Fluoxetine Accumulation and Metabolism as Exposure Biomarker to Better Understand Biological Efects in Gastropods †
Introduction
Materials and Methods
Results and Discussion
References
- Lajeunesse, A.; Gagnon, C.; Sauve, S. Determination of basic antidepressants and their n-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography - Tandem mass spectrometry. Anal Chem 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Gust, M.; Buronfosse, T.; Giamberini, L.; Ramil, M.; Mons, R.; Garric, J. Effects of fluoxetine on the reproduction of two prosobranch mollusks: Potamopyrgus antipodarum and Valvata piscinalis. Environ Pollut 2009, 157, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bringolf, R.B.; Heltsley, R.M.; Newton, T.J.; Eads, C.B.; Fraley, S.J.; Shea, D.; et al. Environmental occurence and reproductive effects of the pharmaceutical fluoxetine in native fresh- water mussels. Environ Toxicol Chem 2010, 29, 1311–1318. [Google Scholar] [PubMed]
- Ramirez, A.J.; Brain, R.A.; Usenko, S.; Mottaleb, M.A.; O’Donnell, J.G.; Stahl, L.L.; et al. Occurrence of pharmaceuticals and per- sonal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem 2009, 28, 2587–2597. [Google Scholar] [PubMed]
- Brooks, B.W.; Chambliss, C.K.; Stanley, J.K.; Ramirez, A.; Banks, K.E.; Johnson, R.D.; et al. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem 2005, 24, 464–469. [Google Scholar] [PubMed]
- Metcalfe, C.D.; Chu, S.G.; Judt, C.; Li, H.X.; Oakes, K.D.; Servos, M.R.; et al. Antidepressants and their metabolites in municipal waste- water, and downstream exposure in an urban watershed. Environ Toxicol Chem 2010, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Metcalfe, C.D. Analysis of paroxe- tine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extrac- tion, mixed mode solid phase extraction cleanup and liquid chromatography-tan- dem mass spectrometry. J Chromatogr A 2007, 1163, 112–118. [Google Scholar] [PubMed]
