The Effect of the Reproductive Cycle on the Bioaccumulation of Heavy Metals and the Induction of Metallothionein in the Polychaete Perinereis cultrifera of the Coastline of El Jadida (Atlantic Coast, Morocco) †
Introduction
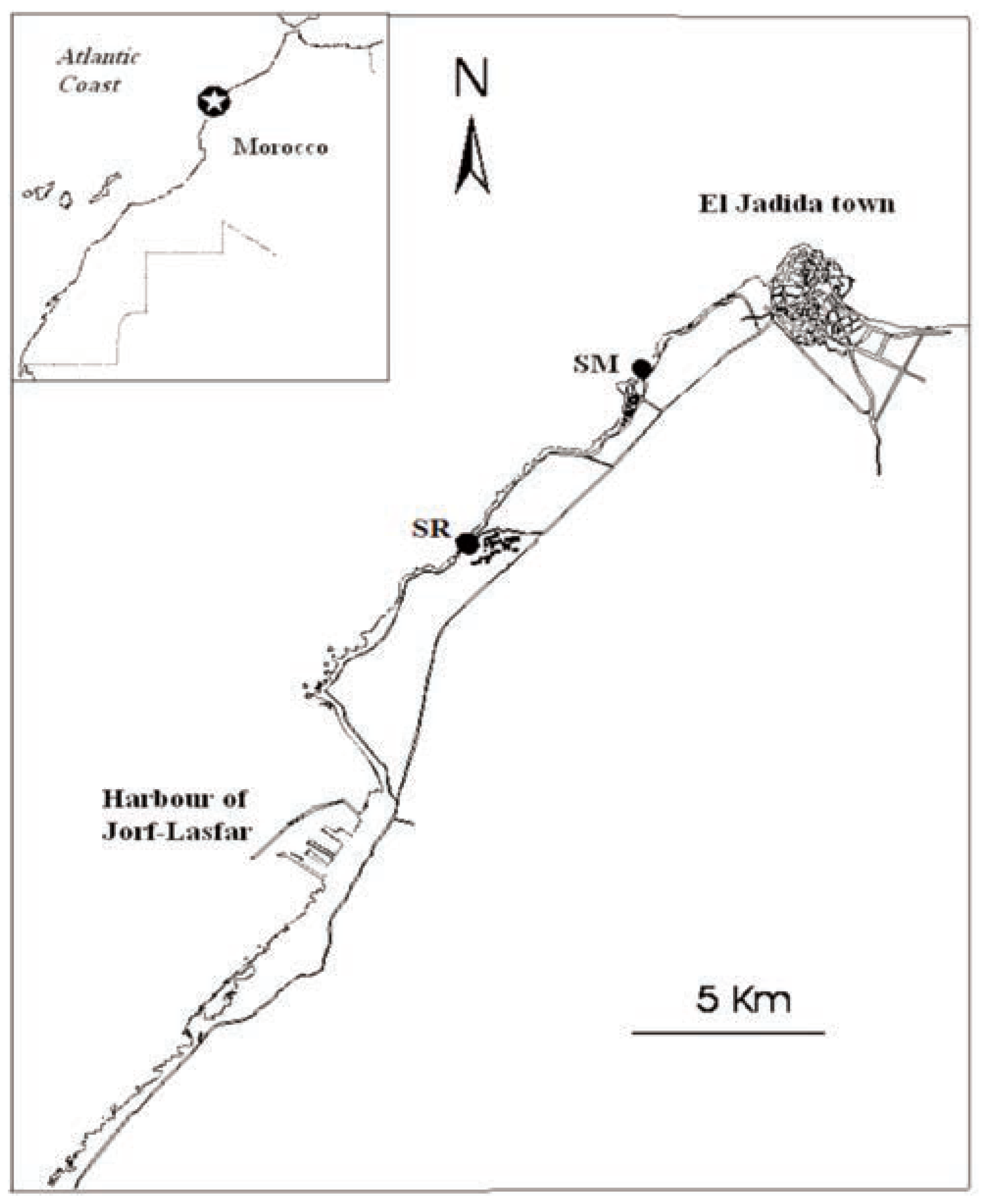
Materials and Methods
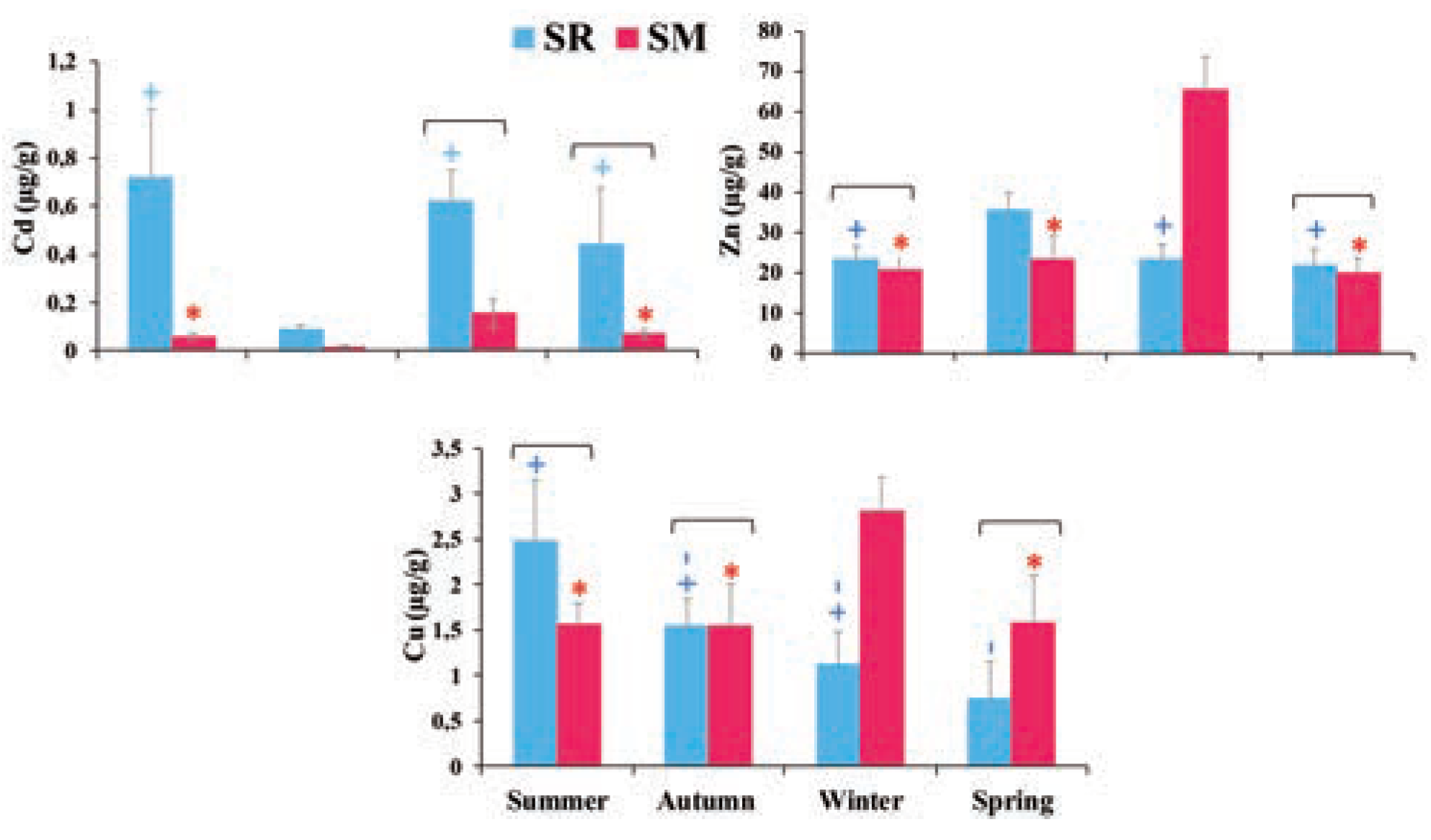
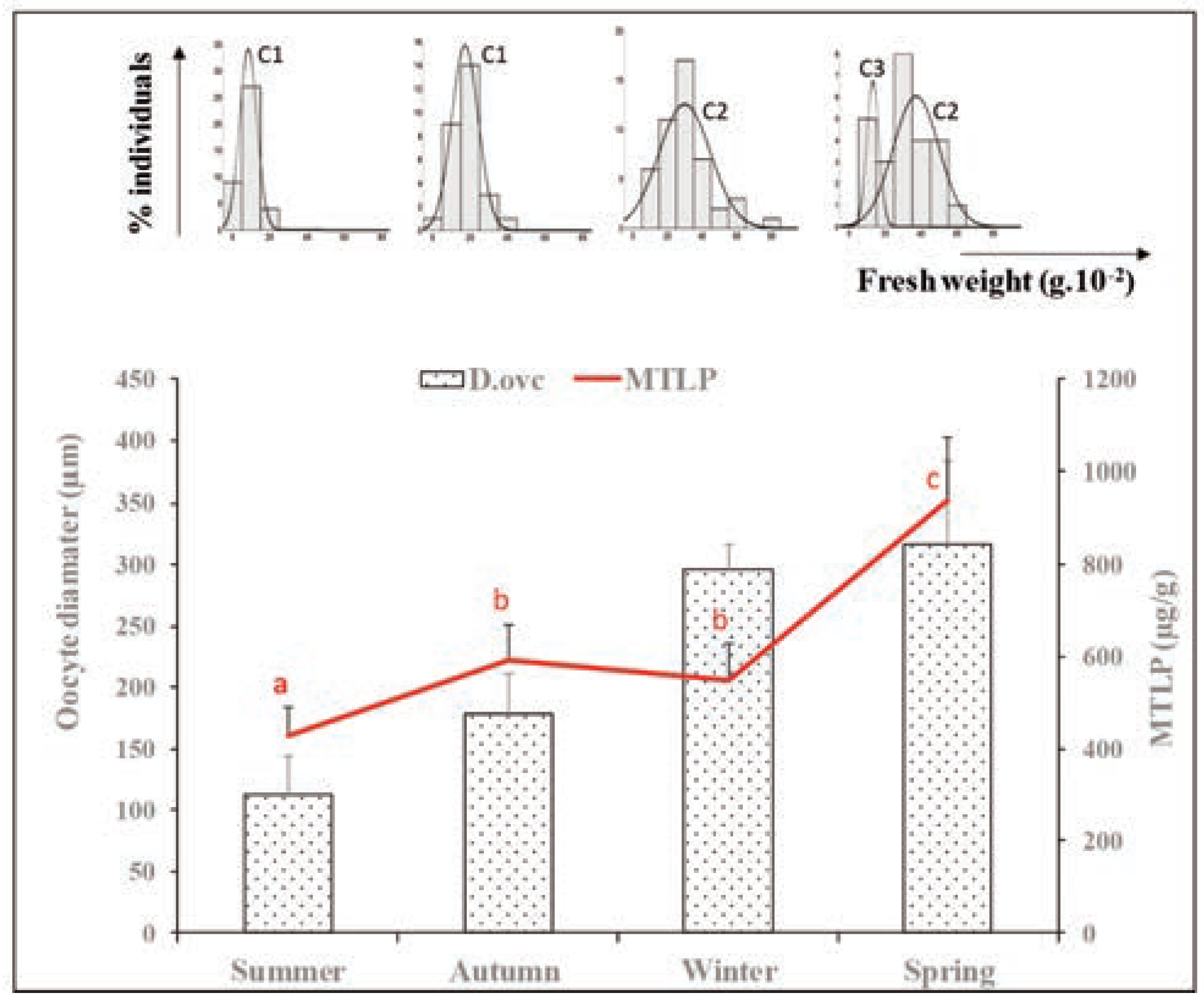
Results and discussion
Conclusions
References
- Le Bris, H.; Glémarec, M. Marine and brackish ecosystems of south Brittany (Lorient and Vilaine Bays) with particular reference to the effect of the turbidity maxima. Estuar. Coast. Shelf Sci. 1996, 42, 737–753. [Google Scholar] [CrossRef]
- Mason, A.Z.; Jenkins, K.D. Metal detoxication in aquatic organisms. In Metal Speciation and Biovailability in Aquatic Systems; Tessier, A., Turner, D.R., Eds.; Wiley & Sons: Chichester, UK, 1995; Volume 3, pp. 469–608. [Google Scholar]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakal, D.B. Principle of Ecotoxicology; Taylor and Francis: London, UK, 1996. [Google Scholar]
