Genotoxic Impact of a Municipal Effluent Dispersion Plume in the Freshwater Mussel Elliptio complanata: An In Situ Study †
Abstract
:Introduction
Materials and Methods
Solvents, chemicals and standards
Sample collection and extraction
Extract clean-up for removal of pigments and interferences
Sample analysis and analytical quality control
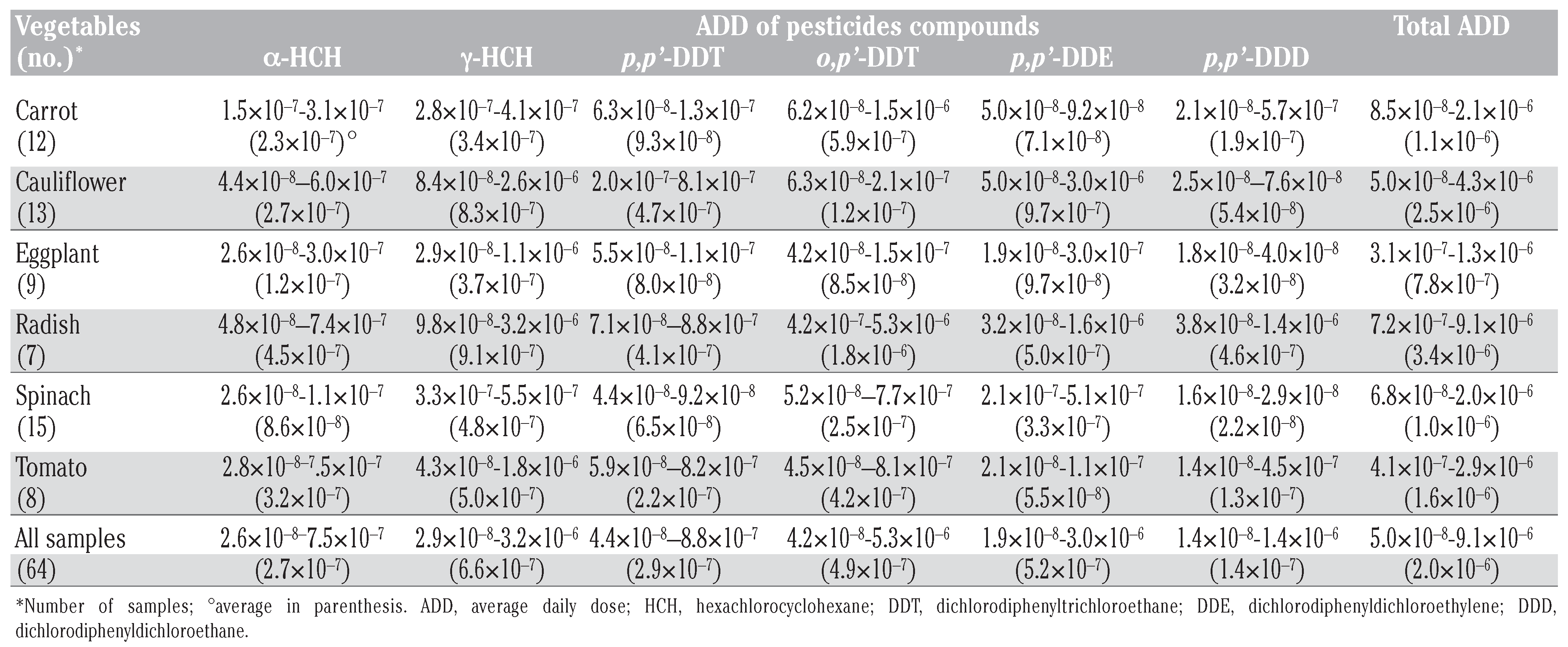
Human health risk assessment
Results
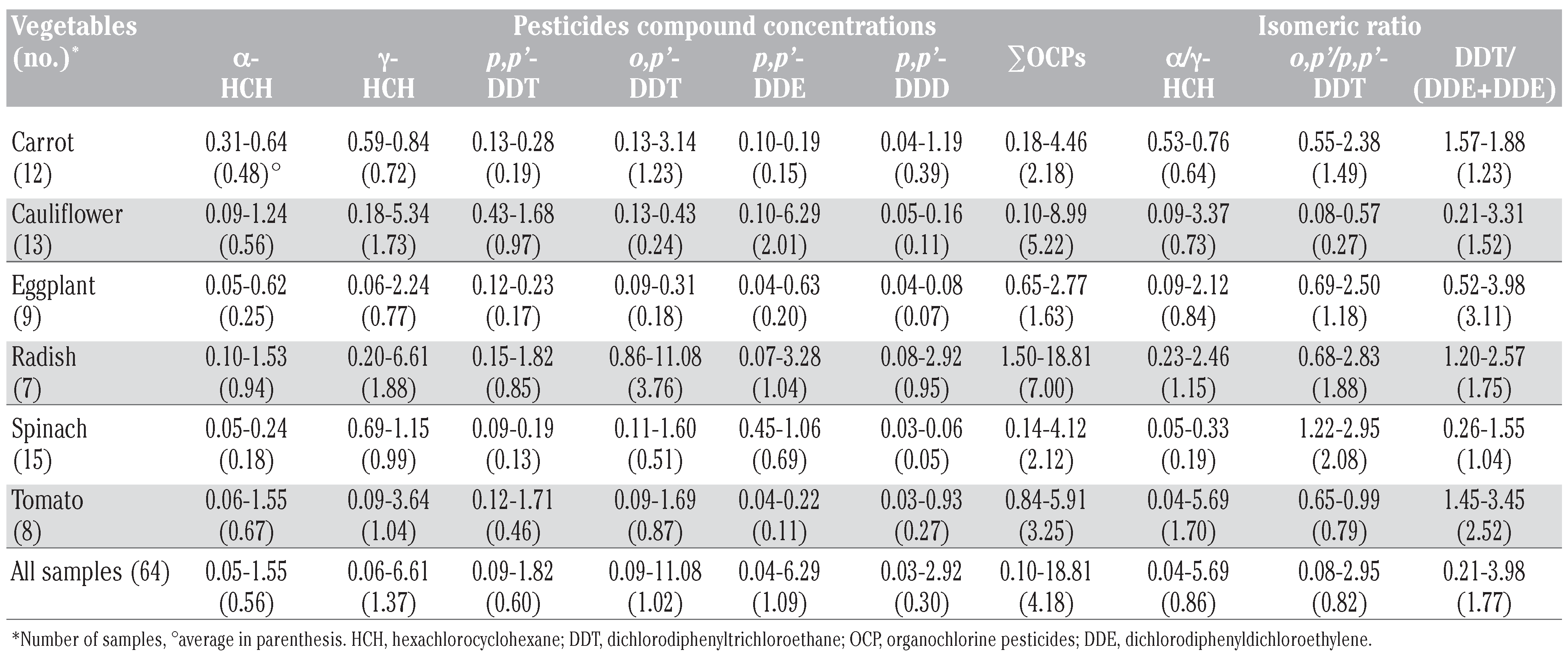
