Patient Centeredness in Orthognathic Surgery
Abstract
1. Introduction
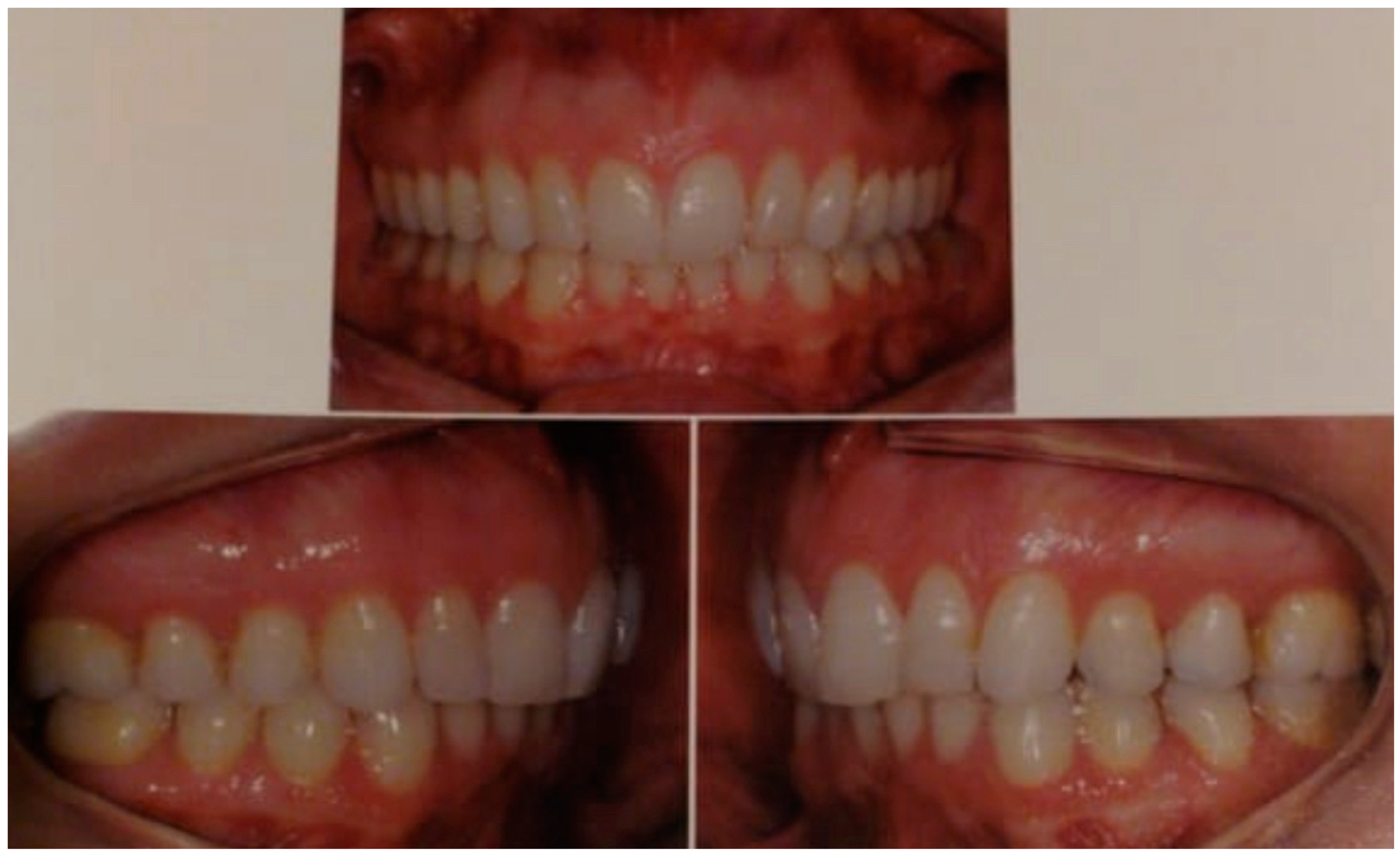
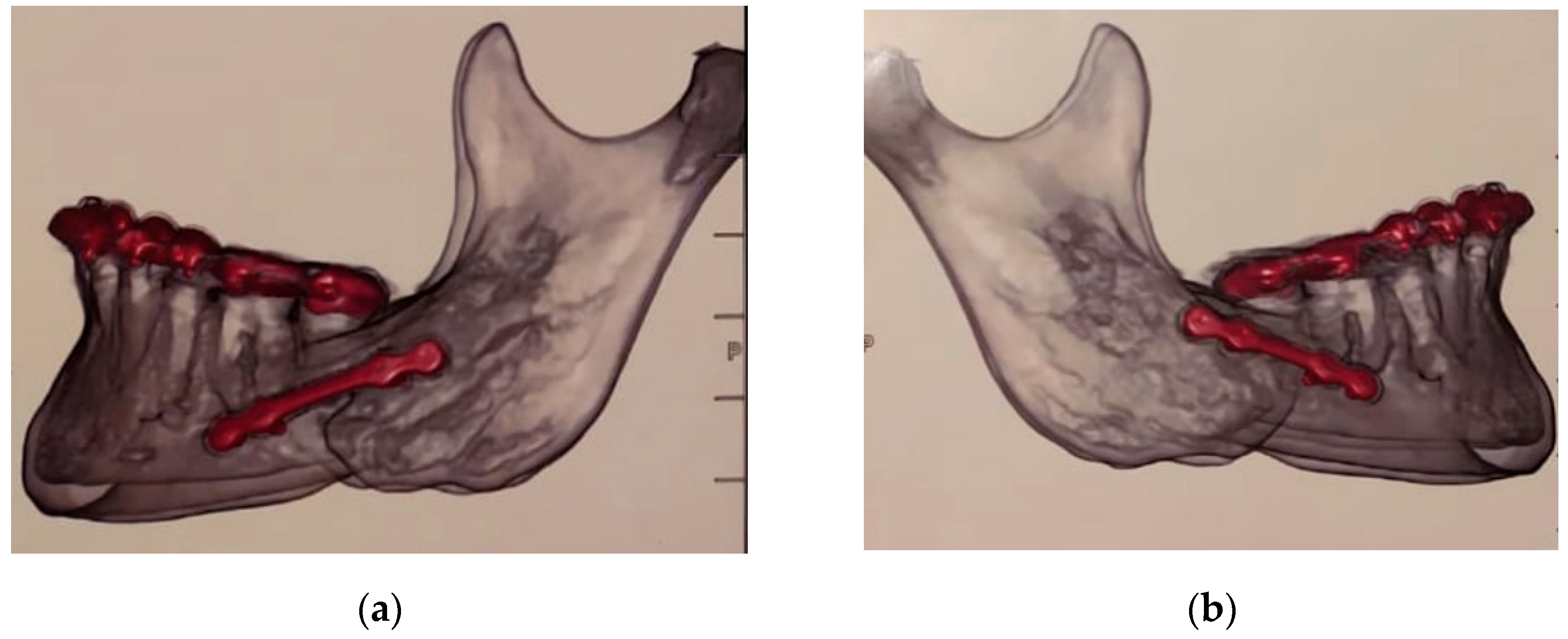
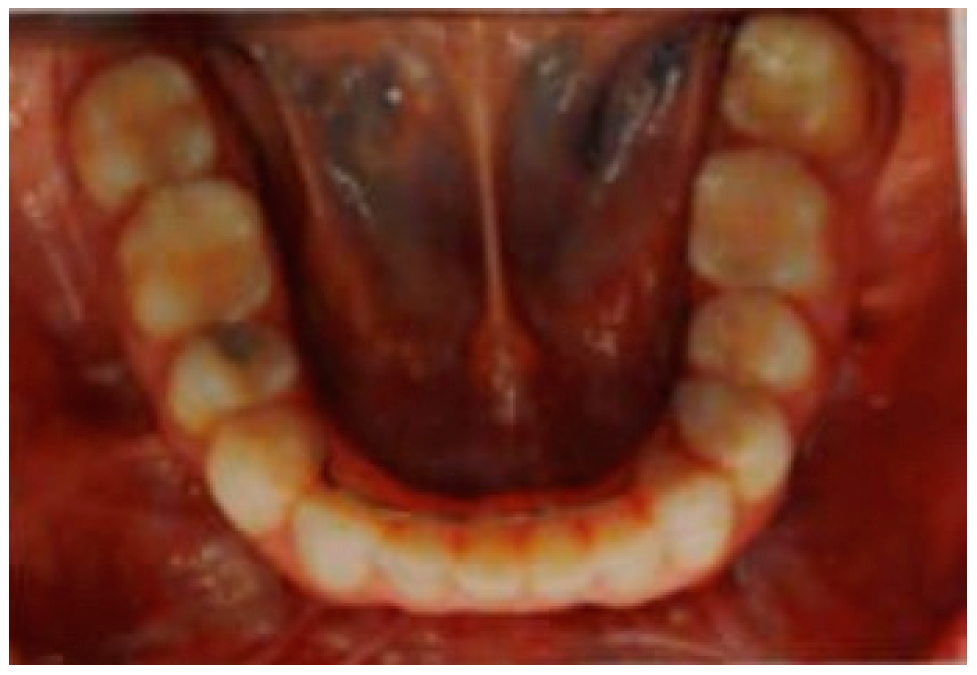
2. Orthognathic Surgery Indicated to Improve Jaw Function
3. Discussion
- postoperatory 3-D imaging shortly after surgery to prove that the screws have not been inserted into the nerve canal;
- either prosthodontic, but most preferably orthodontic measures, to achieve correct and stable occlusion. If prosthodontic treatment is considered, an instrumentally assisted analysis of the bite is necessary;
- in case of persisting pain, removal of the plates after about 6–8 months post-surgery, since they can definitely contribute to painful sensations;
