Biochemical and Histopathological Changes in the Kidney and Adrenal Gland of Rats Following Repeated Exposure to Lambda-Cyhalothrin
Abstract
:Introduction
Materials and Methods
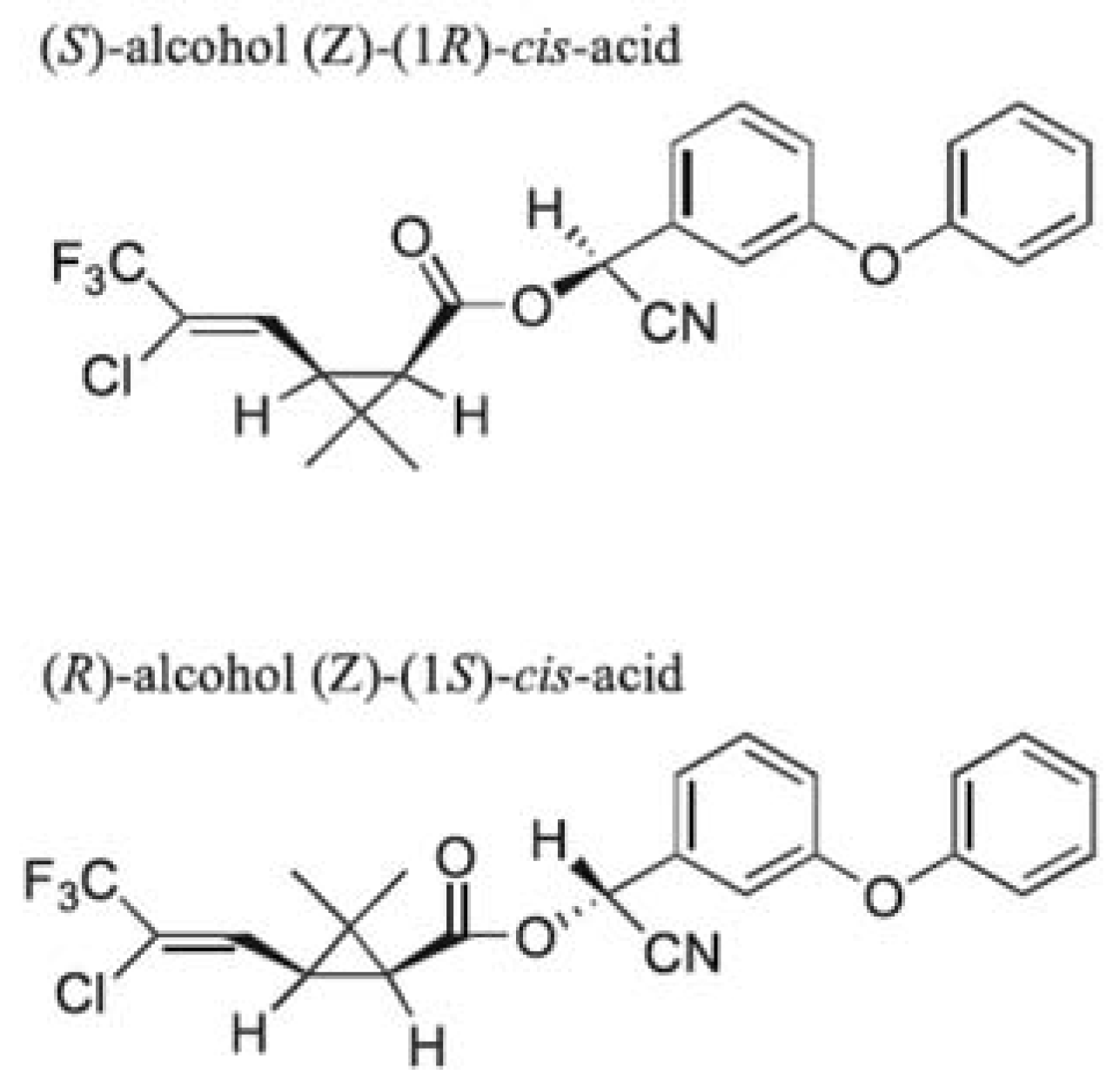
Chemicals
Animals and treatment
Biochemical analysis
Plasma lambda-cyhalothrin concentration
Histopathological examination
Statistical analysis
Results
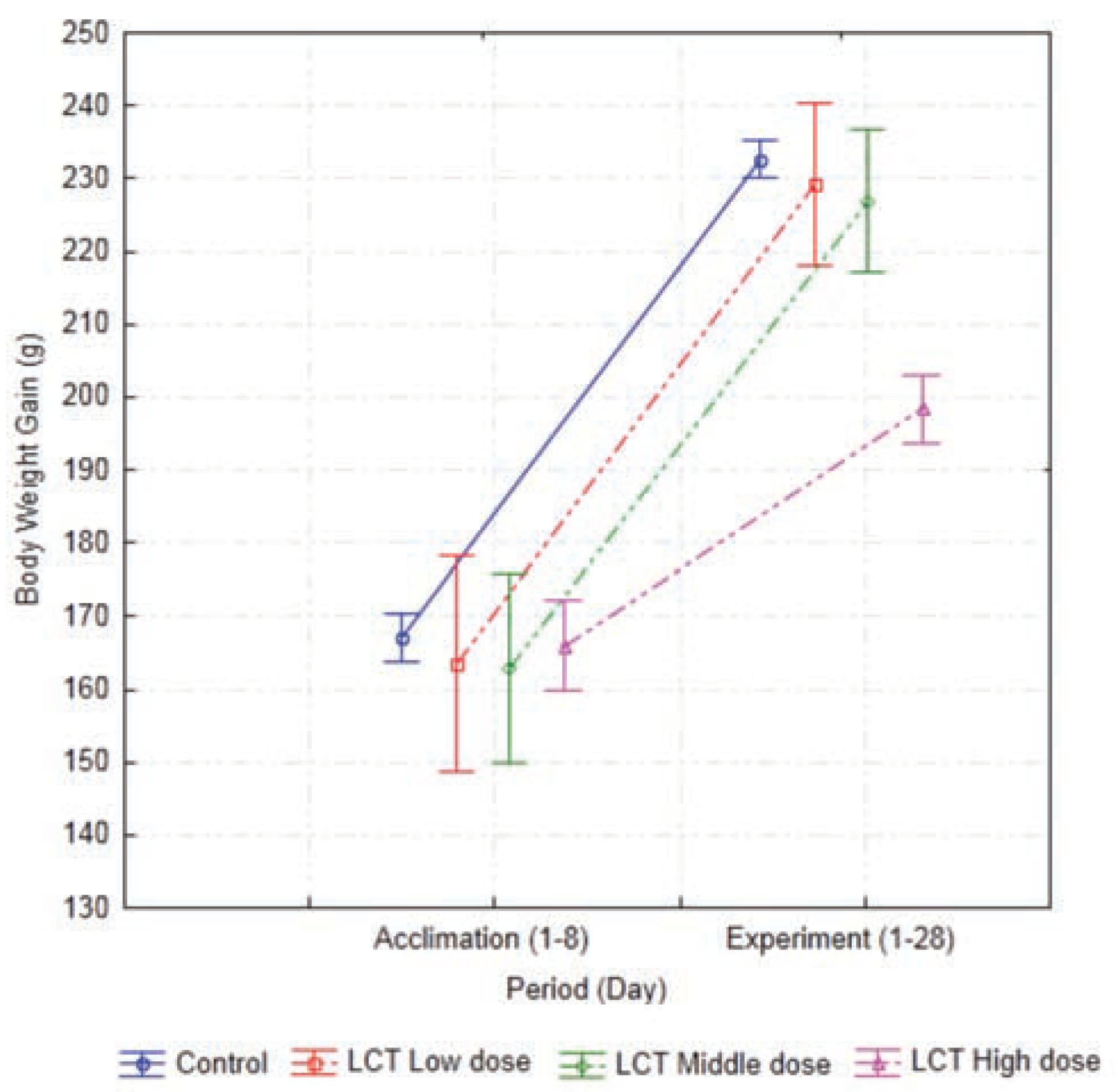
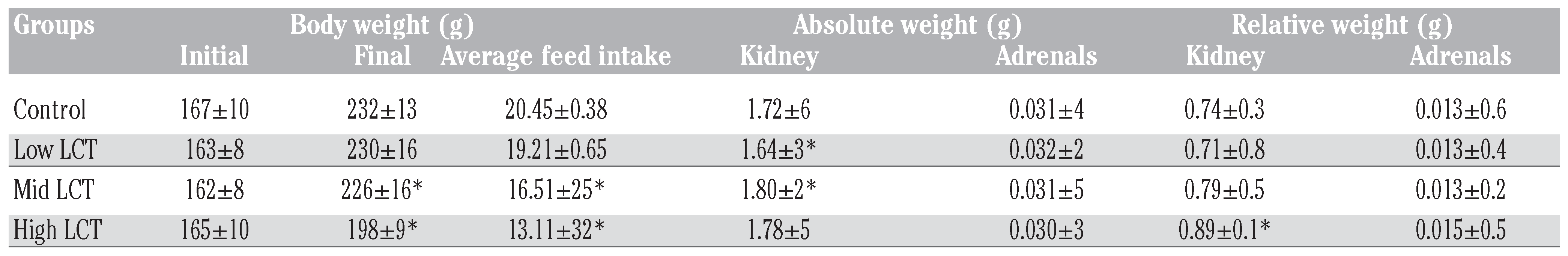
Body weight
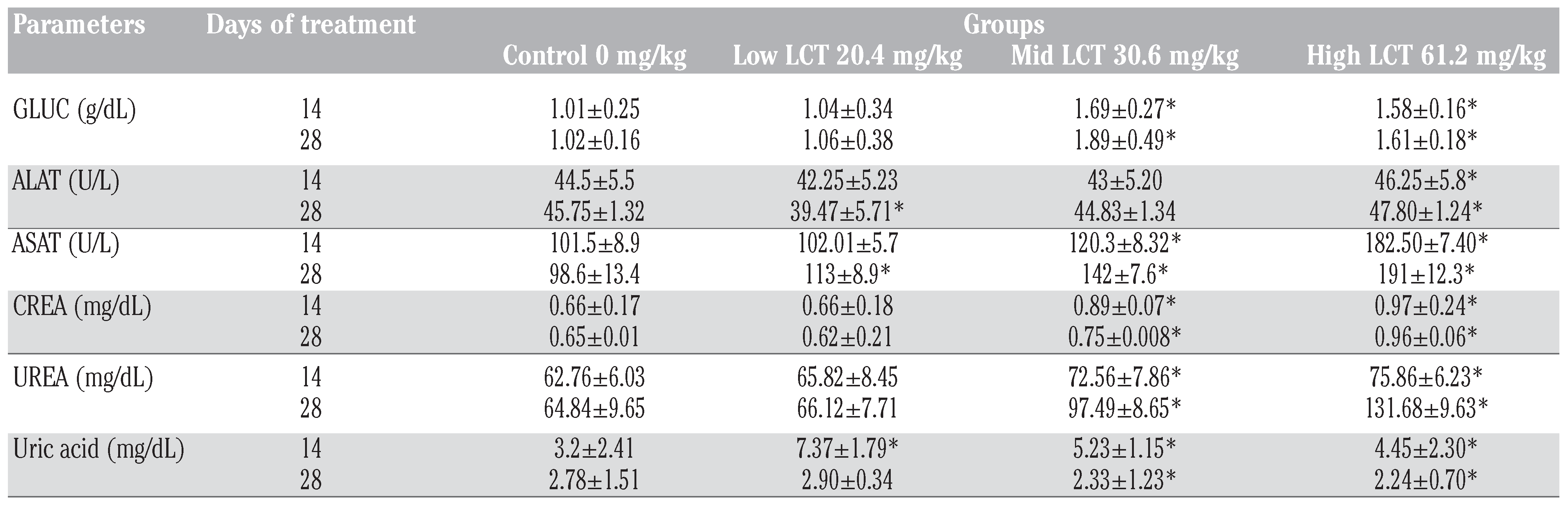
Biochemical results
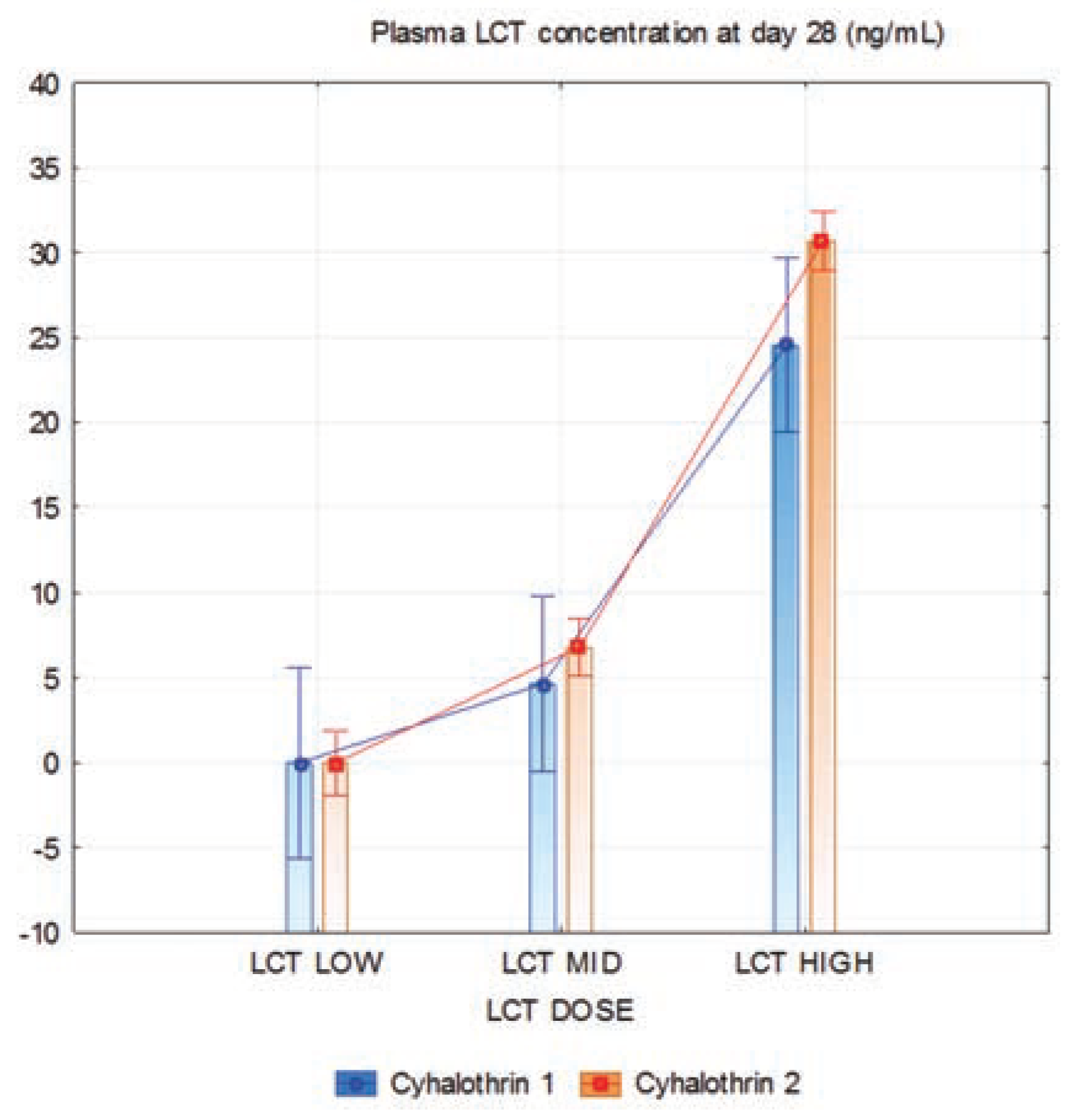
Plasma lambda-cyhalothrin concentration
Histological results
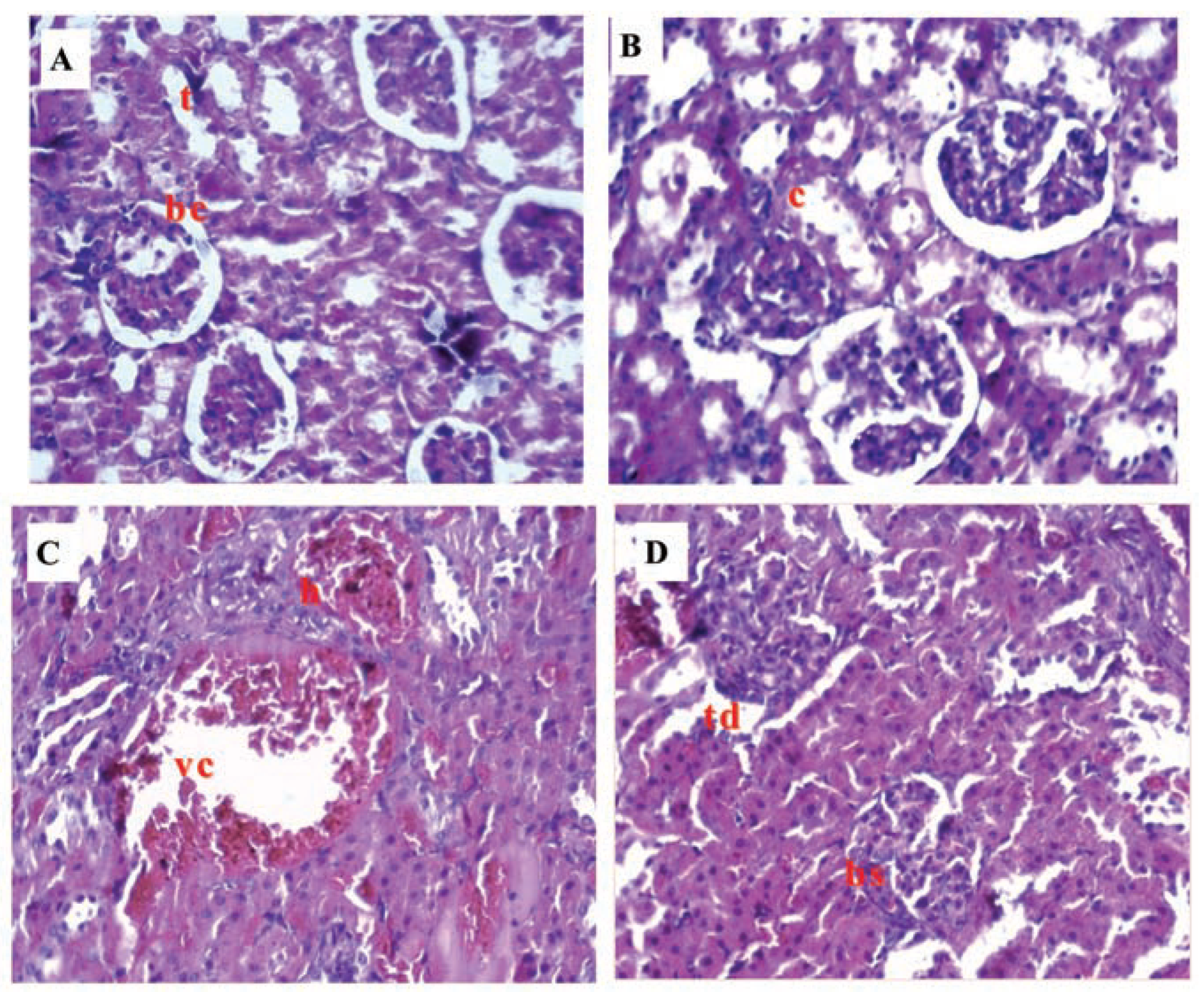
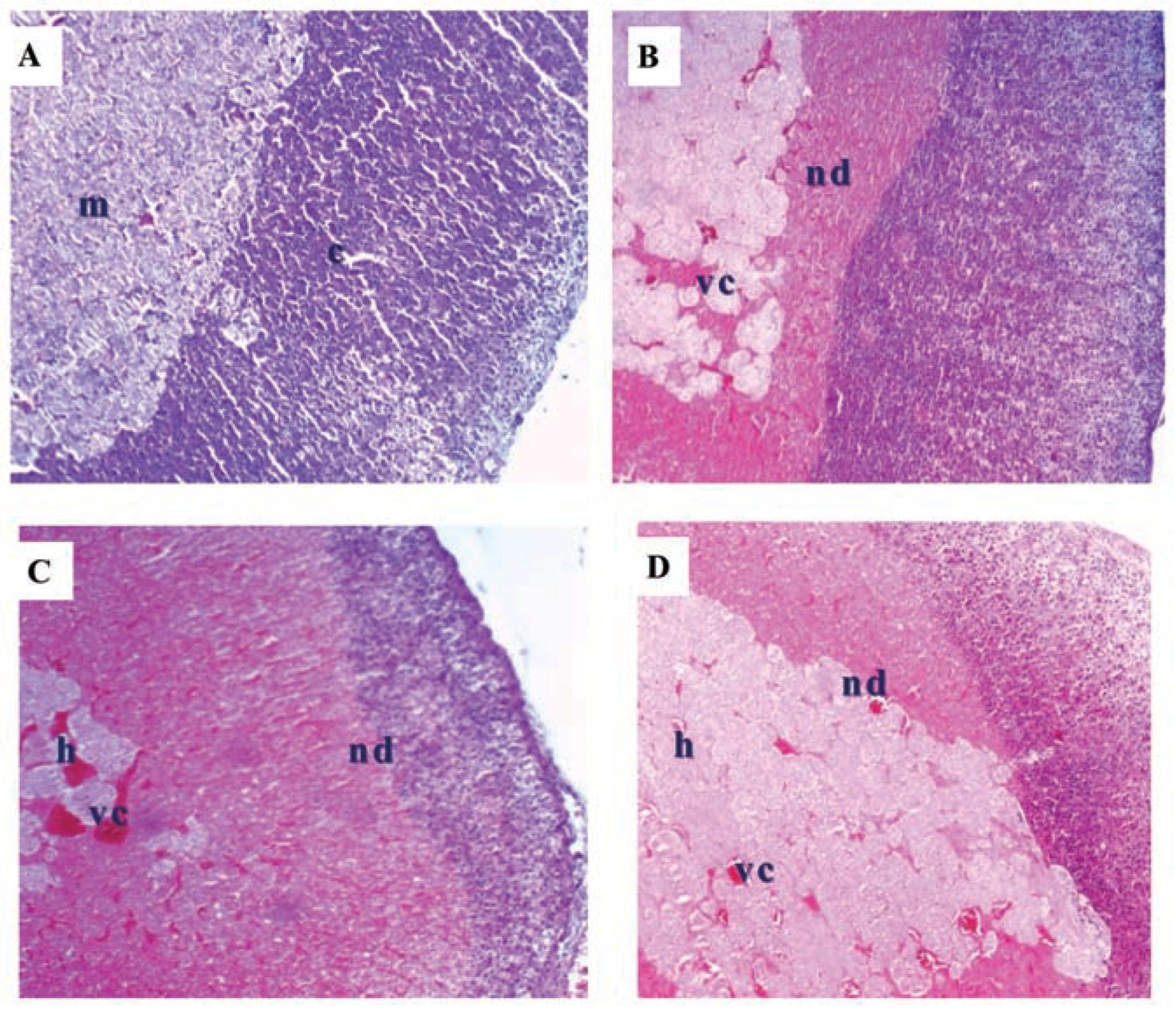
Kidney
Adrenal gland
Discussion
Acknowledgments
Conflicts of Interest
References
- Burrows, H.D.; Canle, M.L.; Santaballa, J.A.; Steenken, S. Reaction pathways and mech- anisms of photodegradation of pesticides. J Photochem Photobiol 2002, B67, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Reffstrup, T.K. Combined actions of pesti- cides in food (Fødevare rapport no. 2002:19); Danish Veterinary and Food Administration: Søborg, 2002. [Google Scholar]