- Paterson, G.; Metcalfe, C.D. Uptake and depuration of the anti-depressant fluoxe- tine by the Japanese medaka (Oryzias latipes). Chemosphere 2008, 74, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, H.; Sekizawa, J.; Kondo, T.; Hirai, N.; Tatarazako, N. The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): Acute toxicity in fish larvae and bioaccumulation in juve- nile fish. Chemosphere 2008, 70, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Gust, M.; Buronfosse, T.; Mons, R.; Andre, C.; Gagne, F.; Garric, J. Is exposure temperature a confounding factor for assessment of reproductive parameters of the New Zealand mudsnail Potamopyrgus antipo- darum? Aquatic Toxicol 2011, 101, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Pazos, A.J.; Sánchez, J.L.; Román, G.; Luz Pérez-Parallé, M.; Abad, M. Seasonal changes in lipid classes and fatty acid composition in the digestive gland of Pecten maximus. Comp Biochem Phys B 2003, 134, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Rouviere, F.; Bulete, A.; Cren-Olive, C.; Arnaudguilhem, C. Multiresidue analysis of aromatic organochlorines in soil by gas chromatography-mass spectrometry and QuEChERS extraction based on water/ dichloromethane partitioning. Compa rison with accelerated solvent extraction. Talanta 2012, 93, 336–344. [Google Scholar] [PubMed]
- Kwon, J.W.; Armbrust, K.L. Laboratory persist- ence and fate of fluoxetine in aquatic envi- ronments. Environ Toxicol Chem 2006, 25, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Oakes, K.D.; Cui, S.; Bragg, L.; Servos, M.R.; Pawliszyn, J. Tissue-specific in vivo bioconcentration of pharmaceuticals in rainbow trout (Oncorhynchus mykiss) Using Space-Resolved Solid-Phase Microextraction. Environ Sci Technol 2010, 44, 3417–3422. [Google Scholar] [CrossRef] [PubMed]
- Fretter, V.; Graham, A. British Prosobranch molluscs. Their fonctional anatomy and ecology; Ray Society: London, 1994. [Google Scholar]