- pain relieving medication/low-level laser application.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhadelia, A.; De Lima, L.; Arreola-Ornelas, H.; Kwete, X.J.; Rodriguez, N.M.; Knaul, F.M. Solving the global crisis in access to pain relief: Lessons from country actions. Am. J. Public Health 2019, 1, 58–60. [Google Scholar] [CrossRef]
- Knaul, F.M.; Farmer, P.E.; Krakauer, E.L.; De Lima, L.; Bhadelia, A.; Kwete, X.J.; Arreola-Ornelas, H.; Gómez-Dantés, O.; Rodriguez, N.M.; A O Alleyne, G.; et al. Alleviating the access abyss in palliative care and pain relief—An imperative universal health coverage: The Lancet Commission report. Lancet 2018, 391, 1391–1454. [Google Scholar] [CrossRef]
- Agbaje, J.; Luyten, J.; Politis, C. Pain complaints in patients undergoing orthognathic surgery. Pain Res. Manag. 2018, 2018, 4235025. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Wolford, L.M.; Perez, D.; Laskin, D.M.; Ellis, E. Does orthognathic surgery cause or cure temporomandibular disorders? A systematic review and meta-analysis. J. Oral Maxillofac. Surg. 2017, 75, 1835–1847. [Google Scholar] [CrossRef]
- Oncology Nursing Society. Available online: https://www.ons.org/ (accessed on 11 April 2020).
- Center for Device and Radiological Health, U.S. Food and Drug Administration. Radiation-Emitting Product Code: NHN. Viewed 15 November 2011. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD_rh/classification.cfm?PCD=NHN (accessed on 11 April 2020).
- The American Chiropractic Association. Available online: http://www.acatoday.org/index.cfm (accessed on 11 April 2020).