- Soderlund, D.M.; Clark, J.M.; Sheets, P.L.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; et al. Mechanisms of pyrethroid neurotoxicity: implications for cumalative risk assess- ment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect 1980, 34, 189–202. [Google Scholar] [CrossRef]
- Righi, D.A.; Palermo-Neto, J. Effects of type II pyrethroids cyhalothrin on peritoneal macrophage activity in rats. Toxicology 2005, 212, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, K.; Muralidhara, P.S.; Rajini, K. Fenvalerate-induced oxidative damage in rat tissues and its attenuation by dietary sesame oil. Food Chem Toxicol 2005, 43, 299–306. [Google Scholar]
- Naumann, K. Synthetic pyrethroid insecti- cides: structures and properties. Chemistry of plant protection; Springer Verlag: Berlin, 1990; Volume 4. [Google Scholar]
- Breckenridge, C.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; et al. Evidence for a separate mechanism of tox- icity for the Type I and the Type II pyrethroid insecticides. NeuroToxicology 2009, 30S, S17–31. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.A.; Ray, D.E. Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicol Sci 2005, 77, 341–6. [Google Scholar] [CrossRef]
- Hossain, M.M.; Richardson, J.R. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci 2011, 122, 512–25. [Google Scholar]
- Ali, Z.Y. Neurotoxic effect of lambda- cyhalothrin, a synthetic pyrethroid pesti- cide: involvement of oxidative stress and protective role of antioxidant mixture. N Y Sci J 2012, 9, 93–103. [Google Scholar]
- Cheng, J.H.; Liu, M.; Yu, Y.; Wang, X.P.; Zhang, H.Q.; Ding, L. Determination of pyrethroids in porcine tissues by matrix solid-phase dispersion extraction and high-perform- ance liquid chromatography. Meat Sci 2009, 82, 407–12. [Google Scholar] [CrossRef]
- Turrillas, F.A.E.; Pastor, A.; Guardia, M.D. Determination of pyrethroid insecticide residues in vegetable oils by using com- bined solid-phases extraction and tandem mass spectrometry detection. Anal Chim Acta 2005, 553, 50–7. [Google Scholar] [CrossRef]
- De-Micco, A.; Cooper, K.R.; Richardson, J.R.; White, L.A. Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol Sci 2010, 113, 177–86. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Ferreira, T.C.R.; Alves, S.A.; Carneiro, R.L.; Lanza, M.R.V. Application of the response surface and desirability design to the Lambda-cyhalothrin degra- dation using photo-Fenton reaction. J Environ Manage 2013, 118, 