Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis
Abstract
1. Introduction
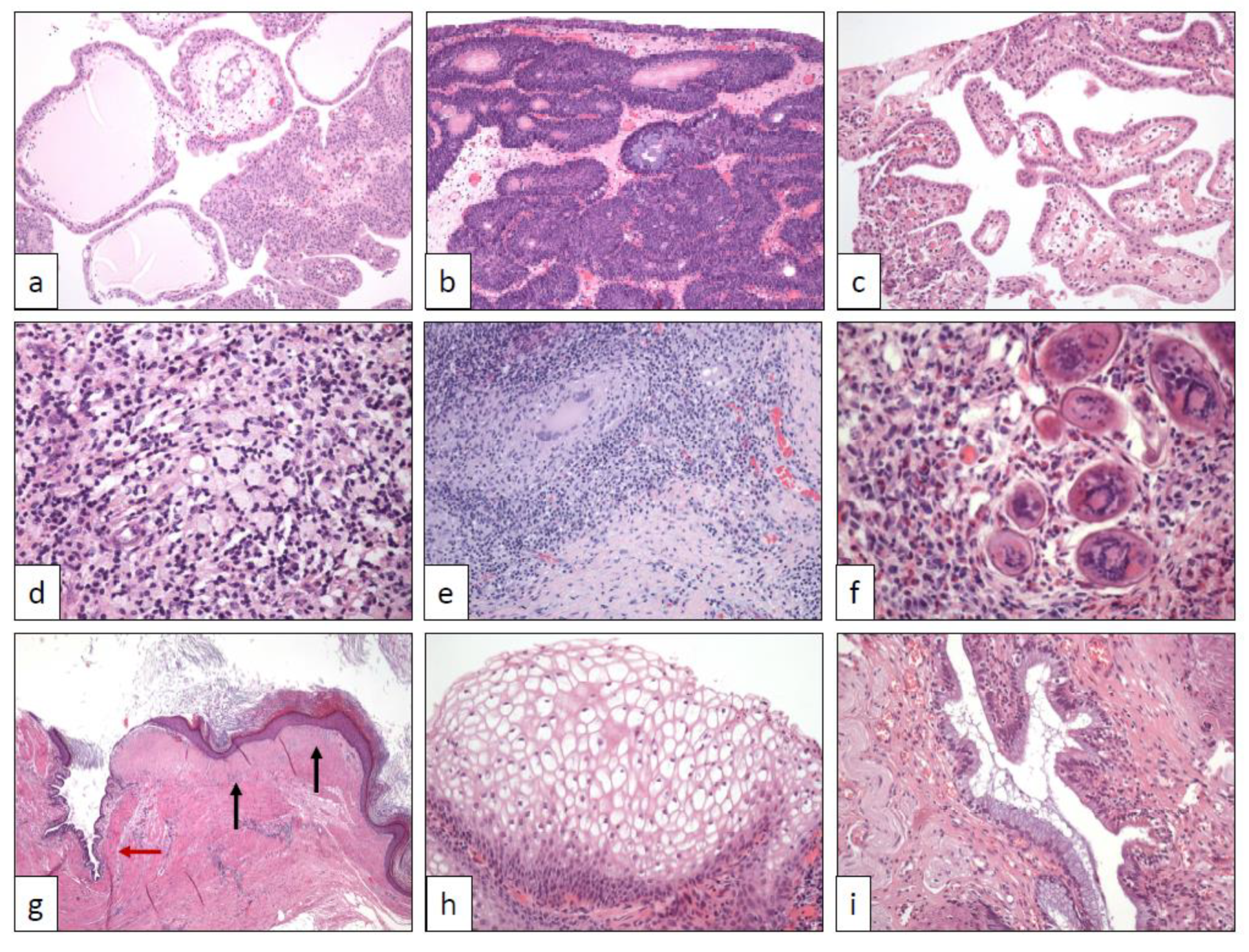
2. Urothelial Papilloma (UP)
3. Inverted Urothelial Papilloma (IUP)
4. Nephrogenic Adenoma (NA)
5. Polypoid Cystitis (PC), Fibroepithelial polyp (FP), Prostatic Polyp (PP), and Verumontanum Cyst (VC)
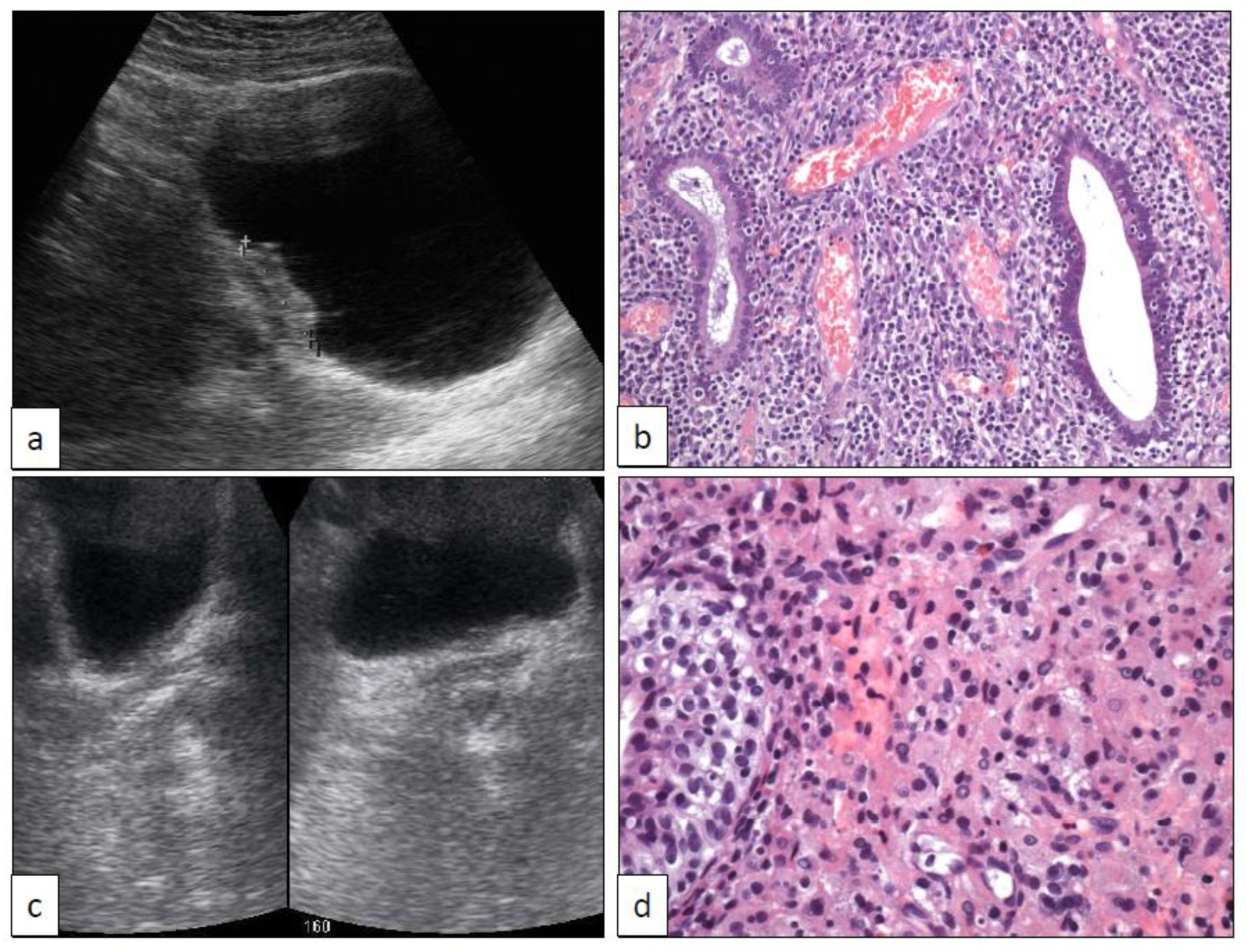
6. Xantho-Granulomatous Inflammation (XI)
7. Bacillus Calmette-Guerin-Induced Inflammatory Reaction (BCGitis)
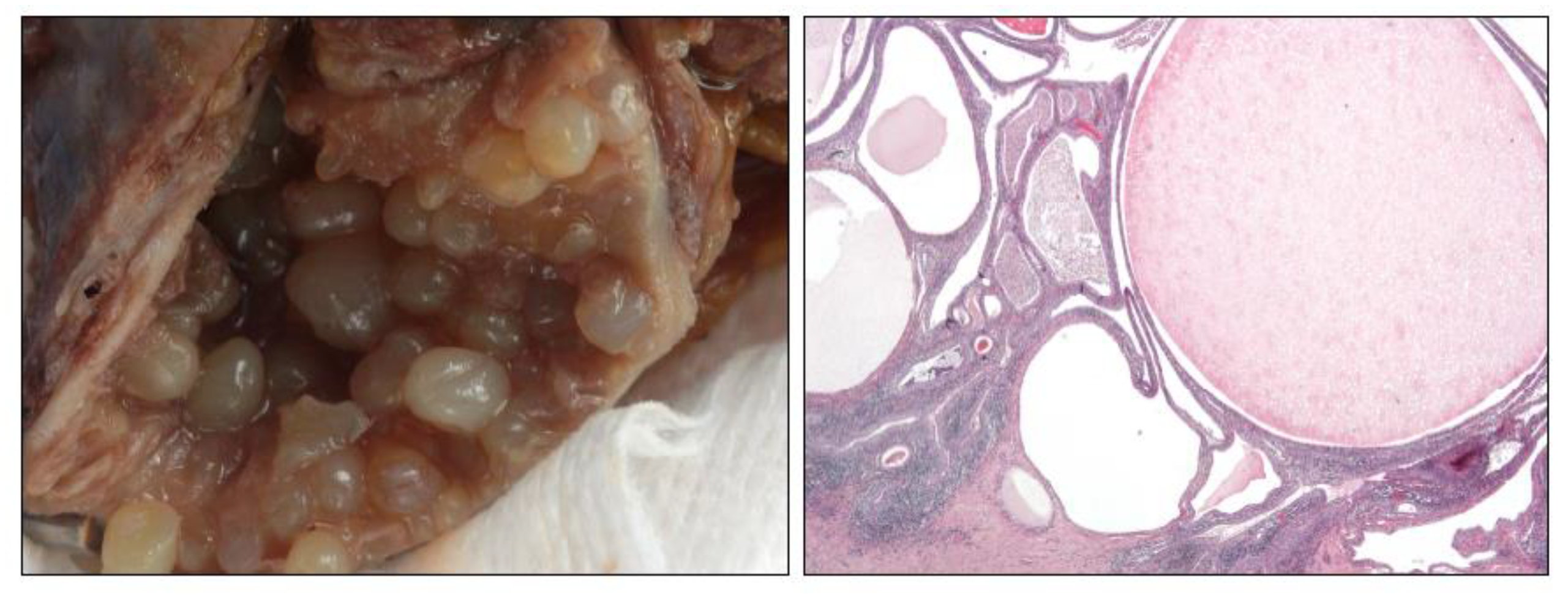
8. Schistosomiasis (SC)
9. Keratinizing Desquamative Squamous Metaplasia (KSM)
10. Post-Radiation Changes (PRC)
11. Vaginal-Type Squamous Metaplasia (VM)
12. Endocervicosis (EC)/Endometriosis (EM) (Müllerianosis)
13. Malakoplakia (MK)
14. Florid von Brunn Nest Proliferation (VB), Cystitis Cystica (CC), and Cystitis Glandularis (CG)
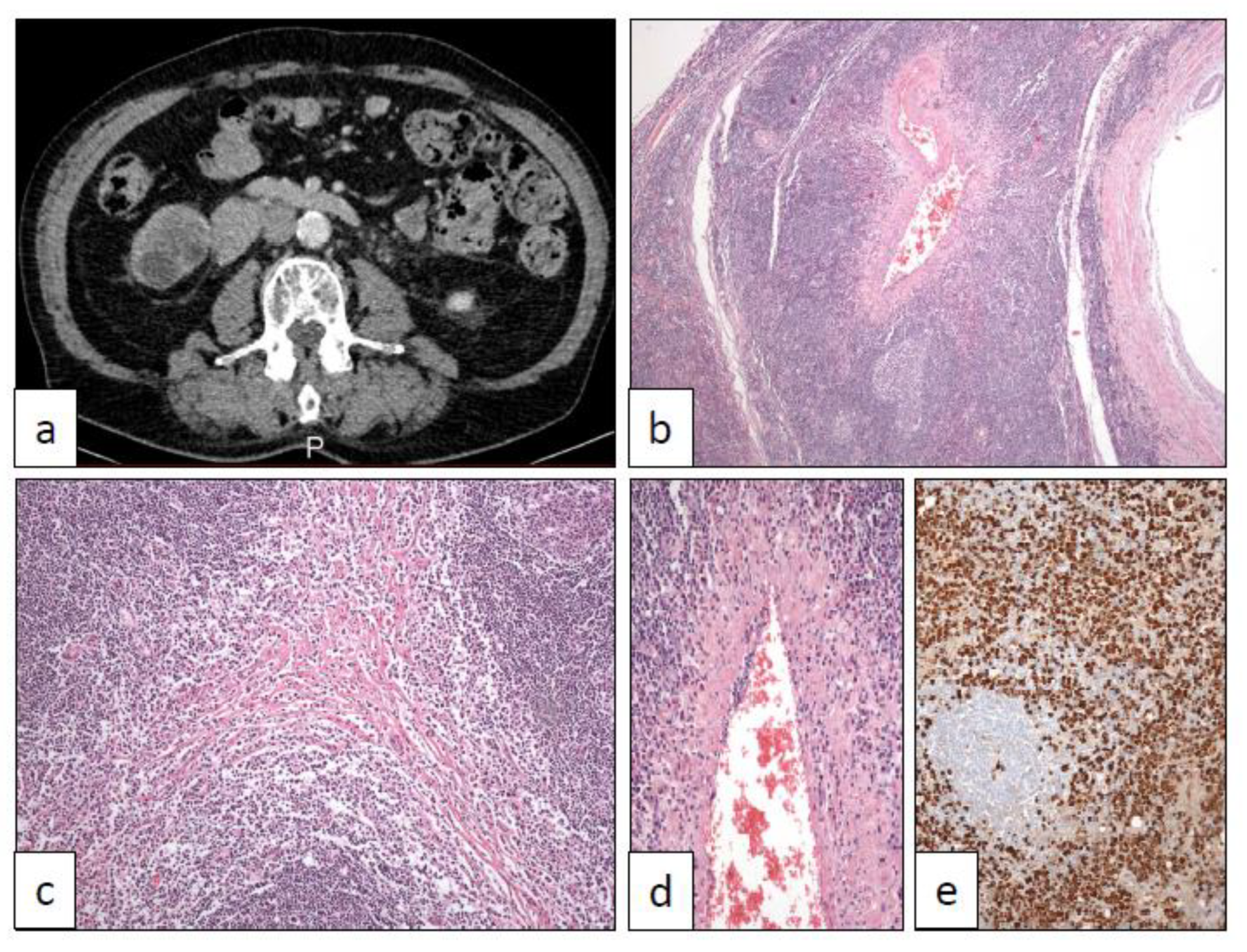
15. IgG4-Related Disease (IGG4)
16. PEComa (PEC)
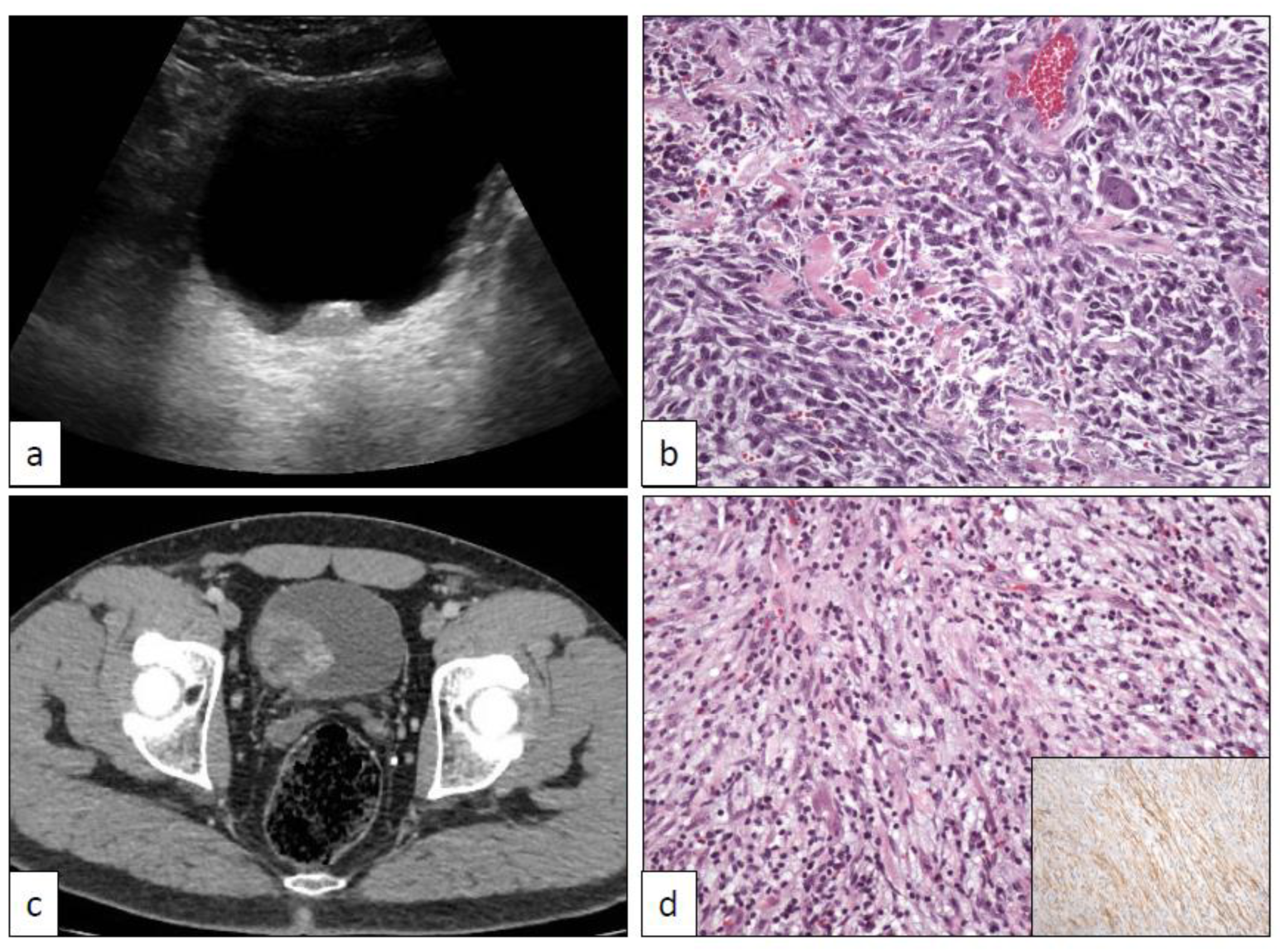
17. Pseudosarcomatous Myofibroblastic Proliferations
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Manini, C.; López, J.I. Unusual faces of bladder cancer. Cancers 2020, 12, 3706. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Reuter, V.R.; Mostofi, F.K. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am. J. Surg. Pathol. 1998, 22, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashir, S.; Yilmaz, A.; Gotto, G.; Trpkov, K. Long term outcome of primary urothelial papilloma: A single institution cohort. Pathology 2014, 46, 37–40. [Google Scholar] [CrossRef]
- Bang, H.; Park, H.; Park, S.; Choi, E.; Cho, M.S.; Sung, S.H.; Choi, S.Y.; Cho, I.M.; Jeong, S.U.; Ro, J.Y. Clinicopathologic study of 60 cases of urothelial neoplasms with inverted growth patterns: Reclassification by international consultation on urologic disease (ICUD) recommendations. Ann. Diagn. Pathol. 2020, 44, 151433. [Google Scholar] [CrossRef]
- Picozzi, S.; Casellato, S.; Bozzini, G.; Ratti, D.; Macchi, A.; Rubino, B.; Pace, G.; Carmignani, L. Inverted papilloma of the bladder: A review and an analysis of the recent literature of 365 patients. Urol. Oncol. 2013, 31, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.E.; Davidson, D.D.; Lopez-Beltran, A.; Montironi, R.; MacLennan, G.T.; Compérat, E.; Idrees, M.T.; Emerson, R.E.; Cheng, L. Human papillomavirus is not an etiologic agent of urothelial inverted papillomas. Am. J. Surg. Pathol. 