- Wong, S.-F.; Wilder-Smith, P. Pilot study of laser effects on oral mucositis in patients receiving chemotherapy. Cancer J. 2002, 8, 247–254. [Google Scholar] [CrossRef]
- Nes, A.G.; Posso, M.B. Patients with moderate chemotherapy-induced mucositis: Pain therapy using low intensity lasers. Int. Nurs. Rev. 2005, 52, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Corti, L.; Chiarion-Sileni, V.; Aversa, S.; Ponzoni, A.; D’Arcais, R.; Pagnutti, S.; Fiore, D.; Sotti, G.; Corti, L. Treatment of chemotherapy-induced oral mucositis with light-emitting diode. Photomed. Laser Surg. 2006, 24, 207–213. [Google Scholar] [CrossRef]
- Abramoff, M.M.F.; Lopes, N.N.F.; Lopes, L.A.; Dib, L.L.; Guilherme, A.; Caran, E.M.; Barreto, A.D.; Lee, M.L.M.; Petrilli, A.S. Low-level laser therapy in the prevention and treatment of chemotherapy-induced oral mucositis in young patients. Photomed. Laser Surg. 2008, 26, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Porto, F.A.; Miraglia, P.; Brunetto, A.L. Low-level infrared laser therapy in chemotherapy-induced oral mucositis: A randomized placebo-controlled trial in children. J. Pediatr. Hematol. Oncol. 2009, 31, 33–37. [Google Scholar] [CrossRef]
- Cauwels, R.G.; Martens, L.C. Low level laser therapy in oral mucositis: A pilot study. Eur. Arch. Paediatr. Dent. 2011, 12, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Arbabi-Kalati, F.; Arbabi-Kalati, F.; Moridi, T. Evaluation of the effect of low level laser on prevention of chemotherapy-induced mucositis. Acta. Med. Iran. 2013, 51, 157–162. [Google Scholar] [PubMed]
- Chermetz, M.; Gobbo, M.; Ronfani, L.; Ottaviani, G.; Zanazzo, G.A.; Verzegnassi, F.; Treister, N.S.; Di Lenarda, R.; Biasotto, M.; Zacchigna, S. Class IV laser therapy as treatment for chemotherapy-induced oral mucositis in onco-haematological paediatric patients: A prospective study. Int. J. Paediatr. Dent. 2014, 24, 441–449. [Google Scholar] [CrossRef]
- Freitas, A.C.; Campos, L.; Brandão, T.B.; Cristófaro, M.; Eduardo, F.D.P.; Luiz, A.C.; Marques, M.M.; Eduardo, C.D.P.; Simões, A. Chemotherapy-induced oral mucositis: Effect of LED and laser phototherapy treatment protocols. Photomed. Laser. Surg. 2014, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Irby, A. Orthognathic surgery risk factors and complications in patients with increasing age. J. Oral. Maxillofac. Surg. 2016, 74, e66–e67. [Google Scholar] [CrossRef]
- Zins, J.E.; Bruno, J.; Moreira-Gonzalez, A.; Bena, J. Orthognathic surgery: Is there a future? Plast. Reconstr. Surg. 2005, 116, 1442–1450. [Google Scholar] [CrossRef]
- Ford, B.P.; Levin, L.M.; Drane, H.B. Trends in orthognathic surgery: A national survey of orthodontists. J. Oral. Maxillofac. Surg. 2016, 72, e45–e46. [Google Scholar] [CrossRef]
- Perillo, L.; Padricelli, G.; Isola, G.; Femiano, F.; Chiodini, P.; Matarese, G. Class II malocclusion dividion 1: A new classification method by cephalometric analysis. Eur. J. Paediatr. Dent. 2012, 13, 192–196. [Google Scholar] [PubMed]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiophatic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar] [PubMed]
- Holt, G.E.; Sarmento, B.; Kett, D.; Goodman, K.W. An unconscious patient with a DNR tattoo. N. Engl. J. Med. 2017, 377, 2192–2193. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R. Genetic Basis of Oral Health Conditions, 1st ed.; Springer: Berlin, Germany, 2020. [Google Scholar]