32–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rai, D.K.; Sharma, B.; Pandey, R.S. λ-cyhalothrin and cypermethrin induced in vivo alterations in the activity of acetyl-cholinesterase in a freshwater fish, Channa punctatus (Bloch). Pestic Bio - chem Phys 2009, 93, 96–9. [Google Scholar] [CrossRef]
- Liu, S.; Pleil, J.D. Human blood and environ- mental media screening method for pesti- cides and polychlorinated biphenyl com- pounds using liquid extraction and gas chromatography–mass spectrometry analysis. J. Chromatogr 2002, 769, 155–67. [Google Scholar]
- FAO/WHO evaluation report based on sub- mission of data from Bharat Rasayan Limited (TC). In FAO specifications and evaluations for agricultural pesticides - Lambda-cyhalothrin; Food and Agriculture, Organization (Ed.) FAO: Rome, 2012; pp. 15–23. Available online: http://www.fao.org/fileadmin/templates/agphome/docu-ments/Pests_Pesticides/Specs/lambda13.pdf.
- Fetoui, H.; Makni, M.; Garoui Zeghal, E.M.N. Toxic effects of lambda-cyhalothrin, a syn- thetic pyrethroids pesticide, on the rat kid- ney: involvement of oxidative stress and protective role of ascorbic acid. Exp Toxicol Pathol 2010, 62, 593–9. [Google Scholar] [CrossRef]