2013, 37, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.; Lopez-Beltran, A.; MacLennan, G.T.; Montironi, R.; Cheng, L. Urothelial lesions with inverted growth patterns: Histogenesis, molecular genetic findings, differential diagnosis and clinical management. BJU Int. 2010, 107, 532–537. [Google Scholar] [CrossRef]
- Patel, P.; Reikie, B.A.; Maxwell, J.P.; Yilmaz, A.; Gotto, G.T.; Trpkov, K. Long-term clinical outcome of inverted urothelial papilloma including cases with focal papillary pattern: Is continuous surveillance necessary? Urology 2013, 82, 857–860. [Google Scholar] [CrossRef]
- Kryvenko, O.N.; Epstein, J.I. Mimickers of urothelial neoplasia. Ann. Diagn. Pathol. 2019, 38, 11–19. [Google Scholar] [CrossRef]
- Santi, R.; Galli, I.C.; Canzonieri, V.; López, J.I.; Nesi, G. Inverted urothelial papilloma of the upper urinary tract: Description of two cases with systematic literature review. Diagn. Pathol. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Akgul, M.; MacLennan, G.T.; Cheng, L. Distinct mutational landscape of inverted urothelial papilloma. J. Pathol. 2019, 249, 3–5. [Google Scholar] [CrossRef]
- Almassi, N.; Pietzak, E.J.; Sarungbam, J.; Tickoo, S.K.; Reuter, V.E.; Solit, D.B.; Al-Ahmadie, H.A. Inverted urothelial papilloma and urothelial carcinoma with inverted growth are histologically and molecularly distinct entities. J. Pathol. 2020, 250, 464–465. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.S.; Zhai, Y.; Cho, K.R.; Dhanasekaran, S.M.; Montgomery, J.S.; Palapattu, G.; Siddiqui, J.; Morgan, T.; Alva, A.; Weizer, A.; et al. HRAS mutations are frequent in inverted urothelial neoplasms. Hum. Pathol. 2014, 45, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Isharwal, S.; Hu, W.; Sarungbam, J.; Chen, Y.B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Sirintrapun, S.J.; Jadallah, S.; Loo, F.L.; et al. Genomic landscape of inverted urothelial papilloma and urothelial papilloma of the bladder. J. Pathol. 2019, 248, 260–265. [Google Scholar] [CrossRef]