Residue levels of hexachlorocyclohexane and dichlorodiphenyltrichloroethane
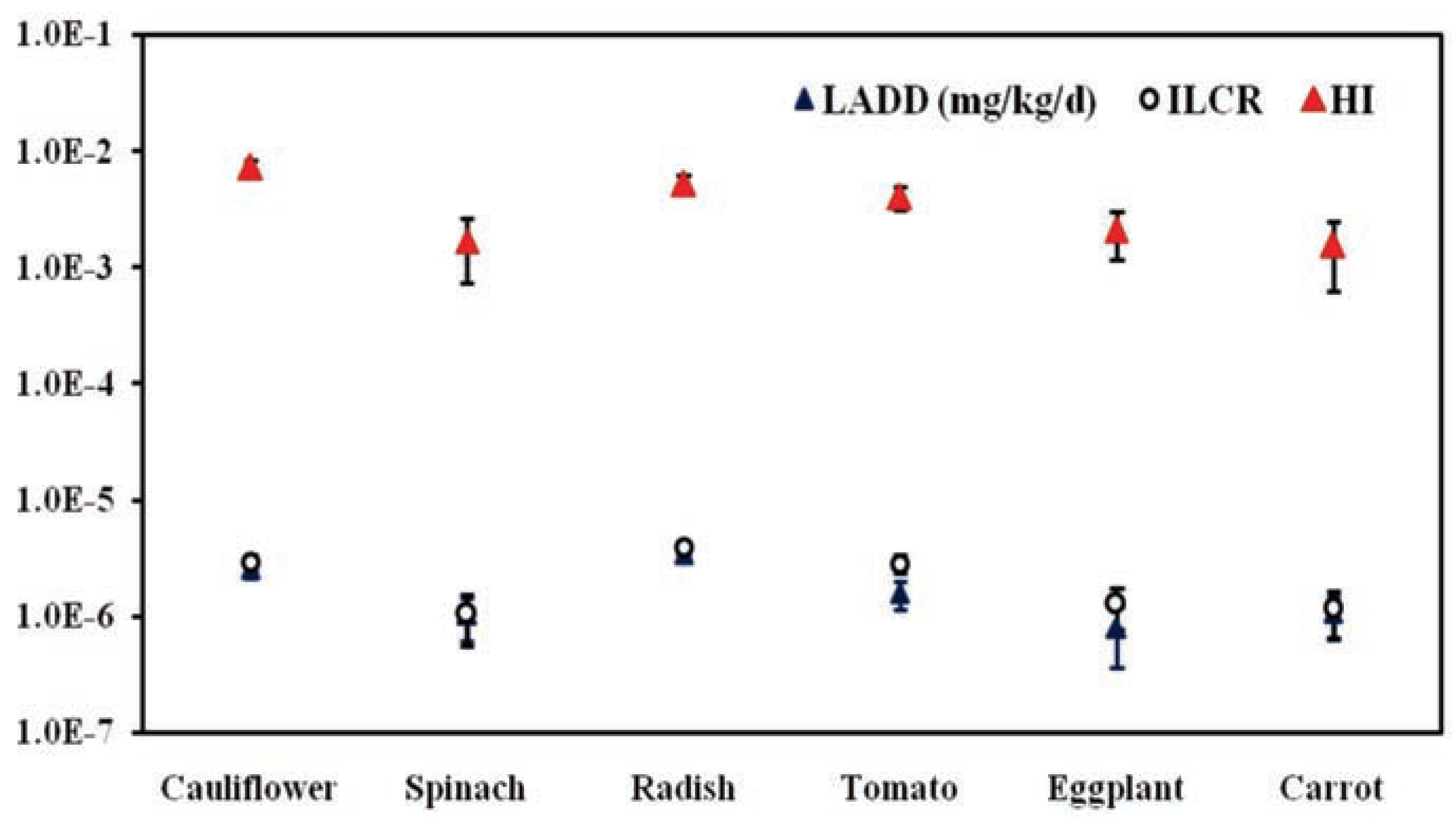
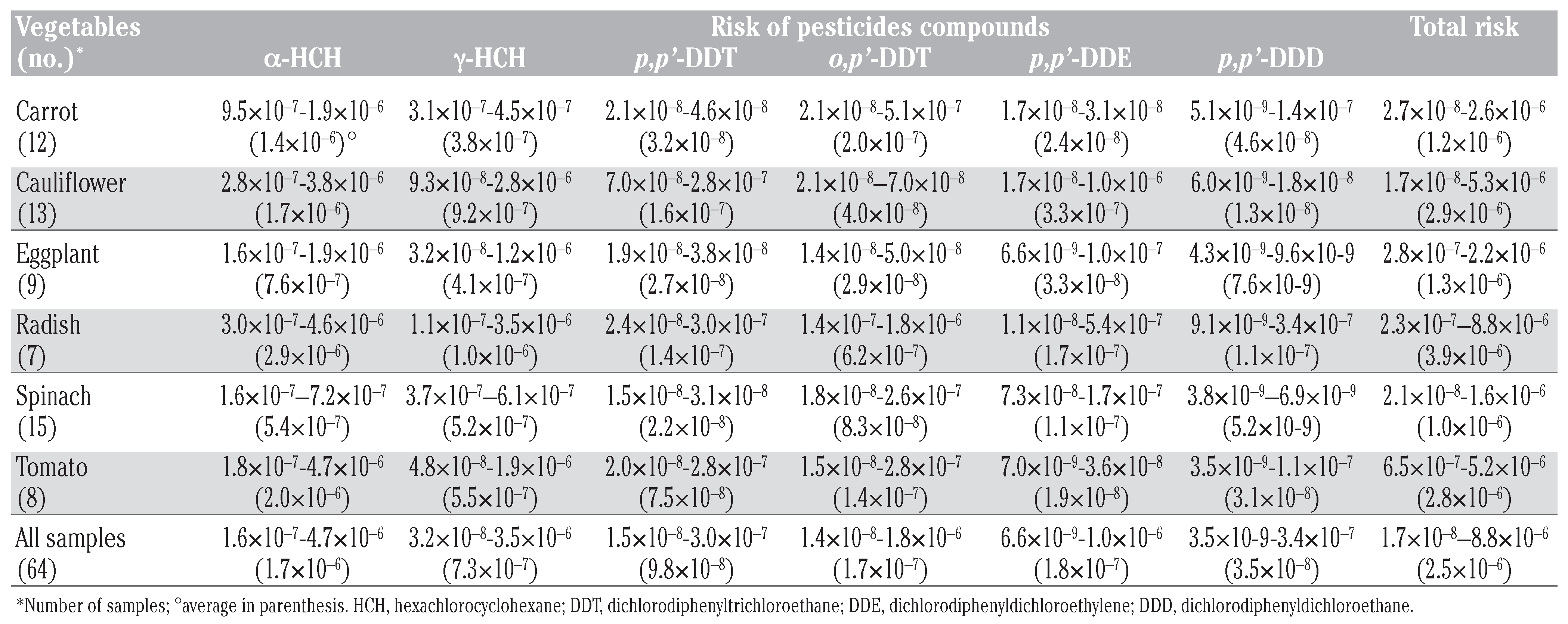
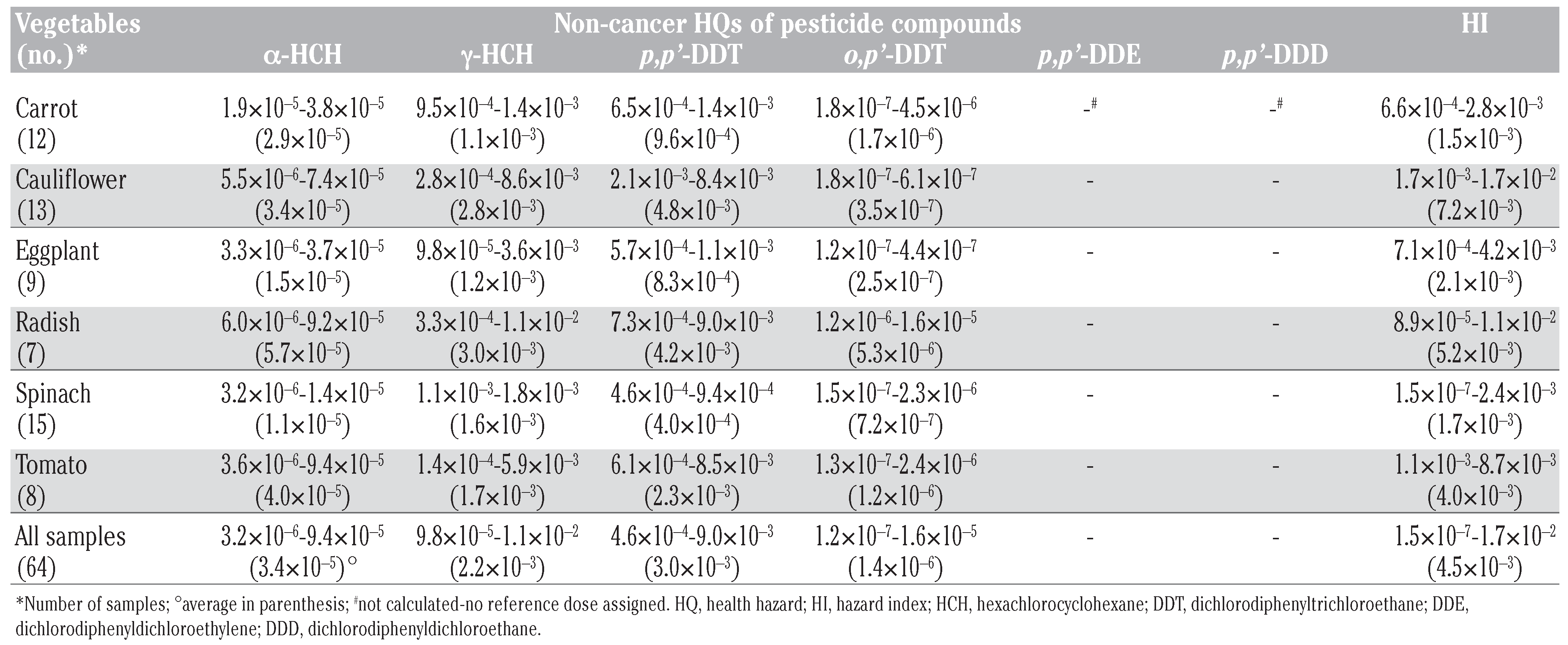
Human health risk estimates
Discussion
Possible sources identification
Human health risk
Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for hexachlorocyclohexanes. Atlanta, GA: U S Department of Health & Human Services Public Health Service, ATSDR; 2005. Available online: http://www.atsdr.cdc.gov/ toxprofiles/tp.asp?id=754&tid=138.