- Yousef, I.Y.; Vitamin, E. Modulates reproduc- tive toxicity of pyrethroid lambda- cyhalothrin in male rabbits. Food Chem Toxicol 2010, 48, 1152–9. [Google Scholar] [PubMed]
- Velmurugan, B.; Selvanayagama, M.; Cengiz, E.I.; Unlu, E. Histopathology of lambda- cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Environ Toxicol Pharmacol 2007, 24, 286–329. [Google Scholar] [CrossRef] [PubMed]
- Muller, J. Aldosterone the minority hor- mone of the adrenal cortex. Steroids 1995, 60, 29. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). Test Guideline for testing of chemicals, Section 4: Health Effects, OECD. Guideline 407, Repeated Dose 28-Day Oral Toxicity Study in Rodents 2003; OECD: Paris, 2003. [Google Scholar]
- Çelik, A.; Mazmancia, B.; Çamlica, Y.; Skin, A.A.; Çömelekoglu, Ü. Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat Res 2003, 539, 91–7. [Google Scholar] [CrossRef]
- Ayla, M.B.; Çamlica, Y.; Çömelekoglu, Ü.; Skin, A.A. Evaluation of cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow by gavage administration. Ecotoxicol Environ Safety 2005, 61, 128–33. [Google Scholar]
- El-Demerdash, F.M. Lambda-cyhalothrin- induced changes in oxidative stress bio- markers in rabbit erythrocytes and allevia- tion effect of some antioxidants. Toxicol In Vitro 2007, 21, 392–7. [Google Scholar] [CrossRef] [PubMed]