- Mazal, P.R.; Schaufler, R.; Altenhuber-Müller, R.; Haitel, A.; Watschinger, B.; Kratzik, C.; Krupitza, G.; Regele, H.; Meisl, F.T.; Zechner, O.; et al. Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients. N. Eng. J. Med. 2002, 347, 653–659. [Google Scholar] [CrossRef]
- López, J.I.; Schiavo-Lena, M.; Corominas-Cishek, A.; Yagüe, A.; Bauleth, K.; Guarch, R.; Hes, O.; Tardanico, R. Nephrogenic adenoma of the urinary tract: Clinical, histological, and immunohistochemical characteristics. Virchows Arch. 2013, 463, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Santi, R.; Angulo, J.C.; Nesi, G.; de Petris, G.; Kuroda, N.; Hes, O.; López, J.I. Common and uncommon features of nephrogenic adenoma revisited. Pathol. Res. Pract. 2019, 215, 152561. [Google Scholar] [CrossRef]
- Sharifai, N.; Abro, B.; Chen, J.F.; Zhao, M.; He, H.; Cao, D. Napsin A is a highly sensitive marker for nephrogenic adenoma: An immunohistochemical study with a specificity test in genitourinary tumors. Hum. Pathol. 2020, 102, 23–32. [Google Scholar] [CrossRef]
- Kwok, J.L.; Eng, M. Polypoid cystitis and bilateral hydronephrosis mimicking urothelial carcinoma. J. Endourol. Case Rep. 2019, 5, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmadie, H.; Gomez, A.M.; Trane, N.; Bove, K.E. Giant botryoid fibroepithelial polyp of bladder with myofibroblastic stroma and cystitis cystica et glandularis. Pediatr. Dev. Pathol. 2003, 6, 179–181. [Google Scholar] [CrossRef]
- Humphrey, P.A. Prostatic-type epithelial polyp of the urethra. J. Urol. 2015, 193, 2095–2096. [Google Scholar] [CrossRef]
- Nork, J.J.; Yap, M.K.; Kaplan, G.W. Verumontanum cyst associated with lower urinary tract symptoms in an adolescent. Urology 2016, 88, 192–194. [Google Scholar] [CrossRef]
- Korkes, F.; Favoretto, R.L.; Bróglio, M.; Silva, C.A.; Castro, M.G.; Perez, M.D. Xanthogranulomatous pyelonephritis: Clinical experience with 41 cases. Urology 2008, 71, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.A.; Williamson, S.R. Xanthogranulomatous ureteritis mimicking ureteral involvement by cancer in a radical cystectomy specimen. Int. J. Surg. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.W.; Fegan, A.W.; Baithun, S.I. Xanthogranulomatous cystitis associated with malignant neoplasms of the bladder. Histopathology 1998, 33, 212–215. [Google Scholar] [CrossRef]
