Does the Bacterial Media Culture Chemistry Affect the Stability of Nanoparticles in Nanotoxicity Assays? †
Introduction
Materials and Methods
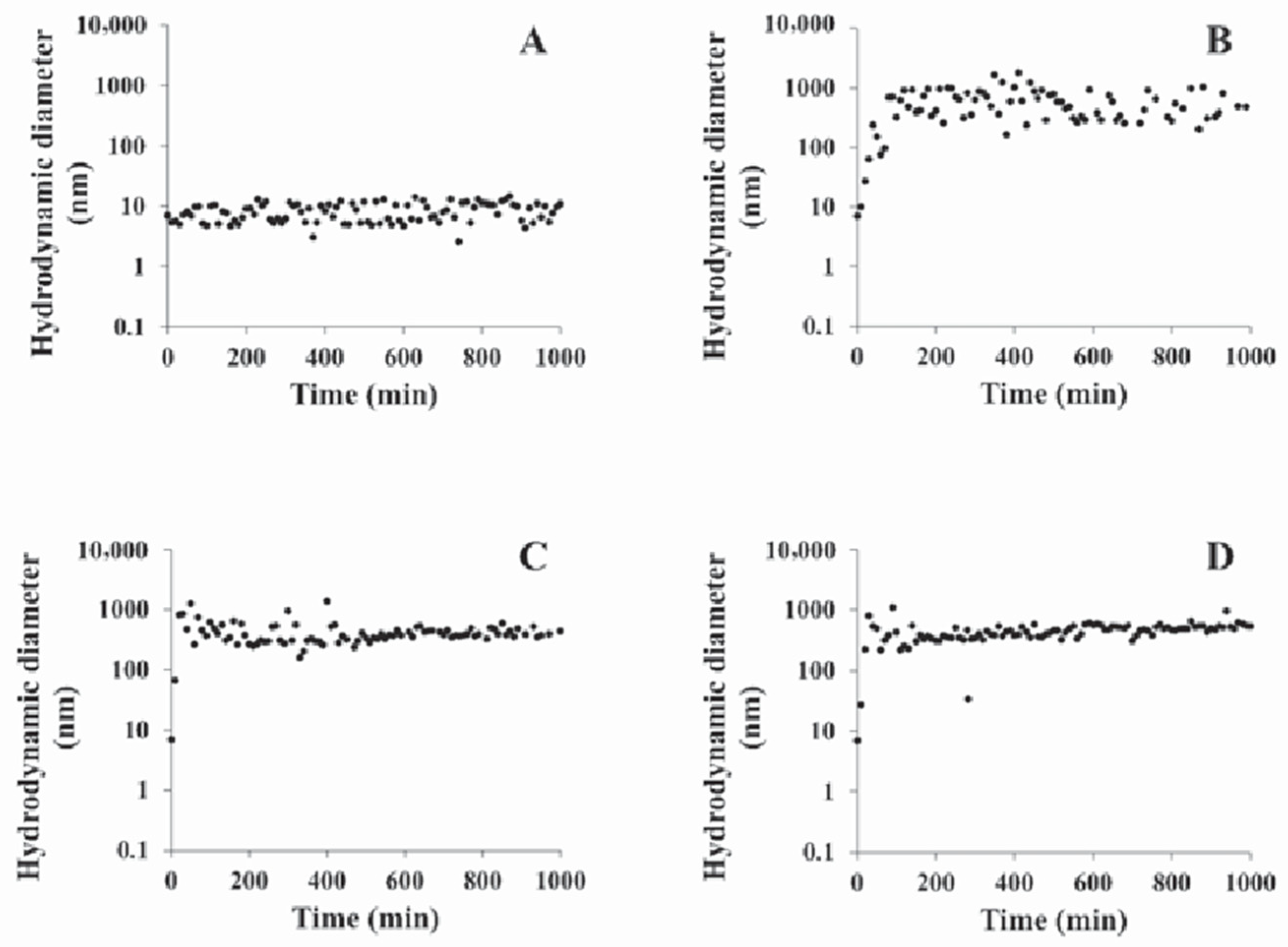
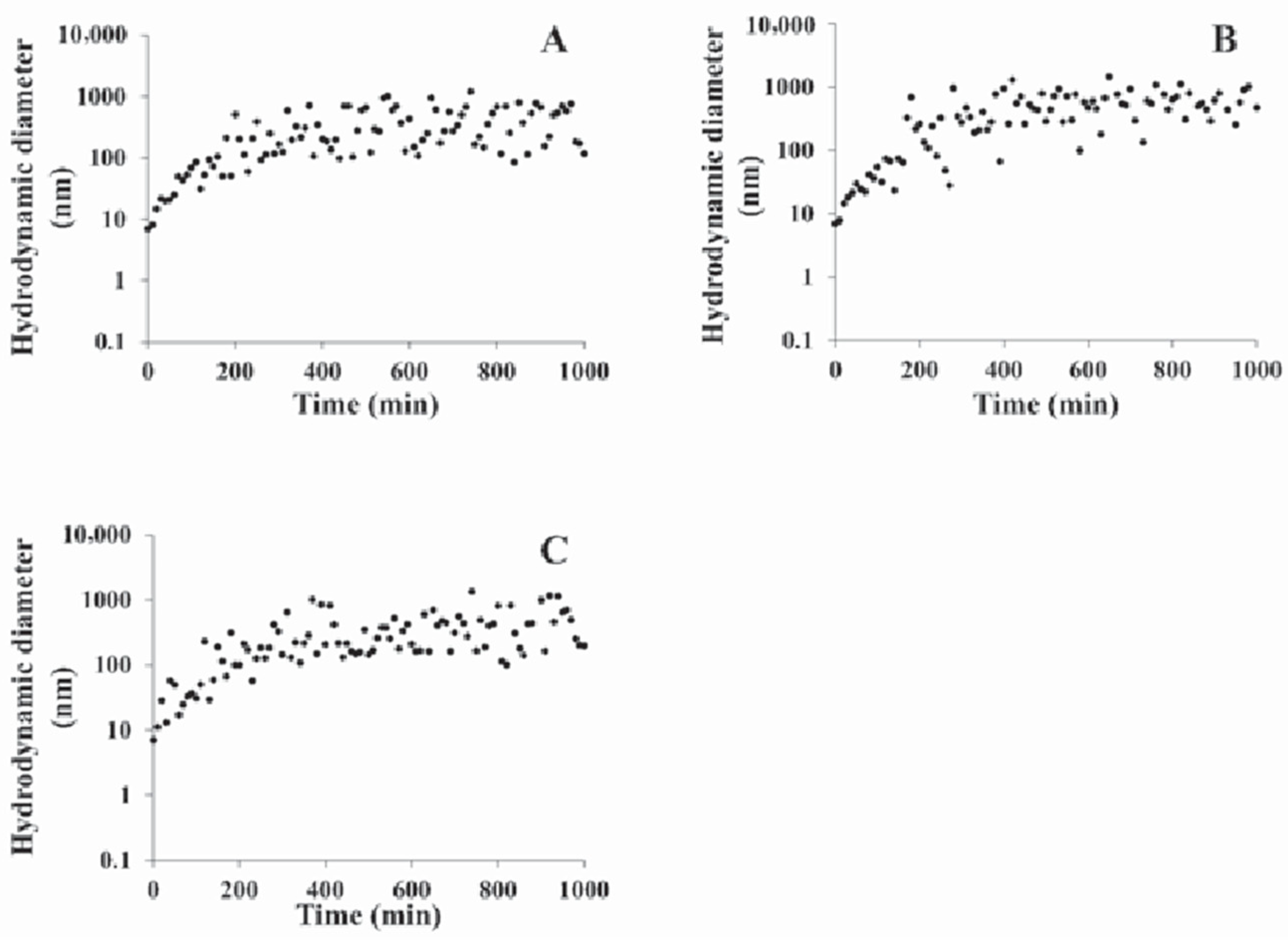
Results and Discussion
Conclusions
Acknowledgments
References
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks), . Nanosilver: safety, health and environmental effects and role in antimicrobial resistance. . Luxembourg; : SCENIHR; ; 2014; . Available from: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_039.pdf.
