Toxicity Effect of Sub-Chronic Oral Administration of Class Bitters®—A Polyherbal Formula on Serum Electrolytes and Hematological Indices in Male Wistar Albino Rats
Abstract
Introduction
Materials and Methods
Herbal sample
Experimental animals
Acute toxicity test
Subchronic oral toxicity study
Samples collection
Statistical analysis
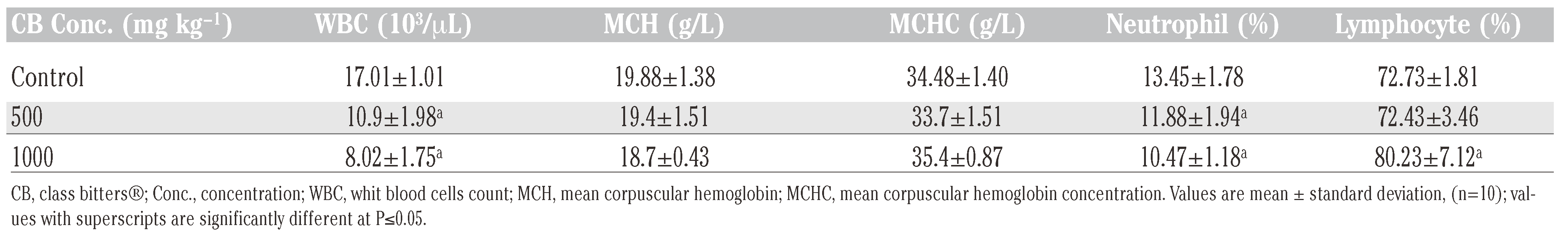
Results
Discussion and Conclusions
Research highlights
Conflicts of Interests
Author Contributions
References
- Bright, J.J. Curcumin and autoimmune disease. Adv Exp Med Biol 2007, 595, 425–51. [Google Scholar] [PubMed]
- Kamboj, V.P. Herbal medicine. Curr Sci 2000, 78, 1–17. [Google Scholar]
- Fahn, A. Plant anatomy, 3rd ed.; Pergamon Press: Oxford, 1989. [Google Scholar]
- Kokwaro, J.O. Medicinal plants of East Africa, 2nd ed.; Kenya Literature: Nairobi, 1993. [Google Scholar]
- Sunday, O.O.; Uguru, M.O.; Ochigbo, E.A. The effect of aqueous extracts on Momordica balsamina on haematological and biochemical parameters in rats. Asian J Pharm Clin Res 2009, 2, 21–5. [Google Scholar]
- Medicines Control Agency (MCA). Safety of herbal medicinal products. July 2002. Available online: https://hfnet.nih.go.jp/usr/ kiso/ninpu-herb/HerbalsSafety Report02_Final.
- De Smet, P.A.G.M. Health risk of herbal remedies. Drug Safety 1995, 13, 81–93. [Google Scholar] [CrossRef]
- Barnes, J.; Mills, S.Y.; Abbot, N.C.; Willoughby, M.; Ernst, E. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face to face interviews with 515 users of herbal remedies. Br J Clin Pharmacol 1998, 45, 496–500. [Google Scholar] [CrossRef]
- Shaw, D.; Leon, C.; Kolev, S.; Murray, V. Traditional remedies and food supplements: a 5-year toxicological study (19911995). Drug Safety 1997, 17, 342–56. [Google Scholar] [CrossRef]
- Adjanahoun, J.E.; Aboubakar, N.; Dramane, K.; Ebot, M.E.; Ekpere, J.A.; Enow-Orock, E.J.; et al. Traditional medicine and pharmacopoeia: contribution to ethnobotanical and floristic studies in Cameroon; Organization of African Unity (OAU)/ Scientific, Technical, and Research Commission (STRC): Yaoundé, 1996. [Google Scholar]
- Noumi, E.; Amvam, Z.P.H.; Lontsi, D. Aphrodisiac plants used in Cameroon. Fitoterapia 1998, 69, 125–34. [Google Scholar]
- Lampiao, F. The role of Mondia whitei in reproduction: a review of current evidence. Internet J Third World Med 2009, 8, (1). [Google Scholar]
- Lompo, M.; Nikiema, J.B.; Guissou, I.P.; Moes, A.J.; Fontaine, J. The topical anti-inflammatory effect of chloroform extracts from Khaya senegalensis stembarks. Phytother Res 1998, 12, 448–50. [Google Scholar] [CrossRef]
- Iwu, M. Hankbook of African medicinal plants, pharmacognostical profile of selected medicinal plants; CRC Press Inc.: Boca Raton, FL, 1993. [Google Scholar]