- Ali, A.M.; Nelvigi, G.G.; Keshavaiah, V.G.; Ratkal, C.S. Extensive xanthogranulomatous cystitis mimicking bladder cancer. Urol. Ann. 2014, 6, 373–375. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.C.; Zheng, L.Q.; Miao, W.L. Xanthogranulomatous cystitis imitating bladder neoplasm: A case report and review of the literature. Int. J. Clin. Exp. Pathol. 2014, 7, 8255–8258. [Google Scholar]
- Jacobsen, A.; Joensen, U.N.; Hammershøj Jensen, P. A case of xanthogranulomatous inflammation of the urethra: Treatment with a steroid-based non-surgical approach. Scand. J. Urol. 2019, 53, 267–268. [Google Scholar] [CrossRef]
- Yeow, Y.; Chong, Y.L. Xanthogranulomatous pyelonephritis presenting as Proteus preperitoneal abscess. J. Surg. Case Rep. 2016, 2016, rjw211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Val-Bernal, J.F.; Castro, F. Xanthogranulomatous pyelonephritis associated with transitional cell carcinoma of the renal pelvis. Urol. Int. 1996, 57, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gilbert, S.M.; Kamat, A.M. Unraveling the mechanism of the antitumor activity of Bacillus Calmette-Guerin. Eur. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Mbanefo, E.C.; Fu, C.L.; Ho, C.P.; Le, L.; Ishida, K.; Hammam, O.; Hsieh, M.H. Interleukin-4 signaling plays a major role in urogenital schistosomiasis-associated bladder pathogenesis. Infect. Immun. 2020, 88, e00669-19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Barnetson, R.J.; Krishna, N.S. Keratinizing squamous metaplasia of the bladder: A review. Urol. Int. 2008, 81, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Boswell, P.D.; Fugitt, B.; Kane, C.J. Keratinizing desquamative squamous metaplasia of the kidney mimicking transitional cell carcinoma. Urology 1998, 52, 512–513. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Reynolds, L.F.; Watterson, J.D.; Lavallée, L.T.; Flood, T.A. Recurrent nephrolithiasis associated with keratinizing desquamative squamous metaplasia. Can. J. Urol. 2016, 23, 8577–8580. [Google Scholar]
- Coode-Bate, J.; Davies, M.C.; Cook, I. Keratinizing desquamative squamous metaplasia in the kidney of a patient with complex spinal cord injury. J. Spinal Cord Med. 2016, 39, 240–242. [Google Scholar] [CrossRef][Green Version]