- Benn, TM; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 2008, 42, 4133–9. [Google Scholar]
- Geranio, L; Heuberger, M; Nowack, B. The behavior of silver nanotextiles during washing. Environ Sci Technol 2009, 43, 8113–18. [Google Scholar]
- Kaegi, R; Sinnet, B; Zuleeg, S; Hagendorfer, H; Mueller, E; Vonbank, R; et al. Release of silver nanoparticles from outdoor facades. Environ Pollut 2010, 158, 2900–5. [Google Scholar]
- Stone, V. ENRHES: Engineered nanoparticles: review of health and environmental safety; Edinburg. 2009. Available from: http://cordis.europa.eu/result/rcn/45841_en.html.
- Doiron, K; Pelletier, E; Lemarchand, K. Impact of polymer-coated silver nanoparticles on marine microbial communities: a microcosm study. Aquat Toxicol 2012, 124-125, 22–7. [Google Scholar]
- Doiron, K; Millour, M; Gagné, J-P; Lemarchand, K. Combined effects of silver nanoparticles and humic and fulvic acids on Vibrio splendidus growth. J Xenobiotics 2014, 4, 4893. [Google Scholar]
- Jin, X; Li, M; Wang, J; Marambio-Jones, C; Peng, F; Huang, X; et al. High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: Influence of specific ions. Environ Sci Technol 2010, 44, 7321–8. [Google Scholar]
- Chambers, BA; Afrooz, ARMN; Bae, S; Aich, N; Katz, L; Saleh, NB; et al. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ Sci Technol 2014, 48, 761–9. [Google Scholar]
- Marambio-Jones, C; Hoek, EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanoparticle Res 2010, 12, 1531–51. [Google Scholar]
- Das, P; Xenopoulos, MA; Williams, CJ; Hoque, ME; Metcalfe, CD. Effects of silver nanoparticles on bacterial activity in natural waters. Environ Toxicol Chem 2012, 31, 122–30. [Google Scholar]
- Das, P; Williams, CJ; Fulthorpe, RR; Hoque, ME; Metcalfe, CD; Xenopoulos, MA. Changes in bacterial community structure after exposure to silver nanoparticles in natural waters. Environ Sci Technol 2012, 46, 9120–8. [Google Scholar]
- Lapresta-Fernández, A; Fernández, A; Blasco, J. Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. Trends Anal Chem 2012, 32, 40–59. [Google Scholar]
- Petosa, AR; Jaisi, D P; Quevedo, IR; Elimelech, M; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ Sci Technol 2010, 44, 6532–49. [Google Scholar]
- Huynh, KA; Chen, KL. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ Sci Technol 2011, 45, 5564–71. [Google Scholar]
- El Badawy, AM; Luxton, T P; Silva, RG; Scheckel, KG; Suidan, MT; Tolaymat, TM. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 2010, 44, 1260–6. [Google Scholar]
- Zhang, Y; Chen, Y; Westerhoff, P; Crittenden, J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 2009, 43, 4249–57. [Google Scholar]
- Levard, C; Hotze, EM; Lowry, GV; Brown, GE. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 2012, 46, 6900–14. [Google Scholar]
©Copyright M. Millour et al., 2015 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Millour, M.; Doiron, K.; Lemarchand, K.; Gagné, J.-P. Does the Bacterial Media Culture Chemistry Affect the Stability of Nanoparticles in Nanotoxicity Assays? J. Xenobiot. 2015, 5, 5772. https://doi.org/10.4081/xeno.2015.5772
Millour M, Doiron K, Lemarchand K, Gagné J-P. Does the Bacterial Media Culture Chemistry Affect the Stability of Nanoparticles in Nanotoxicity Assays? Journal of Xenobiotics. 2015; 5(2):5772. https://doi.org/10.4081/xeno.2015.5772
Chicago/Turabian StyleMillour, M., K. Doiron, K. Lemarchand, and J.-P. Gagné. 2015. "Does the Bacterial Media Culture Chemistry Affect the Stability of Nanoparticles in Nanotoxicity Assays?" Journal of Xenobiotics 5, no. 2: 5772. https://doi.org/10.4081/xeno.2015.5772
APA StyleMillour, M., Doiron, K., Lemarchand, K., & Gagné, J.-P. (2015). Does the Bacterial Media Culture Chemistry Affect the Stability of Nanoparticles in Nanotoxicity Assays? Journal of Xenobiotics, 5(2), 5772. https://doi.org/10.4081/xeno.2015.5772