- Smith, E.M.; Chu, S.G.; Paterson, G.; Metcalfe, C.D.; Wilson, J.Y. Cross-species comparison of fluoxetine metabolism with fish liver microsomes. Chemosphere 2010, 79, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Hartter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 2000, 85, 11–28. [Google Scholar] [PubMed]
- Nalecz-Jawecki, G. Evaluation of the in vitro biotransformation of fluoxetine with HPLC, mass spectrometry and ecotoxico- logical tests. Chemosphere 2007, 70, 29–35. [Google Scholar] [CrossRef] [PubMed]
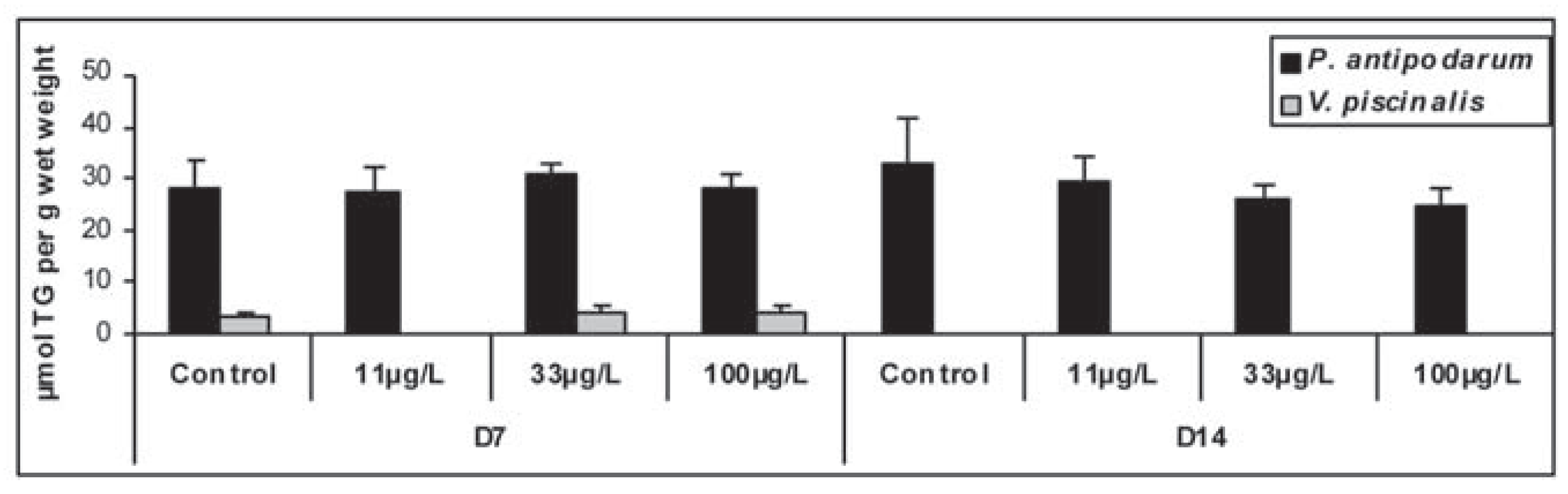
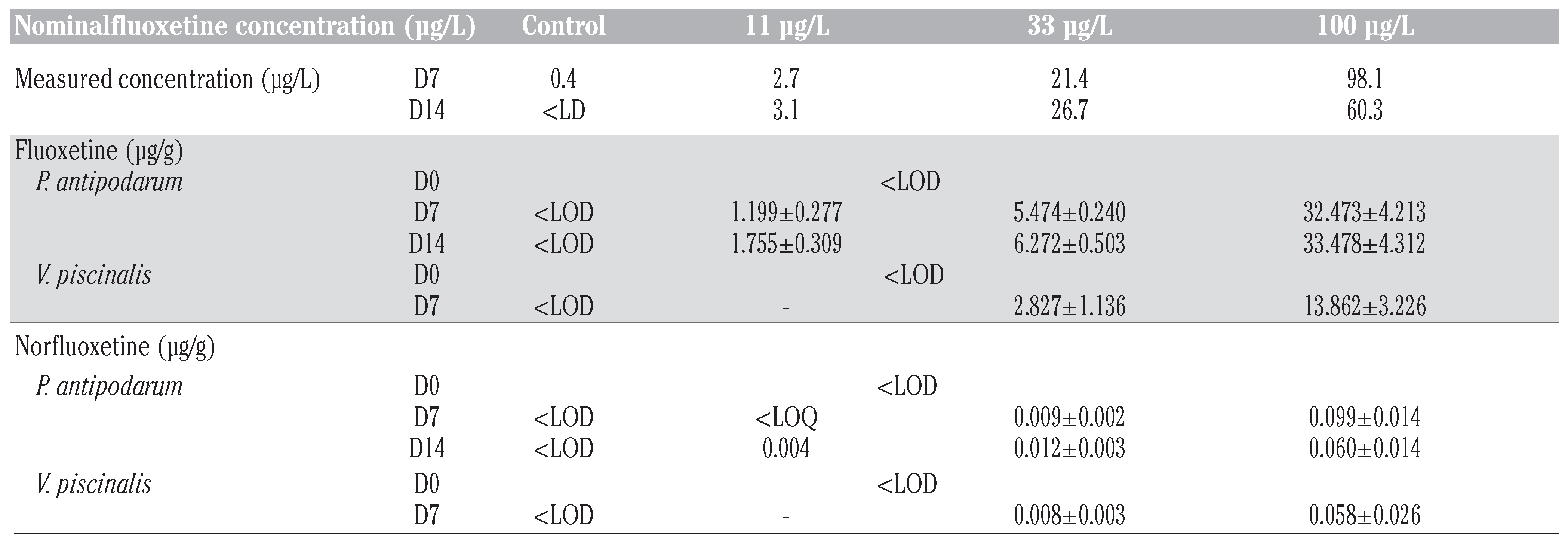 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Copyright C. Lorin-Nebel et al., 2014 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BY-NC 4.0).
Share and Cite
Gust, M.; Cren-Olivé, C.; Bulete, A.; Buronfosse, T.; Garric, J. Fluoxetine Accumulation and Metabolism as Exposure Biomarker to Better Understand Biological Efects in Gastropods. J. Xenobiot. 2013, 3, s1e4. https://doi.org/10.4081/xeno.2013.s1.e4
Gust M, Cren-Olivé C, Bulete A, Buronfosse T, Garric J. Fluoxetine Accumulation and Metabolism as Exposure Biomarker to Better Understand Biological Efects in Gastropods. Journal of Xenobiotics. 2013; 3(s1):s1e4. https://doi.org/10.4081/xeno.2013.s1.e4
Chicago/Turabian StyleGust, M., C. Cren-Olivé, A. Bulete, T. Buronfosse, and J. Garric. 2013. "Fluoxetine Accumulation and Metabolism as Exposure Biomarker to Better Understand Biological Efects in Gastropods" Journal of Xenobiotics 3, no. s1: s1e4. https://doi.org/10.4081/xeno.2013.s1.e4
APA StyleGust, M., Cren-Olivé, C., Bulete, A., Buronfosse, T., & Garric, J. (2013). Fluoxetine Accumulation and Metabolism as Exposure Biomarker to Better Understand Biological Efects in Gastropods. Journal of Xenobiotics, 3(s1), s1e4. https://doi.org/10.4081/xeno.2013.s1.e4




