Spinal Cord Compression as a Consequence of Spinal Plasmacytoma in a Patient with Multiple Myeloma: A Case Report
Abstract
1. Introduction
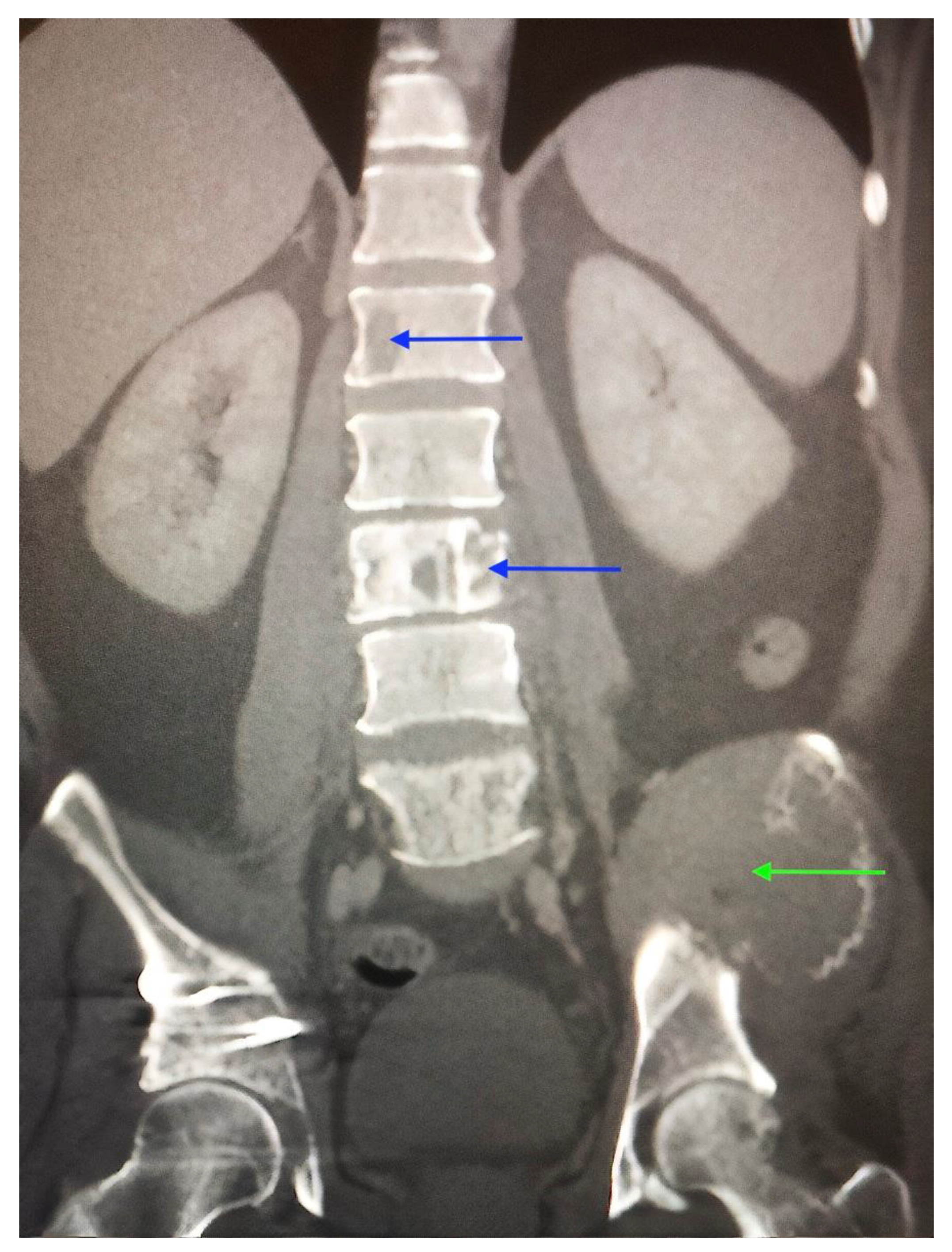
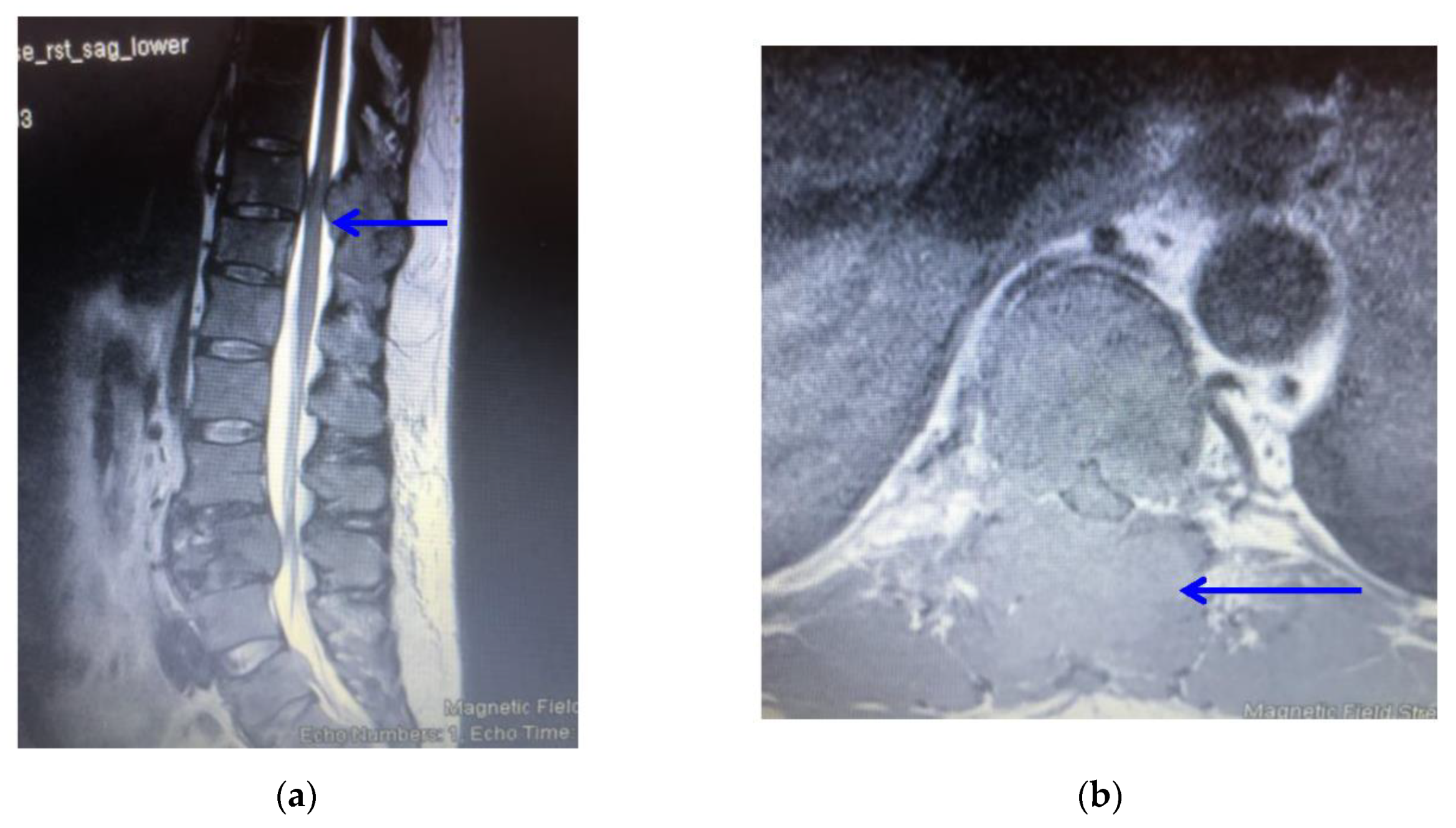
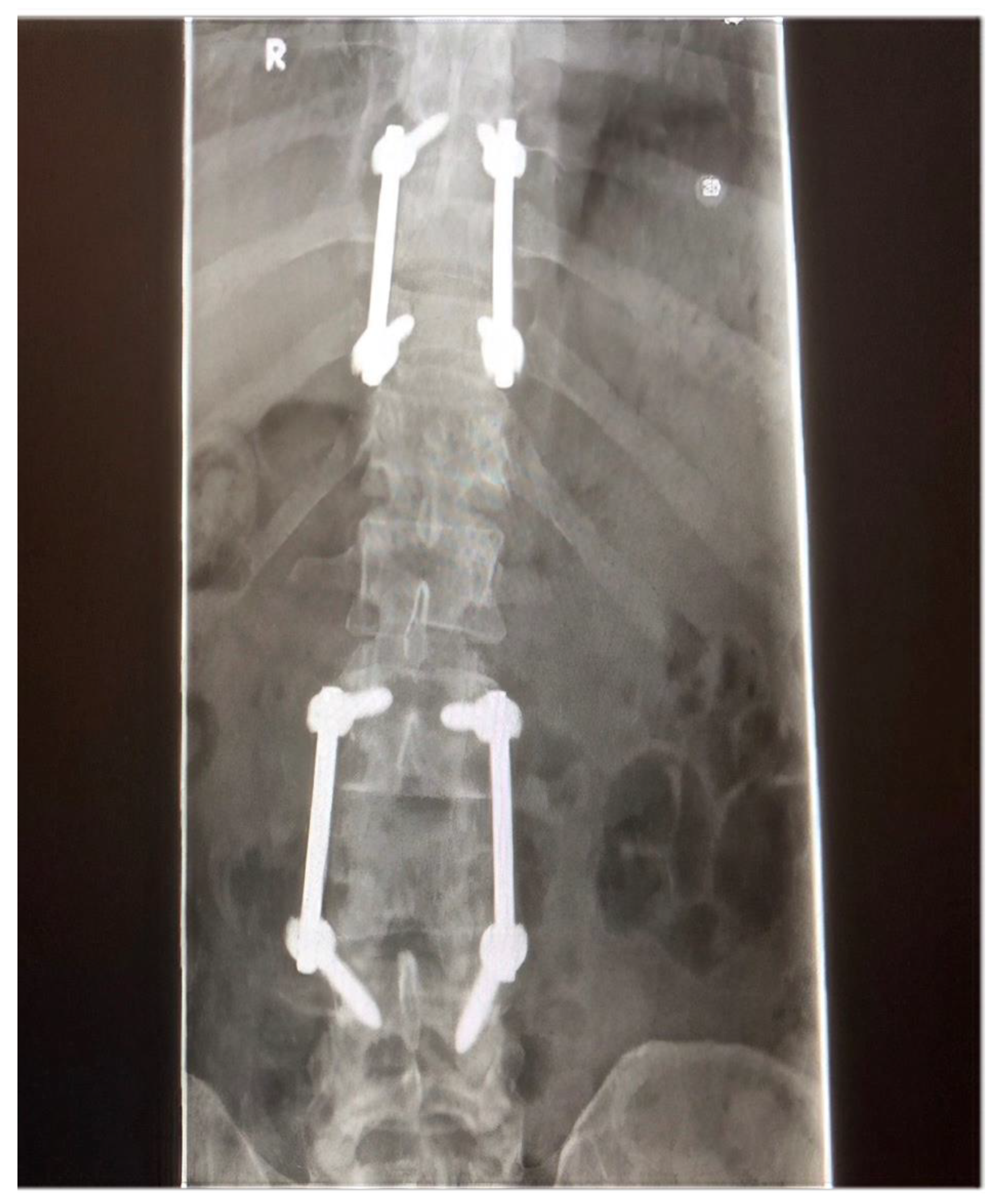
2. Case Report
3. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batson, O.V. The function of the vertebral veins and their role in the spread of metastases: 1940. Clin. Orthop. Relat. Res. 1995, 312, 4–9. [Google Scholar]
- Howell, E.P.; Williamson, T.; Karikari, I.; Abd-El-Barr, M.; Erickson, M.; Goodwin, M.L.; Reynolds, J.; Sciubba, D.M.; Goodwin, C.R. Total en bloc resection of primary and metastatic spine tumors. Ann. Transl. Med. 2019, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I. A Prospective Audit of the Diagnosis, Management and Outcome of Malignant Cord Compression; 97/08; Clinical Research and Audit Group (CRAG): London, UK, 2001. [Google Scholar]
- National Institute for Health and Care Excellence. Metastatic Spinal Cord Compression in Adults: Risk Assessment, Diagnosis and Management: NICE Guidelines (CG75); NICE: London, UK, 2008. [Google Scholar]