- Angulo, J.C.; López, J.I.; Flores, N. Pseudosarcomatous myofibroblastic proliferation of the bladder: Report of two cases and literature review. J. Urol. 1994, 151, 1008–1012. [Google Scholar] [CrossRef]
- Baker, P.M.; Young, R.H. Radiation-induced pseudocarcinomatous proliferations of the urinary bladder: A report of 4 cases. Hum. Pathol. 2000, 31, 678–683. [Google Scholar] [CrossRef]
- Long, E.D.; Shepherd, R.T. The incidence and significance of vaginal metaplasia of the bladder trigone in adult women. Br. J. Urol. 1983, 55, 189–194. [Google Scholar] [CrossRef]
- Henry, L.; Fox, M. Histological findings in pseudomembranous trigonitis. J. Clin. Pathol. 1971, 24, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.J.; Henry, L.; Harris, S.C.; Giri, D.D.; Fox, M.; Underwood, J.C.E. Pseudomembranous trigonitis of the bladder: Hormonal aetiology. J. Clin. Pathol. 1989, 42, 922–926. [Google Scholar] [CrossRef]
- Rogers, S.; Cross, S.S.; Williams, J.L.; Shortland, J.R. Vaginal metaplasia of the urothelium in two non-oestrogenized males. Histopathology 1991, 19, 378–379. [Google Scholar] [CrossRef]
- López, J.I.; Angulo, J.C. Vaginal metaplasia of the bladder associated with ectopic ureterocele. A clinicopathologic study of two cases. Arch. Anat. Cytol. Pathol. 1992, 40, 217–219. [Google Scholar]
- Habiba, M.; Brosens, I.; Benagiano, G. Müllerianosis, endocervicosis, and endosalpingiosis of the urinary tract: A literature review. Reprod. Sci. 2018, 25, 1607–1618. [Google Scholar] [CrossRef]
- Leonardi, M.; Espada, M.; Kho, R.M.; Magrina, J.F.; Millischer, A.E.; Savelli, L.; Condous, G. Endometriosis and the urinary tract: From diagnosis to surgical treatment. Diagnostics 2020, 10, 771. [Google Scholar] [CrossRef]
- Nakaguro, M.; Tsuzuki, T.; Shimada, S.; Taki, T.; Tsuchiyama, M.; Kitamura, A.; Suzuki, Y.; Nakano, Y.; Ono, K. Adenocarcinoma arising in urinary bladder endocervicosis. Pathol. Int. 2016, 66, 108–113. [Google Scholar] [CrossRef]
- Clement, P.B.; Young, R.H. Endocervicosis of the urinary bladder. A report of six cases of a benign müllerian lesion that may mimic adenocarcinoma. Am. J. Surg. Pathol. 1992, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A. Endometriosis, endocervicosis and müllerianosis of the bladder. J. Urol. 2014, 192, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Tsujimoto, M.; Tsubamoto, H.; Komori, S.; Hirota, S. Immunohistochemical phenotype of the urinary bladder endocervicosis: Comparison with normal endocervix and well-differentiated mucinous adenocarcinoma of the uterine cervix. Pathol. Int. 2010, 60, 528–532. [Google Scholar] [CrossRef]