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for DDT, DDE, and DDD; U S Department of Health & Human Services Public Health Service, ATSDR: Atlanta, GA, USA, 2008.
- Wania, F.; Mackay, D. Tracking of distribution of persistent organic pollutants. Environ Sci Technol 1996, 30, 390A–396A. [Google Scholar] [CrossRef] [PubMed]
- UNEP (United Nations Environment Programme). UNEP/POPS/COP.4/38. 8 May 2009. Available online: http://www.POPs.int/.
- van den Berg, H. Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Persp 2009, 117, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Jit, S.; Dadhwal, M.; Kumari, H.; Jindal, S.; Kaur, J.; et al. Evaluation of hexachlorocyclohexane contamination from the last lindane production plant operating in India. Environ Sci Pollut Res 2011, 18, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Government of India. Annual Report 2011-2012, Department of Chemicals & Petrochemicals Ministry of Chemicals & Fertilizers; Government of India: New Delhi, India, 2012.
- Bhattacharyya, A.; Barik, S.R.; Ganguly, P. New pesticide molecules, formulation technology and uses: present status and future challenges. J Plant Prot Sci 2009, 1, 9–15. [Google Scholar]
- UNEP (United Nations Environment Programme). National implementation plans Stockholm Convention on persistent organic pollutants (POPs) (Government of India); Secretariat of the Stockholm Convention: Stockholm, Sweden, 2011; Available online: http://chm.pops.int/.