- Gills, L.S. Ethnomedical uses of plants in Nigeria; University of Benin press: Benin City, 1992; p. 276. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Madati, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Sumuelson, G. Inventory of plants used in traditional medicine in Tanzania. 1. Plants of the families Acanthaceae-Cucurbitaceae. J Ethnopharmacol 1982, 6, 29–60. [Google Scholar] [CrossRef]
- Martey, O.N.K.; Armah, G.E.; Sittie, A.A.; Okine, L.K.N. A chronic toxicity study of the ground root bark of Capparis erythrocarpus (Capparecceae) in malw Sprague-dewley rat. Pak J Biol Sci 2013, 16, 1706–13. [CrossRef] [PubMed]
- Burkhills, H.M. Useful plants of West Tropical Africa. 2nd ed. Royal Botan Garden 1985, 1, 130–2. [Google Scholar]
- Amayaw, Y.; Owusu-Ansah, E. Morphohistological studies of two plants species used in ethnomedicine. J Herbs Spices Med Plants 1998, 5, 60–85. [Google Scholar]
- Somova, L.L.; Shode, F.O.; Moodley, K.; Govender, Y. Cardiovascular and diuretic activity of kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica. J Ethnopharmacol 2001, 77, 165–74. [Google Scholar] [CrossRef]
- Patrick-Iwuanyanwu, K.C.; Wegwu, M.O.; Okiyi, J.K. Hepatoprotective effects of African Loust Bean (Parkia clappertoniana) and Negro Pepper (Xylopia aethiopica) in CCl4-Induced Liver damage in Wistar Albino rats. Int J Pharmacol 2010, 6, 744–9. [Google Scholar] [CrossRef]
- Patrick-Iwaunyanwu, K.C.; Chinaka, D.E.; Gboelo, B.B. Evaluation of acute and subchronic toxicities of class bitters; a polyherbal formula in male albino rats. Pharmacologia 2012, 3, 707–12. [Google Scholar] [CrossRef][Green Version]
- Organization for Economic Co-operation and Development (OECD). Guidelines for the testing of chemicals; OECD: Paris.
- Chandra, P.; Sachan, N.; Ghosh, A.K.; Kishore, K. Acute and Sub-chronic oral toxicity studies of a mineralo-herbal drug amlene on experimental rats. Int J Pharm Res 2010, 1, 15–8. [Google Scholar]
- Ashafa, A.T.; Olunu, O.O. Toxicological evaluation of ethanolic root extract of Morinda lucida (L.) Benth. (Rubiaceae) in male Wistar rats. J Nat Pharm 2011, 2, 108–14. [Google Scholar] [CrossRef]
- Sunmonu, T.O.; Oloyede, O.B. Performance and haematological indices in rats exposed to monocrotophos contamination. Hum Exp Toxicol 2010, 29, 845–50. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; et al. Concordance of toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Ochie, J.; Kolhatkar, A. Medical laboratory science: theory and practice; Tata McGraw-Hill Publishing Company Ltd.: New Delhi, 2008. [Google Scholar]
- Kratz, A.; Ferraro, M.; Sluss, P.M.; Lewandrowski, K.B.; Ellender, S.M.; Peters, C.C.; et al. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 2004, 351, 1548–63. [Google Scholar] [PubMed]
- Chauhan, V.; Kelepouris, E.; Chauhan, N.; Vaid, M. Current concepts and management strategies in chronic kidney disease-mineral and bone disorder. Southern Med J 2012, 105, 479–85. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.B.; Zandi-Nejad, K. Disorders of potassium balance. In Brenner and Rector’s The Kidney, 8th ed; Brenner, B.M., Ed.; ; Elsevier Saunders: Philadelphia, PA, 2008; p. 15. [Google Scholar]
