Introduction
Uranium is ubiquitous in natural waters at concentrations ranging from a few ng/L to low µg/L depending on the composition of sur- rounding rocks and up to 1 mg/L at the vicinity of uraniferous sites [1]. Depleted uranium (DU) is a byproduct of enriched uranium and is used in military, aviation, medical and research applications. Freshwater ecosystems may con- stitute the final receptor areas of DU to which they may be chronically exposed both to its chemical toxicity [2].
Among all physiological processes possibly disturbed by pollutants, the immune system is likely to be one of the more sensitive physio-logical systems [3]. Fish innate immune responses, which are the first line of immune system defence of these organisms may be suppressed by xenobiotics and seem to represent relevant immunotoxic endpoints [4]. Keller et al. [5] reported effects of cadmium, copper and zinc on immune parameters including total white blood cells, macrophage activity, and oxidative burst in several fish species. Mercury and selenite were also proved to be toxic to lysozyme activity and lymphocyte proliferation in blue gourami [6]. Phagocytosis and oxidative burst were also reduced in the presence of cad- mium or mercury in rainbow trout [7]. However, data on effects of DU on fish immune system are scarce.
The objective of this work was to test the potential impacts of DU on zebrafish immune system using several endpoints measured by flow cytometry: cell population, cell mortality, reactive oxygen species (ROS) production, lysosome integrity and phagocytosis activity. Two separate experiments were conducted on zebrafish in order to assess DU effects in vivo on adults and ex vivo on leucocytes.
Materials and Methods
Freshly isolated leucocytes from naïve fish were exposed ex vivo during 17 h to 0, 20, 250, 500 μg DU/L. In in vivo experiment, adults were exposed during 3 days to 20 and 250 µg/L; a control with no DU was also added. Adult ani- mals were sacrificed for sampling after 3 days in order to assess the activity of different immune parameters: cell sub-population, cell mortality (propidium iodide exclusion test), oxidative burst [H2DCFDA before and after stimulation by phorbol 12-myristate 13-acetate (PMA)], lysosomal membrane integrity (LMI) (acridine orange) and phagocytosis (fluores- cent beads). At the end of the ex vivo contam- ination. The same biomarkers were measured on leucocytes exposed to DU, except for LMI. Results were expressed as percentage of posi- tive cells for cell sub-population, mortality and phagocytosis, and as the mean fluorescence intensity for ROS production and LMI.
Results and Discussion
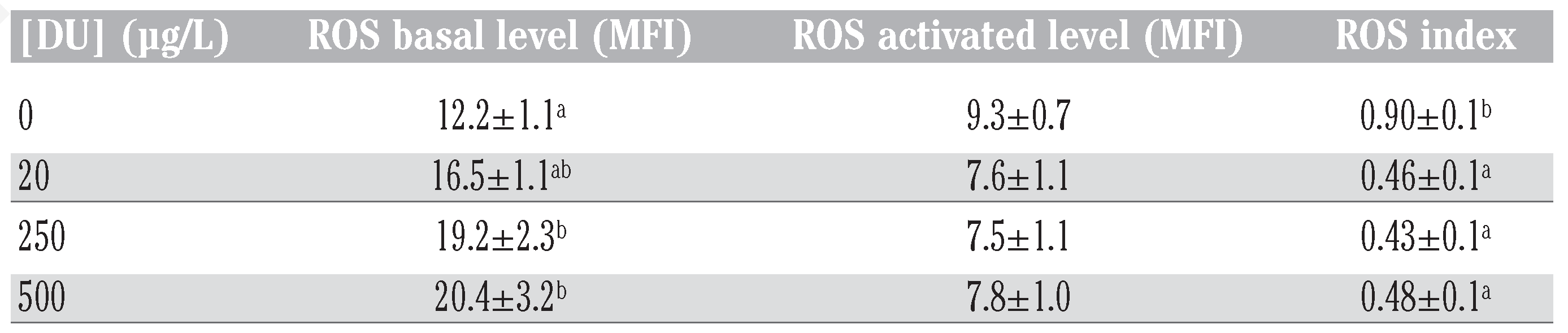
In the ex vivo experiment, ROS basal level was higher in cells exposed to DU for all tested concentrations (Table 1). ROS stimulated level showed no difference between control and DU concentrations (Table 1). ROS stimulation index was therefore lower in cells exposed to DU for all tested concentrations (Table 1). No other significant effect of DU was detected on other parameters tested.

Table 1.
Effects of depleted uranium on leucocyte parameters during the ex vivo experi- ment. Values are means of 20 replicates, except for reactive oxygen species values for tech- nical reasons (n comprised between 8 and 14); standard error is presented.
