Introduction
Paraquat (PQ), a bipyridylium herbicide that is effective as non-selective herbicide when applied to leaves [
1]. Because Paraquat has a redox potential of –446 mv, any reducing agent with sufficient energy can donate an electron to the bipyridylium divalent cation, Paraquat
2+, to form a free radical, paraquat
+. The oxidation of the bipyridylium radical to form the original paraquat
2+ results in the transfer of the electron to oxygen and the formation of superoxides [
2]. Subsequent Haber-Weiss and Fenton reactions yield toxic hydroxyl radicals. Thus the herbicide (paraquat) functions as a catalyst to transfer reducing equivalent to oxygen. These reactive oxygen species (ROS) so formed may escape the electron transport chain and cause damage to cellular components [
3].
In general, Antioxidant systems either prevent these ROS from being formed, or remove them before they can damage vital components of the cell [
4]. Antioxidants are classified into two broad divisions, depending on their solubility in water (hydrophilic) or in lipids (hydrophobic or lipophilic). Water – soluble antioxidants reacts with oxidants in the cell cytosol and the blood plasma, while lipid – soluble ones protects cell membranes from lipid peroxidation [
5]. Though various antioxidants behave synergistically, ascorbic acid (vitamin C) can degrade other antioxidants or reactivate them [
5]. Vitamin C (ascorbate) can directly scavenge oxygen free radicals with and without enzyme catalysts and can indirectly scavenge them by recycling others to their reduced form [
6]. By reacting with activated oxygen more readily than any other aqueous components, vitamin C protects critical macromolecules from oxidative damage [
6].
The relative importance of vitamin C as an antioxidant is the reason this research was centered on how to enhance the antioxidant system in the body to counter the destructive effects of Paraquat. The liver enzyme was so chosen because the liver is the energy store and source of the body.
Materials and Methods
Rats