- Boussios, S.T.; Cooke, D.; Hayward, C.; Kanellos, F.S.; Tsiouris, A.K.; Chatziantoniou, A.A.; Zakynthinakis-Kyriakou, N.; Karathanasi, A. Metastatic Spinal Cord Compression: Unravelling the Diagnostic and Therapeutic Challenges. Anticancer Res. 2018, 38, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Garg, R.K.; Somvanshi, D.S. Spinal tuberculosis: A review. J. Spinal Cord Med. 2011, 34, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Markanday, A. Acute phase reactants in infections: Evidence-based review and a guide for clinicians. Open Forum Infect. Dis. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.P.; Ashford, R.U.; Rao, A.S.; Dickson, R.A. Primary bone tumours of the spine: A 42-year survey from the Leeds Regional Bone Tumour Registry. Eur. Spine J. 2007, 16, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Hayward, C.; Cooke, D.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Karathanasi, A. Spinal Ewing Sarcoma Debuting with Cord Compression: Have We Discovered the Thread of Ariadne? Anticancer Res. 2018, 38, 5589–5597. [Google Scholar] [CrossRef] [PubMed]
- Helweg-Larsen, S.; Sørensen, P.S. Symptoms and signs in metastatic spinal cord compression: A study of progression from first symptom until diagnosis in 153 patients. Eur. J. Cancer 1994, 30, 396–398. [Google Scholar] [CrossRef]