- Lubart, R.; Wollman, Y.; Friedmann, H.; Rochkind, S.; Laulicht, I. Effects of visible and near-infrared lasers on cell cultures. J. Photochem. Photobiol. B 1992, 12, 305–310. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; Lanzafame, R.J. The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts. Photochem. Photobiol. 1994, 59, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Vinck, E.M.; Cagnie, B.J.; Cornelissen, M.J.; Declercq, H.A.; Cambier, D.C. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med. Sci. 2003, 18, 95–99. [Google Scholar] [CrossRef]
- Frigo, L.; Fávero, G.M.; Lima, H.J.; Maria, D.A.; Bjordal, J.M.; Joensen, J.; Iversen, V.V.; Marcos, R.L.; Parizzoto, N.A.; Lopes-Martins, R.A.B. Low-level laser irradiation (InGaAlP-660 nm) increases fibroblast cell proliferation and reduces cell death in a dose-dependent manner. Photomed. Laser Surg. 2010, 28, S151–S156. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Oliveira, C.F.; Kurachi, C.; Hebling, J.; Costa, C.A.D.S. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, J.; Goralczyk, K.; Klawe, J.J.; Lukowicz, M.; Michalska, M.; Goralczyk, B.; Zalewski, P.; Newton, J.L.; Gryko, L.; Zajac, A.; et al. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J. Physiol. Pharmacol. 2013, 64, 387–391. [Google Scholar]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef]
- Agaiby, A.D.; Ghali, L.R.; Wilson, R.; Dyson, M. Laser modulation of angiogenic factor production by T-lymphocytes. Lasers Surg. Med. 2000, 26, 357–363. [Google Scholar] [CrossRef]
- Stadler, I.; Evans, R.; Kolb, B.; Naim, J.O.; Narayan, V.; Buehner, N.; Lanzafame, R.J. In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg. Med. 2000, 27, 255–261. [Google Scholar] [CrossRef]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef]
- Esmaeelinejad, M.; Bayat, M. Effect of low-level laser therapy on the release of interleukin-6 and basic fibroblast growth factor from cultured human skin fibroblasts in normal and high glucose mediums. J. Cosmet. Laser Ther. 2013, 15, 310–317. [Google Scholar] [CrossRef]
- de Sousa, A.P.; Paraguassú, G.M.; Silveira, N.T.; De Souza, J.; Cangussu, M.C.T.; Dos Santos, J.N.; Pinheiro, A.L.B. Laser and LED phototherapies on angiogenesis. Lasers Med. Sci. 2013, 28, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Tsai, J.L.; Wang, Y.H.; Lee, C.-L.; Chen, J.-K.; Huang, M.-H. Low-level laser irradiation promotes cell proliferation and mRNA expression of type I collagen and decorin in porcine Achilles tendon fibroblasts in vitro. J. Orthop. Res. 2009, 27, 646–650. [Google Scholar] [CrossRef]
- Usumez, A.; Cengiz, B.; Oztuzcu, S.; Demir, T.; Aras, M.H.; Gutknecht, N. Effects of laser radiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med. Sci. 2014, 29, 1807–1813. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; Lanzafame, R.J. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg. Med. 1997, 20, 56–63. [Google Scholar] [CrossRef]
- Dadpay, M.; Sharifian, Z.; Bayat, M.; Bayat, M.; Dabbagh, A. Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J. Photochem. Photobiol. B 2012, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, H. Evaluation of low level laser therapy in reducing diabetic polyneuropathy related pain and sensorimotor disorders. Acta Med. Iran. 2013, 51, 543–547. [Google Scholar]
- Baxter, G.D.; Walsh, D.M.; Allen, J.M.; Lowe, A.S.; Bell, A.J. Effects of low intensity infrared laser irradiation upon conduction in human median nerve in vivo. Exp. Physiol. 1994, 79, 227–234. [Google Scholar] [CrossRef]