A total of 100 male albino rats (Rattus nor egicus), weighing between 180-220 g [average body weight (bw) of 0.2±0.02 kg], obtained from the animal house of the Department of Pharmacology and Toxicology, Collage of Health Sciences, University of Port Harcourt, Choba, Rivers State, Nigeria, were fed ad libitum with animal pelletized finisher feed with negligible vitamin c content, and allowed to acclimatize for two weeks in metabolic cages before the commencement of the study.
Paraquat
A percentage of 20 w/v Dizmazone® (Paraquat solution) from Dizengoff W.A. Ltd. (Lagos, Nigeria) sealed in an opaque plastic container, with a shelf-life of two years.
Vitamin C
Four bottles of Mason Natural® - pure Vitamin C (100 mg) caplets were employed. They are products of Mason Vitamins, Inc. (Miami Lakes, FL, USA).
Method
Two mL of sub-lethal doses of the toxicant (PQ) was intraperitoneally (i.p.) administered to the animals, under anaesthetics [
7], in different dosed subgroups – A
1, A
2 (0 g/kg); B
1,B
2 (0.02 g/kg); C
1,C
2 (0.04 g/kg) and D
1,D
2 (0.06 g/kg) – on bi-weekly basis over a period of three months (in simulation of contamination from polluted feed, water or air), while the control animals (subgrouped A
1, A
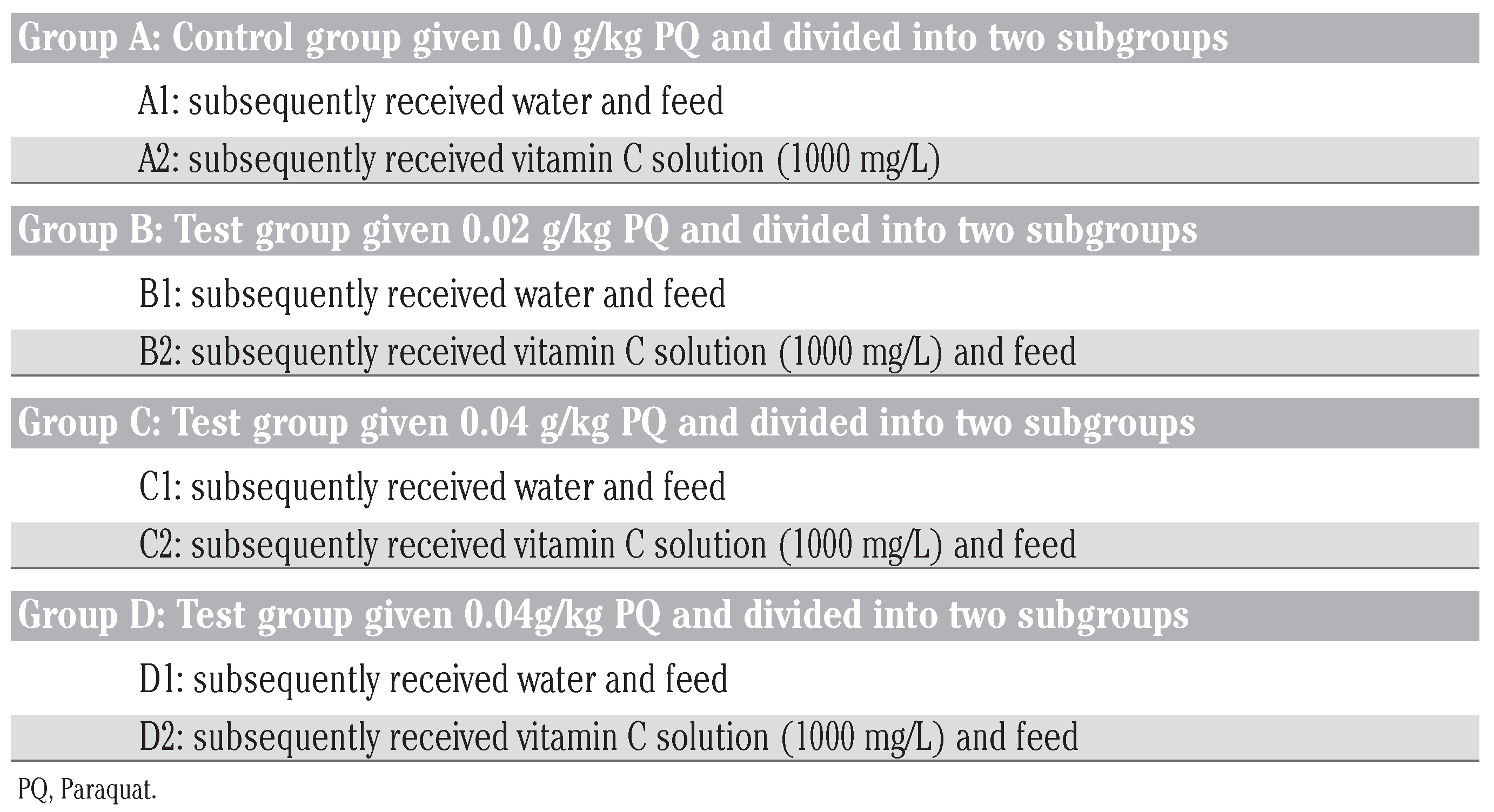
2) received 2 mL of normal saline (0.98 %) likewise in conformity with international standard in checking injection site reaction. The subgrouped animals were designated into: i) for non vitamin C treatment; and ii) for vitamin C treatment as indicated in
Table 1.
This study was conducted under eight sub-groups (A1, A2, B1, B2, C1, C2, D1 and D2). All the subgroups had 12 animals each with A1 and A2 being the control subgroups that received no Paraquat, A2, B2, C2 and D2 were placed on vitamin C – they were made to drink from water bottles with glass sipper tubes that contained vitamin c solution (200 mg/L), A1, B1, C1 and D1 were the subgroups that received ordinary drinking water with negligible vitamin C content. The water and vitamin c solution bottles were refilled at least trice daily irrespective of the volume of water or vitamin c remaining in the in-use bottles. It will be note worthy to state that at month 3, the food and water consumption by the rats were affected by PQ intoxication, mainly the subgroups dosed 0.06 g/kg bw without vitamin c treatment (D1).