- Snedeker, S.M. Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin. Environ Health Perspect 2001, 109, 35–47. [Google Scholar]
- Ejaz, S.; Akram, W.; Lim, C.W.; Lee, J.J.; Hussain, I. Endocrine disrupting pesticides: a leading cause of cancer among rural people in Pakistan. Exper Oncol 2004, 26, 98–105. [Google Scholar]
- Cox, S.; Niskar, A.S.; Narayan, K.M.V.; Marcus, M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: hispanic health and nutrition examination survey, 1982-1984. Environ Health Perspect 2007, 115, 1747–1752. [Google Scholar] [CrossRef]
- Lee, D.H.; Steffes, M.; Jacob, D.R., Jr. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ Health Perspect 2007, 115, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.H.; Bouaziz, A.; et al. Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Robison, A.K.; Sirbasku, D.A.; Stancel, G.M. DDT supports the growth of an estrogen- responsive tumor. Toxicol Lett 1985, 27, 109–113. [Google Scholar] [CrossRef]
- Nakata, H.; Kawazoe, M.; Arizono, K.; Abe, S.; Kitano, T.; Shimada, H.; et al. Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissue from China: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxocol 2002, 43, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Petersen, B.; et al. Pesticide residues in food-acute dietary exposure. Pest Manage Sci 2004, 60, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Wendie, L.; Claeysa, W.L.; Schmit, J.F.; Bragard, C.; Maghuin-Rogister, G.; Pussemier, L.; et al. Exposure of several Belgian consumer groups to pesticide residues through fresh fruit and vegetable consumption. Food Control 2011, 22, 508–516. [Google Scholar]
- Samanta, S. Organochlorine pesticide residue studies in fish of the River Ganges in West Bengal. Pesticide Res J 2006, 18, 104–108. [Google Scholar]
- Aktar, M.W.; Paramasivam, M.; Sengupta, D.; Purkait, S.; Ganguly, M.; Banerjee, S. Impact assessment of pesticide residues in fish of Ganga River around Kolkata in West Bengal. Environ Monit Assess 2009, 157, 97–104. [Google Scholar] [CrossRef]
- Devanathan, G.; Subramanian, A.; Someya, M.; Sudaryanto, A.; Isobe, T.; Takahashi, S.; et al. Persistent organochlorines in human breast milk from major metropolitan cities in India. Environ Poll 2008, 157, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Someya, M.; Ohtake, M.; Kunisue, T.; Subramanian, A.; Takahashi, S.; Chakraborty, P.; et al. Persistent organic pollutants in breast milk of mothers residing around an open dumpsite in Kolkata, India: specific dioxin-like PCB levels and fish as a potential source. Environ Int 2009, 36, 27–35. [Google Scholar] [CrossRef]
- Guzzella, L.; Roscioli, C.; Vigano, L.; Saha, M.; Sarkar, S.K.; Bhattacharya, A. Evaluation of the concentration of HCH, DDT, HCB, PCB and PAH in the sediments along the lower stretch of Hugli estuary, West Bengal, northeast India. Environ Int 2005, 31, 523–534. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Binneli, A.; Riva, C.; Parolini, M.; Chatterjee, M.; Bhattacharya, A.K.; et al. Organochlorine pesticide residues in sediment cores of Sundarban wetland, north- eastern part of Bay of Bengal, India, and their ecotoxicological significance. Arch Environ Contam Toxicol 2008, 55, 358–371. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Bhattacharya, B.D.; Bhattacharya, A.; Chatterjee, M.; Alam, A.; Satpathy, K.K.; et al. Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ Int 2008, 34, 1062–1071. [Google Scholar] [CrossRef]