- Sulman, A.; Goldman, H. Malacoplakia presenting as a large bladder mass. Urology 2002, 60, 163. [Google Scholar] [CrossRef]
- Lee, S.L.J.; Teo, J.K.; Limj, S.K.T.; Hema, P.S.; Mancer, K. Coexistence of malakoplakia and papillary urothelial carcinoma of the urinary bladder. Int. J. Surg. Pathol. 2015, 23, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Tian, W.; Zhuge, J.; Zheng, X.; Huang, T.; Cai, D.; Zhang, D.; Yang, X.J.; Argani, P.; Fallon, J.T.; et al. Distinguishing nested variants of urothelial carcinoma from benign mimickers by TERT promoter mutation. Am. J. Surg. Pathol. 2015, 39, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H. What do the IgG4-related disease (IgG4-RD) classification criteria tell us about the nature of IgG4-RD? Presse Med. 2020, 49, 104020. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, K.M. IgG4-related inflammatory pseudotumor of the renal pelvis involving renal parenchyma, mimicking malignancy. Diagn. Pathol. 2016, 11, 12. [Google Scholar] [CrossRef]
- Lei, W.; Xin, J.; Shao, C.; Mao, M.; Zhu, C.; Wu, C.; Jin, L. IgG4-related kidney disease mimicking malignant ureter tumor. Case report and literature review. Medicine (Baltimore) 2016, 95, e2550. [Google Scholar] [CrossRef]
- Zhong, W.; Kam, J.; Beattle, K.; Yuminaga, Y.; Ferguson, R.; Ko, R. A rare case of ureteral IgG4 disease masquerading as urothelial carcinoma. Urology 2018, 118, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Kufukihara, R.; Niwa, N.; Mizuno, R.; Ohara, K.; Mikami, S.; Kikuchi, E.; Oya, M. Immunoglobulin G4-related disease arising from the bladder wall. Urol. Int. 2019, 103, 488–490. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, G.; Cheng, W. Renal pelvis involvement of immunoglobulin G4-related disease mimicking malignancy on 18F-FDG PET/CT. Clin. Nucl. Med. 2019, 44, 767–768. [Google Scholar] [CrossRef]
- Gehring, C.; Starkebaum, G.A.; Voelzke, B.B.; Liew, J.W. Immunoglobulin G4-related disease of the urinary bladder. Rheumatology (Oxford) 2020, 59, 907–908. [Google Scholar] [CrossRef]