On monthly intervals, four animals per subgroup were selected, anaesthetized with gaseous isoflurane anesthetic machine, the induction chamber was prefilled with 4% isoflurane and oxygen (0.6 L/min). The rats were placed in the induction chamber and observed for signs of lateral recumbence, (steady breathing and no attempt to right itself when the induction chamber is slightly tilted), only then it becomes anaesthetized enough for transfer to the mask on the rodent breathing circuit. Open the lid of the induction chamber and quickly check for absence of the pedal reflex [
8], if present, 10 mL of blood sample were collected using cardiac puncture procedures [
7].
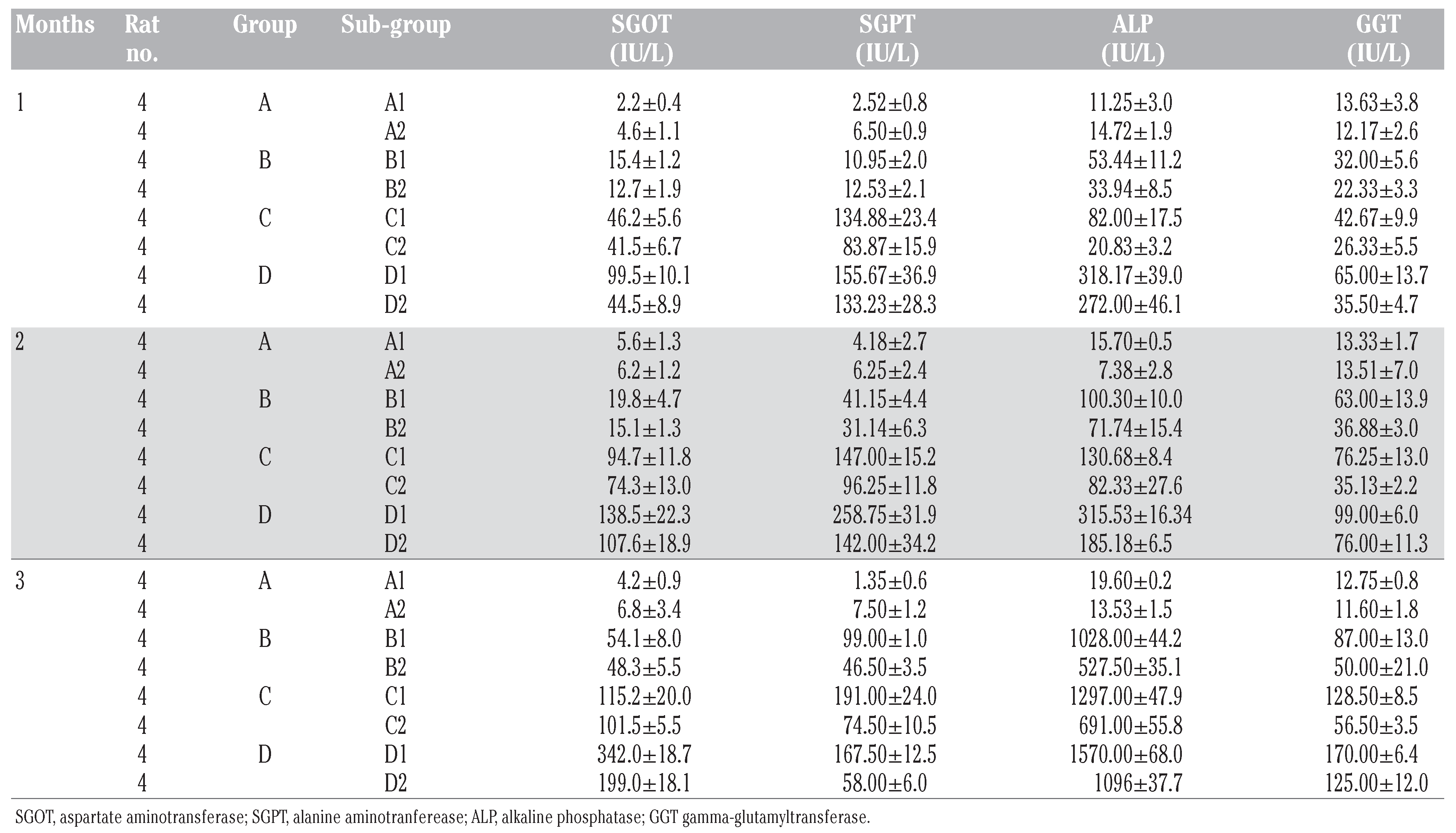
The samples were processed and centrifuge, with the serum separated and labeled accordingly; stored frozen until needed for the estimation of enzyme activity using Kodac Autoanalyser machine. Using the study pattern below, the results were as shown in
Table 1 and
Table 2 and in
Figure 1,
Figure 2,
Figure 3 and
Figure 4.