- Pratera, M.R.; Gogal, J.R.A.R.; Blaylockb, B.L.; Longstrethc, J.; Holladaya, S.D. Single-dose topical exposure to the pyrethroid insecti- cide, permethrin in C57BL/6N mice: effects on thymus and spleen. Food Chem Toxicol 2002, 40, 1863–73. [Google Scholar] [CrossRef]
- Ball, L.M.; Chhabra, R.S. Intestinal absorption of nutrients in rats treated with 2, 3, 7, 8- tetrachlorodi benz-p-dioxin (TCDD). J Toxicol Environ Health 1981, 8, 629–36. [Google Scholar] [CrossRef]
- Kilian Delport, E.R.; Bornman, M.S.; De Jager, C. Simultaneous exposure to low concen- trations of dichlorodiphenyltrichloroe- thane, deltamethrin, nonylphenol and phy- toestrogens has negative effects on the reproductive parameters in male Sprague- Dawley rats. Andrologia 2007, 39, 128–35. [Google Scholar] [CrossRef] [PubMed]
- Coles, E.H. Veterinary clinical pathology; W.B. Saunders: Philadelphia, PA, 1986. [Google Scholar]
- Almdal, T.P.; Vilstrup, H. Strict insulin treat- ment normalizes the organic nitrogen con- tents and the capacity of urea-N synthesis in experimental diabetes in rats. Diabetologica 1988, 31, 114–8. [Google Scholar] [CrossRef] [PubMed]
