.; van der Hout-van der Jagt, M.B.; Oei, S.G.; Mischi, M.; Vullings, R.; van Laar, J.O.E.H. Changes in Maternal Heart Rate Variability in Response to the Administration of Routine Obstetric Medication in Hospitalized Patients: Study Protocol for a Cohort Study (MAMA-Heart Study). Clin. Pract. 2021, 11, 13-25. https://doi.org/10.3390/clinpract11010004
Bester M, Moors S, Joshi R, Nichting TJ, van der Hout-van der Jagt MB, Oei SG, Mischi M, Vullings R, van Laar JOEH. Changes in Maternal Heart Rate Variability in Response to the Administration of Routine Obstetric Medication in Hospitalized Patients: Study Protocol for a Cohort Study (MAMA-Heart Study). Clinics and Practice. 2021; 11(1):13-25. https://doi.org/10.3390/clinpract11010004
Chicago/Turabian StyleBester, Maretha, Suzanne Moors, Rohan Joshi, Thomas J. Nichting, M. Beatrijs van der Hout-van der Jagt, S. Guid Oei, Massimo Mischi, Rik Vullings, and Judith O. E. H. van Laar. 2021. "Changes in Maternal Heart Rate Variability in Response to the Administration of Routine Obstetric Medication in Hospitalized Patients: Study Protocol for a Cohort Study (MAMA-Heart Study)" Clinics and Practice 11, no. 1: 13-25. https://doi.org/10.3390/clinpract11010004
APA StyleBester, M., Moors, S., Joshi, R., Nichting, T. J., van der Hout-van der Jagt, M. B., Oei, S. G., Mischi, M., Vullings, R., & van Laar, J. O. E. H. (2021). Changes in Maternal Heart Rate Variability in Response to the Administration of Routine Obstetric Medication in Hospitalized Patients: Study Protocol for a Cohort Study (MAMA-Heart Study). Clinics and Practice, 11(1), 13-25. https://doi.org/10.3390/clinpract11010004