Animal care
We do affirm that in carrying out this research that The Nigerian Institutional and National Guide for the care and use of laboratory animals were followed.
Data computation
The Excel (2007) window’s package and two-way analysis of variance (ANOVA) statistical methods were used for the result analysis. With levels of significance measured at P≤0.05 and 0.001, respectively.
Results
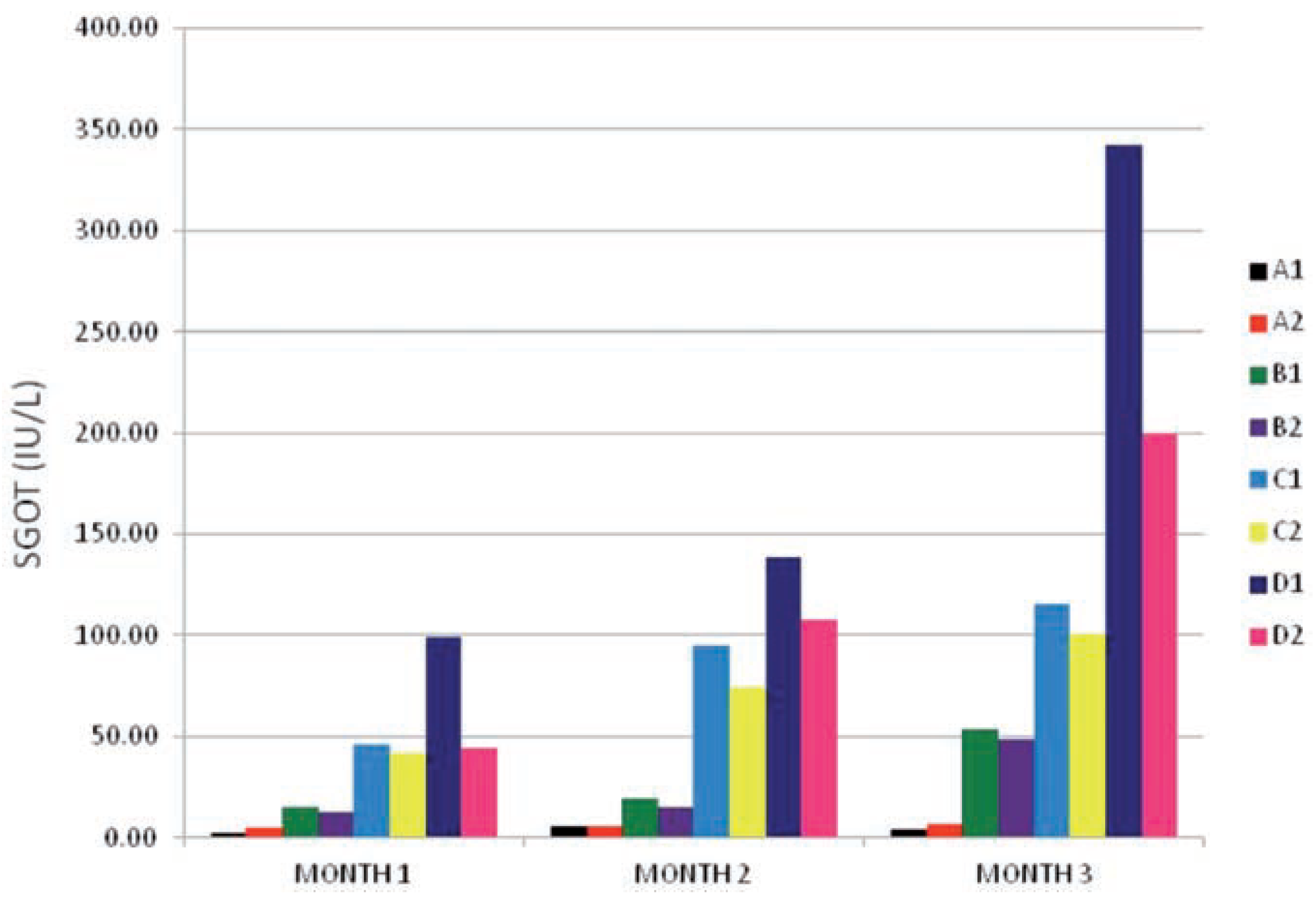
The SGOT activity of all the subgroups is shown in
Figure 1. The enzyme activity values was found to have increased on the test subgroups (B
1, B
2, C
1, C
2, D
1 and D
2) when compared to the control subgroups (A
1 and A
2). And the increases was found to be directly related to the dose and exposure time effects (P≤0.001). As the time of exposure moved from month 1 down to month 3 the enzyme activity of the PQ only treated subgroups (B
1, C
1 and D