- Zhang, G.; Chakraborty, P.; Li, J.; Sampathkumar, P.; Balasubramanian, T.; Kathiresan, K.; et al. Passive atmospheric sampling of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in urban, rural, and wetland sites along the coastal length on India. Environ Sci Technol 2008, 42, 8218–8223. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Zhang, G.; Li, J.; Xu, Y.; Liu, X.; Tanabe, S.; et al. Selected organochlorine pesticides in the atmosphere of major Indian cities: levels, regional versus local variations, and sources. Environ Sci Technol 2010, 44, 8038–8043. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.L.; Qi, S.; Chakraborty, P.; Zhang, G.; Yadav, I.C. Passive air sampling of organochlorine pesticides in a northeastern state of India, Manipur. J Environ Sci 2011, 23, 808–815. [Google Scholar] [CrossRef]
- Singh, B.; Gupta, A. Monitoring of pesticide residues in farmgate and market samples of vegetables in a semiarid, irrigated area. Bull Environ Contam Toxicol 2002, 68, 747–751. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Kumar, R.; Kathpal, T.S. Monitoring of seasonal vegetables for pesticide residues. Environ Monit Assess 2002, 74, 263–270. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, R.; Madan, V.K.; Singh, R.; Singh, J.; Kathpal, T.S. Magnitude of pesticide contamination in winter vegetables from Hissar, Haryana. Environ Monit Assess 2003, 87, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I. Pesticide residues in vegetables in and around Delhi. Environ Monit Assess 2003, 86, 265–271. [Google Scholar] [CrossRef]
- Bhanti, M.; Taneja, A. Monitoring of organochlorine pesticide residues in summer and winter vegetables from Agra, India – a case study. Environ Monit Asses 2005, 10, 341–346. [Google Scholar] [CrossRef]
- Ranga Rao, G.V.; Sahrawat, K.L.; Ch Rao, S.; Das, B.; Reddy, K.K.; Bharath, B.S.; et al. Insecticide residues in vegetable crops growth in Kothapalli watershed, Andhra Pradesh, India: a case study. Indian J Dryland Agric Res Dev 2009, 24, 21–27. [Google Scholar]
- Srivastava, A.K.; Trivedi, P.; Srivastava, M.K.; Lohani, M.; Srivastava, L.P. Monitoring of pesticide residues in market basket samples of vegetable from Lucknow City, India: QuEChERS method. Environ Monit Assess 2011, 176, 465–472. [Google Scholar] [CrossRef]
- Bankar, R.; Ray, A.K.; Kumar, A.; Adeppa, K.; Puri, S. Organochlorine pesticide residues in vegetables of three major markets in Uttar Pradesh, India. Acta Biologica Indica 2012, 1, 77–80. [Google Scholar]
- Kumar, B.; Mukherjee, D.P. Organochlorine residues in vegetables. Int J Veg Sci 2012, 18, 121–136. [Google Scholar] [CrossRef]
- Gowda, S.R.A.; Somashekar, R.K. Evaluation of pesticide residues in farmgate samples of vegetables in Karnataka, India. Bull Environ Contam Toxicol 2012, 89, 626–632. [Google Scholar] [CrossRef]
- Kumari, B.; Kathpal, T.S. Monitoring of pesticide residues in vegetarian diet. Environ Monit Assess 2009, 151, 19–26. [Google Scholar] [PubMed]
- Brock, J.W.L.; Melnyk, J.; Caudill, S.P.; Needham, L.L.; Bond, A.E. Serum levels of several organochlorine pesticides in farmers correspond with dietary exposure and local use history. Toxicol Ind Health 1998, 14, 275–289. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk assessment guidance for superfund. Human health evaluation manual (Part A); EPA 540-1-89-002; United States Environmental Protection Agency: Washington, DC, USA, 1989.
- Morra, P.; Bagli, S.; Spadoni, G. The analysis of human health risk with a detailed procedure operating in a GIS environment. Environ Int 2006, 32, 444–454. [Google Scholar] [CrossRef]
- USEPA. Human health risk assessment; 2012. Available online: http://www.epa.gov/ reg3hwmd/risk/human.