- Kamo, M.; Nozaki, T.; Muraishi, N.; Hattori, K.; Suzuki, K.; Okada, M.; Yamamura, J. CT findings of upper urinary tract lesions in IgG4-related disease: Comparison with urothelial carcinoma. AJR Am. J. Roentgenol. 2020, 215, 406–412. [Google Scholar] [CrossRef]
- Deshpande, V. The pathology of IgG4-related disease: Critical issues and challenges. Semin. Diagn. Pathol. 2012, 29, 191–196. [Google Scholar] [CrossRef]
- Martignoni, G.; Pea, M.; Reghellin, D.; Zamboni, G.; Bonetti, F. Perivascular epithelioid cell tumor (PEComa) in the genitourinary tract. Adv. Anat. Pathol. 2007, 14, 36–41. [Google Scholar] [CrossRef]
- Planelles, M.; Macías, L.; Peiró, G.; Bulimbasic, S.; Hes, O.; Robles, A.; Michal, M.; Davidson, W.; López, J.I. Rheb/mTOR/p70s6k cascade and TFE3 expression in conventional and sclerosing PEComas of the urinary tract. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Cheng, M.Y.; Ng, K.F.; Liu, C.Y.; Wang, J.S.; Chai, C.I.; Huang, S.H.; Chen, P.C.H.; Ho, D.M.T. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): Genetic evidence for the relationship of PEComa with angiomyolipoma. J. Pathol. 2008, 214, 387–393. [Google Scholar] [CrossRef]
- Hornick, J.L.; Fletcher, C.D.M. Sclerosing PEComa: Clinicopathologic analysis of a distinctive variant with a predilection for the retroperitoneum. Am. J. Surg. Pathol. 2008, 32, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Bunde, P.J.; Montironi, R.; López-Beltrán, A.; Zhang, S.; Wang, M.; MacLennan, G.T.; Cheng, L. Malignant perivascular epithelioid cell neoplasm (PEComa) of the urinary bladder with TFE3 gene rearrangement: Clinicopathologic, immunohistochemical and molecular features. Am. J. Surg. Pathol. 2013, 37, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Alquati, S.; Gira, F.A.; Bartoli, V.; Contini, S.; Corradi, D. Low-grade myofibroblastic proliferations of the urinary bladder. Arch. Pathol. Lab. Med. 2013, 137, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Santana, A.; Sanchez-Chapado, M. Bilateral keratinizing desquamative squamous metaplasia of the upper urinary tract. J. Urol. 1997, 158, 1908–1909. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Pulinthanathu, R. Malignant transformation of inflammatory myofibroblastic tumor of urinary bladder: A rare case scenario. Bladder 2019, 6, e39. [Google Scholar] [CrossRef]