1) increased two- to three- folds that of the subgroups that received vitamin C in addition to PQ insult (B
2, C
2 and D
2) at the same level of significance (P≤0.001). indicating that an interaction exists between dose of PQ/vitamin C given and time of exposure.The test subgroups with the highest dose 0.06 g/kg bw (D
1 and D
2) gave an explicit picture on how vitamin c effected an improved enzyme activity when compared to the one on PQ insult only. With D
1 being higher than two-times D
2 at months 1 and 3.
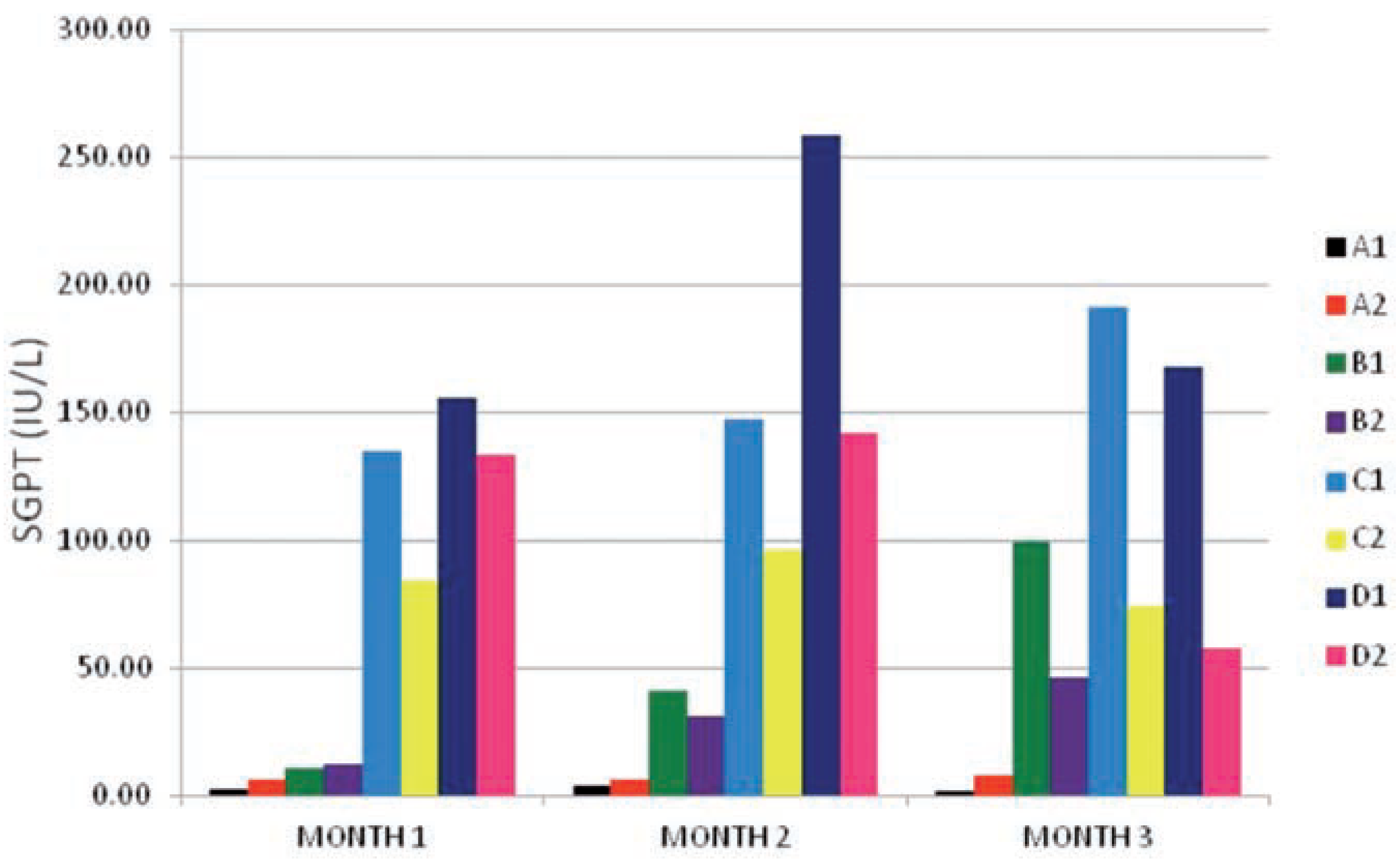
The results of the SGPT enzyme activity values as shown in
Figure 2 were similar to the ones obtained in SGOT (
Figure 1) values above. There existed an interaction between dose given and time of exposure effects at P≤0.001 level of significance. The test subgroups (B
1, B
2, C
1, C
2, D
1 and D
2) were highly elevated when compared to the control subgroups (A