- Yang, Y.; Li, D.; Mu, D. Levels, seasonal variations and sources of OCPs in ambient air of Guangzhou, China. Atm Environ 2008, 42, 677–687. [Google Scholar] [CrossRef]
- Willet, L.; Ulrich, E.M.; Hites, H.A. Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 1998, 32, 2197–2207. [Google Scholar] [CrossRef]
- Malaiyandi, M.; Shah, S. Evidence of photoisomerization of hexachlorocyclohexane isomers in the ecosphere. J Environ Sci Health 1980, A19, 887–910. [Google Scholar] [CrossRef]
- Walker, K.; Vallero, D.A.; Lewis, R.G. Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environ Sci Technol 1999, 33, 4373–4378. [Google Scholar] [CrossRef]
- Hoai, P.M.; Ngoc, N.T.; Minh, N.H.; Viet, P.H.; Berg, M.; Alder, A.C.; et al. Recent levels of organochlorine pesticides and polychlorinated biphenyls in sediments of the sewer system in Hanoi, Vietnam. Environ Poll 2010, 158, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Macmillan, A.; Scholtz, M.T. Global HCH usage with 10×10 longitude/latitude resolution. Environ Sci Technol 1996, 30, 3525–3533. [Google Scholar] [CrossRef]
- Spencer, W.; Cliath, M.M. Volatility of DDT and related compounds. J Agric Food Chem 1972, 20, 645–649. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). DDT and ifs derivatives- environmental aspects (Environmental Health Criteria 83); World Health Organization: Geneva, Switzerland, 1989.
- Travis, C.C.; Arms, A.D. Bioconcentration of organics in beef, milk, and vegetation. Environ Sci Technol 1988, 22, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Atlas, E.; Giam, C.S. Ambient concentration and precipitation scavenging of atmospheric organic pollutants. Water Air Soil Pollut 1988, 38, 19–36. [Google Scholar] [CrossRef]
- Talekar, N.S.; Sun, L.T.; Lee, E.M.; Chen, J.S. Persistence of some insecticides in subtropical soils. J Agric Food Chem 1977, 25, 348–352. [Google Scholar] [CrossRef]
- Battu, R.S.; Singh, B.; Kang, B.K. Contamination of liquid milk and butter with pesticide residues in the Ludhiana district of Punjab state, India. Ecotoxicol Environ Safe 2004, 9, 324–331. [Google Scholar] [CrossRef]
- Sharma, V.P. Malaria and poverty in India. Curr Sci 2003, 84, 513–515. [Google Scholar]
- European Commission. Commission Regulation (EC) No 149/2008 of 29 January 2008 amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV setting maximum residue levels for products covered by Annex I thereto (Text with EEA relevance). In: Official Journal L 58, 1/3/2008, pp 1-398. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008R0149:EN:NOT.
- FSSAI (Food Safety Standards Authority of India). Food safety and standards regulations; 2011. Available online: http://www. fssai.gov.in.
- WHO. Inventory of IPCS and other WHO pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPR) through 2009; World Health Organization: Geneva, Switzerland, 2009. Available online: http://www.who.int.
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
©Copyright B. Kumar et al., 2013 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BYNC 3.0).
Share and Cite
Lacaze, E.; Devaux, A.; Bony, S.; Bruneau, A.; André, C.; Pelletier, M.; Gagné, F. Genotoxic Impact of a Municipal Effluent Dispersion Plume in the Freshwater Mussel Elliptio complanata: An In Situ Study. J. Xenobiot. 2013, 3, e6. https://doi.org/10.4081/xeno.2013.s1.e6
Lacaze E, Devaux A, Bony S, Bruneau A, André C, Pelletier M, Gagné F. Genotoxic Impact of a Municipal Effluent Dispersion Plume in the Freshwater Mussel Elliptio complanata: An In Situ Study. Journal of Xenobiotics. 2013; 3(s1):e6. https://doi.org/10.4081/xeno.2013.s1.e6
Chicago/Turabian StyleLacaze, E., A. Devaux, S. Bony, A. Bruneau, C. André, M. Pelletier, and F. Gagné. 2013. "Genotoxic Impact of a Municipal Effluent Dispersion Plume in the Freshwater Mussel Elliptio complanata: An In Situ Study" Journal of Xenobiotics 3, no. s1: e6. https://doi.org/10.4081/xeno.2013.s1.e6
APA StyleLacaze, E., Devaux, A., Bony, S., Bruneau, A., André, C., Pelletier, M., & Gagné, F. (2013). Genotoxic Impact of a Municipal Effluent Dispersion Plume in the Freshwater Mussel Elliptio complanata: An In Situ Study. Journal of Xenobiotics, 3(s1), e6. https://doi.org/10.4081/xeno.2013.s1.e6