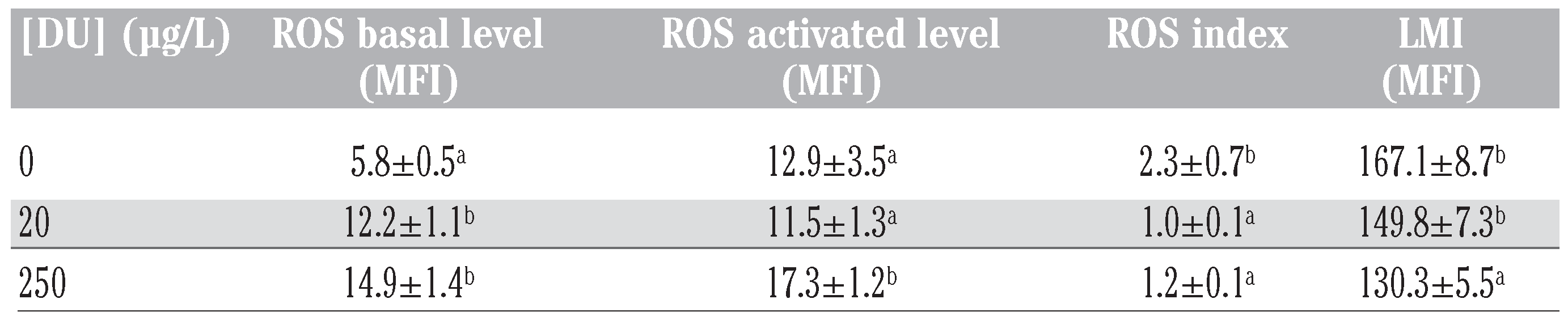
In the in vivo experiment, ROS basal level was higher in fish exposed to DU for all tested concentrations (Table 2). ROS stimulated level was higher in fish exposed to 250 μg DU/L (Table 2). ROS stimulation index was there- fore lower in cells exposed to DU for all tested concentrations (Table 2). LMI was lower in fish exposed at 250 μg DU/L (Table 2). No other significant effect of DU was detected on other parameters tested.

Table 2.
Effects of depleted uranium on leucocyte parameters during the in vivo experi- ment. Values are means of 20 replicates, except for controls values (n=18); standard error is presented.
LMI decreased in fish exposed to 250 µg DU/L after 3 days. In earthworms, lysosomal stability decreased with increasing concentra- tions of U or DU in soils [8]. In rat hepatocytes, DU induced lysosomal membrane rupture [9,10]. Therefore, our study confirmed that DU can affect the lysosomal membrane integrity in adult zebrafish.
DU increased ROS basal level with no modi- fication on ROS PMA-stimulated level, leading to a reduction of ROS stimulation index in both in vivo and ex vivo experiments for all concentration tested. In rats, uranyl acetate and DU increased ROS basal level in kidney mitochondria [9,10,11]. In a previous study, we showed an increase of ROS fold induction index in living whole kidneys removed from zebrafish exposed to 20 μg DU/L during 28 days [12]. It appears that DU induces the ROS basal production in zebrafish kidney leuco- cytes, showing a similarity to the mechanism of action of uranium known in mammals. This phenomenon could lead to an oxidative stress (inflammation) on the whole organism.
In conclusion, our study examined the effects of DU on immune parameters in zebrafish, Danio rerio for the first time. We showed that at environmentally-relevant con- centrations of DU, an increase in ROS basal production, decrease of ROS stimulation by PMA and lysosomal membrane integrity in kid- ney leucocytes were produced. We conclude that DU could pose of risk to fish health in con- taminated environments.
References
- Bonin, B.; Blanc, P.L. L’uranium dans le milieu naturel, des origines jusqu’à la mine. In Métivier H, editor. L’uranium de l’environnement à l’homme; EDP Sciences: Les Ulis, 2001; pp. 7–41. [Google Scholar]
- van Dam, R.A.; Humphrey, C.L.; Martin, P. Mining in the Alligator Rivers Region, northern Australia: Assessing potential and actual effects on ecosystem and human health. Toxicology 2002, 181–182, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Fournier, M.; Coderre, D.; Banska, W.; Krzystyniak, K. Environmental immunotox- icology. Peakall, D., Ed.; In Animals bio- markers as pollution indicators; Chapman and Hall: London, 1992; pp. 167–189. [Google Scholar]
- Bols, N.C.; Brubacher, J.L.; Ganassin, R.C.; Lee, L.E. Ecotoxicology and innate immunity in fish. Develop Comparat Immunol 2001, 25, 853–873. [Google Scholar]
- Keller, J.M.; Meyer, J.N.; Mattie, M.; Augspurger, T.; Rau, M.; Dong, J.; et al. Assessment of immunotoxicology in wild populations: review and recommendations. Rev Toxicol 1999, 3, 167–212. [Google Scholar]
- Low, K.W.; Sin, Y.M. Effects of mercuric chlo- ride and sodium selenite on some immune responses of blue gourami, Trichogaster trichopterus (Pallus). Sci Total Environ 1998, 214, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dardon, J.; Voccia, I.; Hontela, A.; Chilmonczyk, S.; Dunier, M.; et al. Immuno -modulation by heavy metals tested individ- ually or in mixtures in rainbow trout (Oncorhynchus mykiss) exposed in vivo. Environ Toxicol Chem 1999, 18, 1492–1497. [Google Scholar] [CrossRef]
- Giovanetti, A.; Fesenko, S.; Cozzella, M.L.; Asencio, L.D.; Sansone, U. Bioaccumulation and biological effects in the earthworm Eisenia fetida exposed to natural and depleted uranium. J Environ Radioact 2010, 101, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Ghashang, M.; Ettehadi, H.A.; Ghalandari, R. A search for cellular and molecular mechanisms involved in deplet- ed uranium toxicity. Environ Toxicol 2006, 21, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Shaki, F.; Tanbakosazan, F.; Ghalandari, R.; Hossein Ali, E.; Dahaghin, E. Protective effects of fungal beta-(1’3)-D- glucan against oxidative stress cytotoxici- ty induced by depleted uranium in isolated rat hepatocytes. Hum Exper Toxicol 2011, 30, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Hosseini, M.J.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of depleted urani- um on isolated rat kidney mitochondria. Biochim Biophys Acta 2012, 1820, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, B.; Cavalie, I.; Camilleri, V.; Adam- Guillermin, C. Effects of depleted uranium on oxidative stress, detoxification, and defence parameters of zebrafish Danio rerio. Arch Environ Contam Toxicol 2013, 64, 140–150. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Copyright C. Lorin-Nebel et al., 2014 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BY-NC 4.0).