1 and A
2) from month 1 to month 3 especially the subgroups dosed 0.04 g/kg and g/kg bw (C
1, C
2, D
1 and D
2 at month 1) and (B
1, B
2, C
1, C
2, D
1 and D
2 at months 2 and 3). The within group comparison indicated a well defined reduction in the values of the SGPT enzyme activity of the vitamin c treated subgroups (B
2, C
2 and D
2) when compared to the subgroups on PQ insult only (B
1, C
1 and D
1) from month 1 to month 3. This results was in line with the results of the SGOT enzyme activity values obtained in
Figure 1, showing that vitamin c has an ameliorative effect on intracellular PQ toxicity.
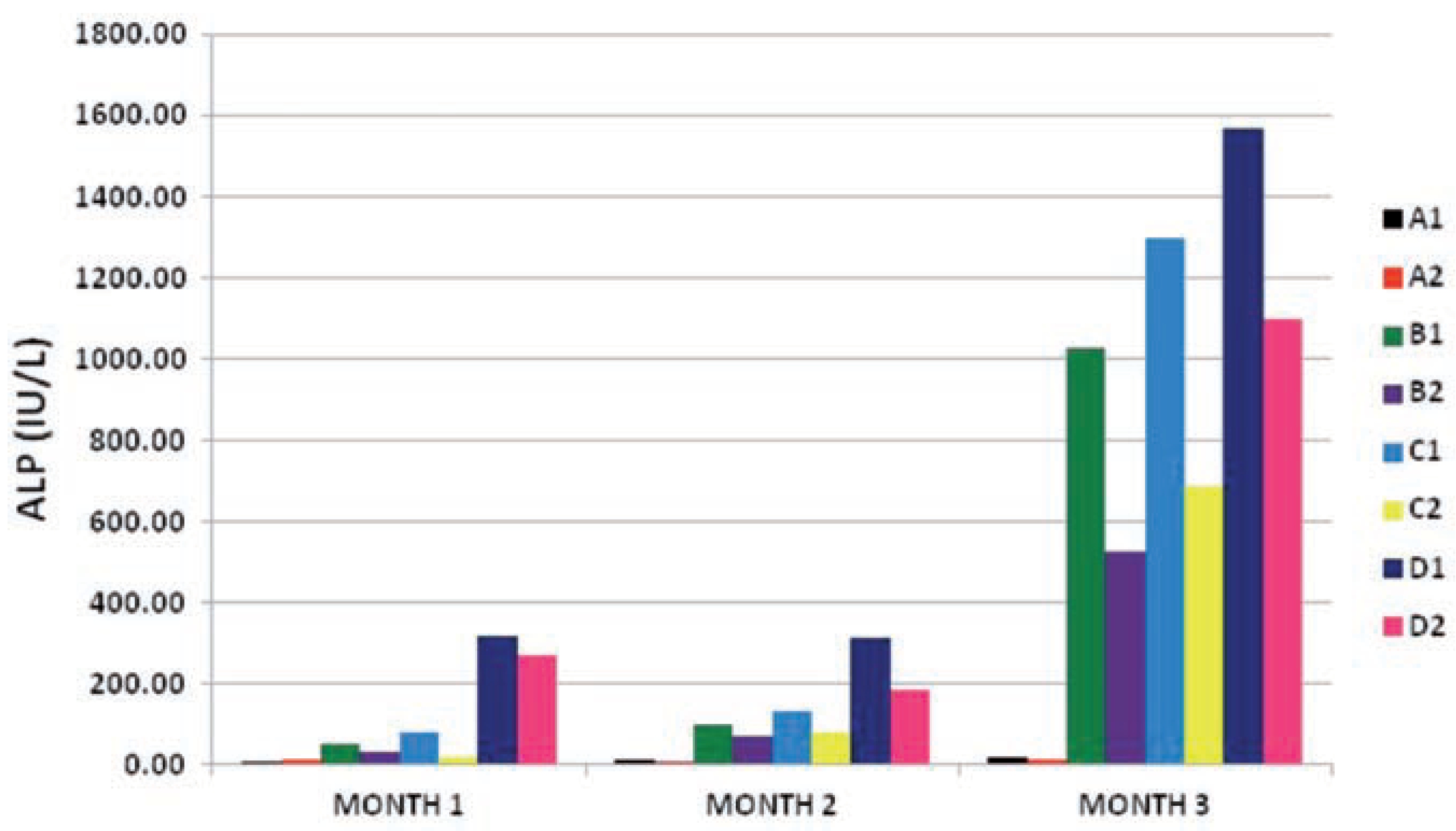
The results obtained from ALP enzyme activity on the subgroups were quite remarkable. At month 1, the test subgroups with lower doses of PQ (B1, B2, C1 and C2) showed slight elevation of ALP activity when compared to the control subgroups (A1 and A2) at P≤0.05, while the subgroups that received the highest dose of 0.06g PQ/kg bw (D1 and D2) had an elevated ALP activity at P≤0.01 when compared to the control subgroups. As the time of exposure increased from month 1 to month 3 the level of enzyme activity of the test subgroups increased the more to a level that was quite high at month 3 (P≤0.001). In all this, we found out that the vitamin c treated test sub-groups had lower ALP enzyme activity values than the subgroups under the same PQ insult only (B2<B1, C2<C1, D2<D1) at P≤0.05 (month 1), P≤0.01 (month 2) and P≤0.001 (month 3) respectively. This result showed that an interaction existed between the dose of PQ given and time of exposure of the toxic insult, and also there was a relationship between the effect of the vitamin c given with the dose of PQ given and the time of exposure.