- Nair, C.; Panikkar, S.; Ray, A. How not to miss metastatic spinal cord compression. Br. J. Gen. Pract. 2014, 64, 596–598. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence by the National Collaborating Centre for Cancer (UK). Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression (CG75); Chapter 6.2: Treatments for Painful Spinal Metastases and Prevention of MSCC; NICE: Cardiff, UK, 2008. [Google Scholar]
- Savage, P.; Sharkey, R.; Kua, T.; Schofield, L.; Richardson, D.; Panchmatia, N.; Papanastasopoulos, P.; Williams, M.; Falconer, A.; Power, D.; et al. Malignant spinal cord compression: NICE guidance, improvements and challenges. QJM Int. J. Med. 2014, 107, 277–282. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence by the National Collaborating Centre for Cancer (UK). Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression (CG75); Chapter 5: Choice of Imaging; NICE: Cardiff, UK, 2008. [Google Scholar]
- Quraishi, N.A.; Esler, C. Metastatic spinal cord compression. BMJ 2011, 342, d2402. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence by the National Collaborating Centre for Cancer (UK). Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression (CG75); Chapter 6.6: Surgery for MSCC; NICE: Cardiff, UK, 2008. [Google Scholar]
- Kee-Yong, H.; Young-Hoon, K.; Hyun-Woo, K. Multiple Myeloma and Epidural Spinal Cord Compression: Case Presentation and a Spine Surgeon’s Perspective. J. Korean Neurosurg. Soc. 2013, 54, 151–154. [Google Scholar]