- Proppe, K.H.; Scully, R.E.; Rosai, J. Postoperative spindle cell nodules of genitourinary tract resembling sarcomas: A report of eight cases. Am. J. Surg. Pathol. 1984, 8, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nochomovitz, L.E.; Orenstein, J.M. Inflammatory pseudotumor of the urinary bladder–possible relationship to nodular fasciitis: Two case reports, cytologic observations, and ultrastructural observations. Am. J. Surg. Pathol. 1985, 9, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sebastin, J.A.S.; Smith, S.C.; Perry, K.D.; Gupta, N.S.; Alanee, S.; Carskadon, S.; Chitale, D.A.; Palanisamy, N.; Williamson, S.R. Pseudosarcomatous myofibroblastic proliferations of the genitourinary tract are genetically different from nodular fasciitis and lack USP6, ROS1 and ETV6 gene rearrangements. Histopathology 2018, 73, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.M.; Demicco, E.G.; Dal Cin, P.; Hirsch, M.S.; Fletcher, C.D.M.; Jo, V.Y. Pseudosarcomatous myofibroblastic proliferations of the urinary bladder are neoplasms characterized by recurrent FN1-ALK fusions. Mod. Pathol. 2020. [Google Scholar] [CrossRef]
- Bertz, S.; Stöhr, R.; Gaisa, N.T.; Wullich, B.; Hartmann, A.; Agaimy, A. TERT promoter mutation analysis as a surrogate to morphology and immunohistochemistry in problematic spindle cell lesions of the urinary bladder. Histopathology 2020, 77, 949–962. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manini, C.; Angulo, J.C.; López, J.I. Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis. Clin. Pract. 2021, 11, 110-123. https://doi.org/10.3390/clinpract11010017
Manini C, Angulo JC, López JI. Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis. Clinics and Practice. 2021; 11(1):110-123. https://doi.org/10.3390/clinpract11010017
Chicago/Turabian StyleManini, Claudia, Javier C. Angulo, and José I. López. 2021. "Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis" Clinics and Practice 11, no. 1: 110-123. https://doi.org/10.3390/clinpract11010017
APA StyleManini, C., Angulo, J. C., & López, J. I. (2021). Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis. Clinics and Practice, 11(1), 110-123. https://doi.org/10.3390/clinpract11010017