- Fetoui, H.; Garoui, E.M.; Zegha, I.E. Lambda- cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. Exp Toxicol Pathol 2009, 61, 189–96. [Google Scholar] [PubMed]
- Yousef, M.I.; El-Demerdash, F.M.; Kamel, K.I.; Al-Salhen, K.S. Changes in some hemato- logical and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicol 2003, 189, 223–34. [Google Scholar] [CrossRef]
- Piner, P.; Üner, N. Oxidative and apoptotic effects of lambda-cyhalothrin mod-ulated by piperonyl butoxide in the liver of Oreochromis niloticus. Environ Toxicol Pharmacol 2012, 33, 414–20. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Lu, Z.D.; Wei, M.; Yang, L.; Kong, H.M. NMR-based metabolomics approach to study the toxicity of lambda-cyhalothrin to goldfish (Carassius auratus). Aquat Toxicol 2014, 146, 82–92. [Google Scholar] [CrossRef]
- Wolansky, M.J.; Gennings, C.; Crofton, K.M. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol Sci 2006, 89, 271–7. [Google Scholar] [CrossRef] [PubMed]
- Lofty, A.E.A.A.; Abd El-Aleem, H.H. Monir. Determination of insecticides malathion and lambda-cyhalothrin residues in zuc- chini by gas chromatography. Bulletin of Faculty of Pharmacy, Cairo University. 2013, 51, 255–60. [Google Scholar]