- Avadhani, A.; Shetty, A.P.; Rajasekaran, S. Isolated extraosseous epidural myeloma presenting with thoracic compressive myelopathy. Spine J. 2010, 10, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Lofton, L. Rehabilitation and treatment of spinal cord tumors. J. Spinal Cord Med. 2013, 36, 4–11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, R.J. Spinal Cord Compression as a Consequence of Spinal Plasmacytoma in a Patient with Multiple Myeloma: A Case Report. Clin. Pract. 2021, 11, 124-130. https://doi.org/10.3390/clinpract11010018
Trivedi RJ. Spinal Cord Compression as a Consequence of Spinal Plasmacytoma in a Patient with Multiple Myeloma: A Case Report. Clinics and Practice. 2021; 11(1):124-130. https://doi.org/10.3390/clinpract11010018
Chicago/Turabian StyleTrivedi, Rishi Jayesh. 2021. "Spinal Cord Compression as a Consequence of Spinal Plasmacytoma in a Patient with Multiple Myeloma: A Case Report" Clinics and Practice 11, no. 1: 124-130. https://doi.org/10.3390/clinpract11010018
APA StyleTrivedi, R. J. (2021). Spinal Cord Compression as a Consequence of Spinal Plasmacytoma in a Patient with Multiple Myeloma: A Case Report. Clinics and Practice, 11(1), 124-130. https://doi.org/10.3390/clinpract11010018