- Chow, R.T.; David, M.A.; Armati, P.J. 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root ganglion neurons: Implications for the analgesic effects of 830 nm laser. J. Peripher. Nerv. Syst. 2007, 12, 28–39. [Google Scholar] [CrossRef]
- Klein, T.; Magerl, W.; Hopf, H.C.; Sandkühler, J.; Treede, R.-D. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J. Neurosci. 2004, 24, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Iwasaka, H.; Okuda, K.; Noguchi, T. GaAlAs (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Lasers Surg. Med. 2007, 39, 797–802. [Google Scholar] [CrossRef]
- Erthal, V.; da Silva, M.D.; Cidral-Filho, F.J.; Dos Santos, A.R.S.; Nohama, P. ST36 laser acupuncture reduces pain-related behavior in rats: Involvement of the opioidergic and serotonergic systems. Lasers Med. Sci. 2013, 28, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Alpasian, C. Orofacial pain and fibromyalgia pain: Being aware of comorbid conditions. World J. Rheumatol. 2015, 5, 45–49. [Google Scholar] [CrossRef]
- Westervelt, A. The medical research gender gap: How excluding women from clinical trials is hurting our health. The Guardian, 1 May 2015; 1. [Google Scholar]
- Dusenbery, M. The surprising reason we lack so much knowledge about women’s health. Forbes, 24 August 2018; 1–4. [Google Scholar]
- Ailes, E.C.; Dawson, A.L.; Lind, J.N.; Gilboa, S.M.; Frey, M.T.; Broussard, C.S.; Honein, M.A. Opioid prescription claims among women of reproductive age—United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 37–41. [Google Scholar]
- White, P.F.; Lazo, O.L.E.; Galeas, L.; Cao, X. Use of electroanalgesia and laser therapies as alternatives to opioids for acute and chronic pain management. F1000Research 2017, 6, 2161. [Google Scholar] [CrossRef]
- Sá de Lira, A.L.; Fontenele, M.K.V. Relationship between pathological occlusal changes and the signs and symptoms of temporomandibular dysfunction. Turk. J. Orthod. 2020, 33, 210–215. [Google Scholar] [CrossRef]
- Hagemeier, N.E. Intriduction to the opioid epidemic: The economic burden on the healthcare system and impact on quality of life. Am. J. Manag. Care 2018, 24, S200–S206. [Google Scholar] [PubMed]
- Chou, R.; Turner, J.A.; Devine, E.B.; Hansen, R.N.; Sullivan, S.D.; Blazina, I.; Dana, T.; Bougatsos, C.; Deyo, R.A. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015, 162, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.G.; Rosenheck, R.A. Association of current and past opioid use disorders with health-related quality of life and employment among US adults. Drug Alcohol Depend. 2019, 199, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Blevins, C.E.; Abrantes, A.M.; Kurth, M.E.; Gordon, A.L.; Stein, M.D. Quality of life and well-being following inpatient and partial hospitalization treatment for opioid use disorder. Arch. Psychiatr. Nurs. 2018, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.R.; Prinz, M.C.O. Patient Centeredness in Orthognathic Surgery. Clin. Pract. 2021, 11, 92-100. https://doi.org/10.3390/clinpract11010014
Vieira AR, Prinz MCO. Patient Centeredness in Orthognathic Surgery. Clinics and Practice. 2021; 11(1):92-100. https://doi.org/10.3390/clinpract11010014
Chicago/Turabian StyleVieira, Alexandre R., and Maria C. O. Prinz. 2021. "Patient Centeredness in Orthognathic Surgery" Clinics and Practice 11, no. 1: 92-100. https://doi.org/10.3390/clinpract11010014
APA StyleVieira, A. R., & Prinz, M. C. O. (2021). Patient Centeredness in Orthognathic Surgery. Clinics and Practice, 11(1), 92-100. https://doi.org/10.3390/clinpract11010014