- Feo, M.L.; Eljarrat, E.; Barcelo, D. Determination of pyrethroid insecticides in environmental samples. Trends Anal Chem 2010, 29, 692–705. [Google Scholar]
 |
 |
© Copyright H. Khaldoun Oularbi, 2014. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BYNC 3.0).
Share and Cite
Khaldoun Oularbi, H. Biochemical and Histopathological Changes in the Kidney and Adrenal Gland of Rats Following Repeated Exposure to Lambda-Cyhalothrin. J. Xenobiot. 2014, 4, 2240. https://doi.org/10.4081/xeno.2014.2240
Khaldoun Oularbi H. Biochemical and Histopathological Changes in the Kidney and Adrenal Gland of Rats Following Repeated Exposure to Lambda-Cyhalothrin. Journal of Xenobiotics. 2014; 4(1):2240. https://doi.org/10.4081/xeno.2014.2240
Chicago/Turabian StyleKhaldoun Oularbi, Hassina. 2014. "Biochemical and Histopathological Changes in the Kidney and Adrenal Gland of Rats Following Repeated Exposure to Lambda-Cyhalothrin" Journal of Xenobiotics 4, no. 1: 2240. https://doi.org/10.4081/xeno.2014.2240
APA StyleKhaldoun Oularbi, H. (2014). Biochemical and Histopathological Changes in the Kidney and Adrenal Gland of Rats Following Repeated Exposure to Lambda-Cyhalothrin. Journal of Xenobiotics, 4(1), 2240. https://doi.org/10.4081/xeno.2014.2240