- Rouhi, A.; Sif, J.; Fersiwi, A.; Gillet, P.; Deutch, B. Reproduction and population dynamics of Perinereis cultrifera (Polychaeta: Nereididae) of the Atlantic coast, El Jadida, Morocco. Cah. Biol. 2008, 49, 151–216. [Google Scholar]
- Sif, J.; Rouhi, A.; Gillet, P.; Moncef, M. Diversité et écologie des Annélides Polychètes du littoral atlantique de la région d’El Jadida, Maroc. Bull. Inst. Sci. Rabat. Sect. Sci. De La Vie 2012, 34, 95–106. [Google Scholar]
- Amiard-Triquet, C.; Caurant, F. Les formes physicochimiques de stockage des métaux chez les organismes marins. Analusis 1994, 22, 24–26. [Google Scholar]
- Viarengo, A.; Nott, J.A. Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comp. Biochem. Physiol. 1993, 140C, 355–372. [Google Scholar] [CrossRef]
- Geffard, A.; Amiard-Triquet, C.; Amiard, J.C.; Mouneyrac, C. Temporal variations of met-allothionein and metal concentrations in the digestive gland of oysters Crassostrea gigas from a clean and a metal-rich sites. Biomarkers 2001, 6, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Orlando, E. Mytillus galloprovincialis as a bioindicator of lead pollution: Biological variable variables and cellular responses. Sci. Total Environ. 1993, 2, 1283–1292. [Google Scholar] [CrossRef]
- Blaise, C.; Gagné, F.; Pellerin, J.; Hansen, P.D.; Trottier, S. Molluscan shellfish biomarker study of the Quebec, Canada, Saguenay fjord with the softshell clam Mya arenaria. Environ. Toxicol. 2002, 17, 170–186. [Google Scholar] [CrossRef] [PubMed]

©Copyright A. Rouhi et al., 2016 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BYNC 4.0).
Share and Cite
Rouhi, A.; Sif, J.; Elyadari, Y. The Effect of the Reproductive Cycle on the Bioaccumulation of Heavy Metals and the Induction of Metallothionein in the Polychaete Perinereis cultrifera of the Coastline of El Jadida (Atlantic Coast, Morocco). J. Xenobiot. 2016, 6, 6588. https://doi.org/10.4081/xeno.2016.6588
Rouhi A, Sif J, Elyadari Y. The Effect of the Reproductive Cycle on the Bioaccumulation of Heavy Metals and the Induction of Metallothionein in the Polychaete Perinereis cultrifera of the Coastline of El Jadida (Atlantic Coast, Morocco). Journal of Xenobiotics. 2016; 6(2):6588. https://doi.org/10.4081/xeno.2016.6588
Chicago/Turabian StyleRouhi, A., J. Sif, and Y. Elyadari. 2016. "The Effect of the Reproductive Cycle on the Bioaccumulation of Heavy Metals and the Induction of Metallothionein in the Polychaete Perinereis cultrifera of the Coastline of El Jadida (Atlantic Coast, Morocco)" Journal of Xenobiotics 6, no. 2: 6588. https://doi.org/10.4081/xeno.2016.6588
APA StyleRouhi, A., Sif, J., & Elyadari, Y. (2016). The Effect of the Reproductive Cycle on the Bioaccumulation of Heavy Metals and the Induction of Metallothionein in the Polychaete Perinereis cultrifera of the Coastline of El Jadida (Atlantic Coast, Morocco). Journal of Xenobiotics, 6(2), 6588. https://doi.org/10.4081/xeno.2016.6588