- Shirley, D.G.; Capasso, G.; Unwin, R.J. Renal physiology. In Principles of clinical nephrology, 2nd ed.; Johnson, R., Feehally, J., Eds.; eds.; Mosby International: Philadelphia, PA, 2003; pp. 21–30. [Google Scholar]
- Draper, H.H.; Polensek, I.; Hadley, M.; Mcgirr, I.G. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids 1988, 19, 836–43. [Google Scholar] [CrossRef] [PubMed]
- Ashafa, A.T.; Sunmonu, T.O.; Afolayan, A.J. Effects of leaf and berry extracts of Phytolacca dioica L. on haematological and weight parameters of Wistar rats. Afr J Pharm Pharmacol 2011, 5, 150–4. [Google Scholar] [CrossRef]
- Guyton, C.A.; Hall, J.E. Textbook of medical physiology, 10th ed.; W.B Saunders Co.: London, 2000; pp. 264, 382, 389, 730–731, 797–802. [Google Scholar]
- Kumar, P.; Clark, M. Clinical medicine, 5th ed.; W.B Saunders Co.: London, 2003; p. 592. [Google Scholar]
- McLellan, S.A.; McLellan, D.B.L.; Walsh, T.S. Anaemia and red blood cell transfusion in the critically ill patient. Blood Rev 2003, 17, 195–208. [Google Scholar] [CrossRef]
- Iniaghe, O.M.; Egharevba, O.; Oyewo, E.B. Effect of aqueous leaf extract of Acalypha wilkesiana on hematological parameters in male Wistar albino rats. Br J Pharm Res 2013, 3, 465–71. [Google Scholar] [CrossRef]
- Chernecky, C.C.; Berger, B.J. Laboratory tests and diagnostic procedures, 3rd ed.; W. B. Saunders Co.: Philadelphia, PA, 2001. [Google Scholar]
 |
 |
 |
©Copyright K.C. Patrick-Iwuanyanwu and K.W. Nkpaa, 2015 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BYNC 3.0).
Share and Cite
Patrick-Iwuanyanwu, K.C.; Nkpaa, K.W. Toxicity Effect of Sub-Chronic Oral Administration of Class Bitters®—A Polyherbal Formula on Serum Electrolytes and Hematological Indices in Male Wistar Albino Rats. J. Xenobiot. 2015, 5, 5369. https://doi.org/10.4081/xeno.2015.5369
Patrick-Iwuanyanwu KC, Nkpaa KW. Toxicity Effect of Sub-Chronic Oral Administration of Class Bitters®—A Polyherbal Formula on Serum Electrolytes and Hematological Indices in Male Wistar Albino Rats. Journal of Xenobiotics. 2015; 5(1):5369. https://doi.org/10.4081/xeno.2015.5369
Chicago/Turabian StylePatrick-Iwuanyanwu, Kingsley C., and Kpobari W. Nkpaa. 2015. "Toxicity Effect of Sub-Chronic Oral Administration of Class Bitters®—A Polyherbal Formula on Serum Electrolytes and Hematological Indices in Male Wistar Albino Rats" Journal of Xenobiotics 5, no. 1: 5369. https://doi.org/10.4081/xeno.2015.5369
APA StylePatrick-Iwuanyanwu, K. C., & Nkpaa, K. W. (2015). Toxicity Effect of Sub-Chronic Oral Administration of Class Bitters®—A Polyherbal Formula on Serum Electrolytes and Hematological Indices in Male Wistar Albino Rats. Journal of Xenobiotics, 5(1), 5369. https://doi.org/10.4081/xeno.2015.5369