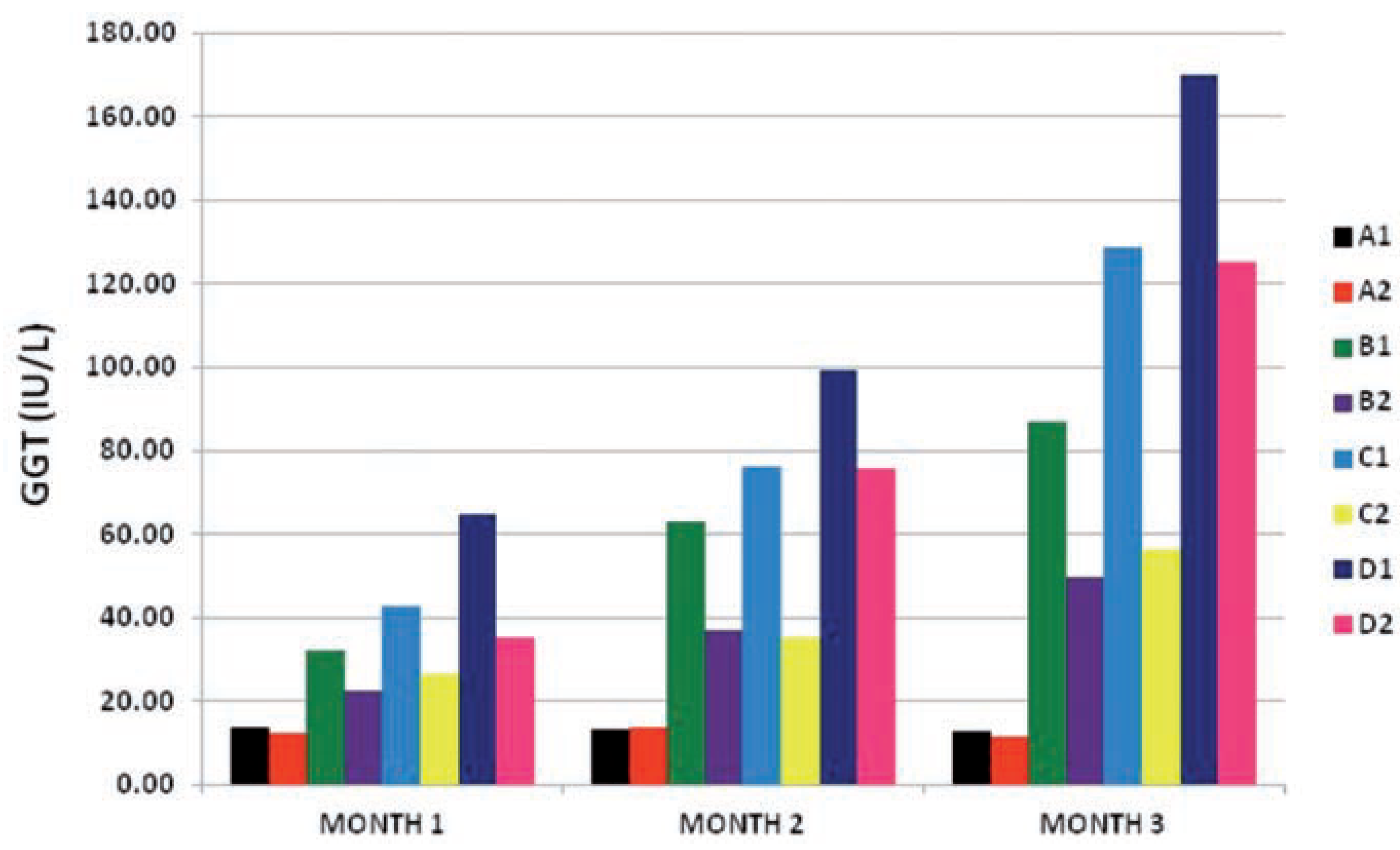
At month 1 the GGT enzyme activity values of the test subgroups were increasing in direct relation to the increase in dose of the toxicant (PQ) at P≤0.001, and this relationship continued to increase the enzyme activity even as the exposure time increased from month 1 to month 3 at P≤0.001. Also the vitamin c treated subgroups (B2, C2 and D2) all had much lower enzyme activity values than their conterpart under PQ insult only (B1, C1 and D1) at P≤0.001 from month 1 through to month 3. This also suports the existence of an interaction between the dose of PQ given and time of exposure of the toxic insult, and also a relationship between the effect of the vitamin c given with the dose of PQ given and the time of exposure.
Discussion
PQ studies and the toxicity effects of this chemical on the organs of the body, including its mechanism of toxicity have been reported [
9]. Furthermore, several reports presented PQ as a hepatotoxin [
10,
11,
12,
13].
Following the findings from the acute toxicity study, where it was observed that toxicity effects existed with increasing dose and time of exposure of PQ (i.p.) insult on the liver cells [
14]. A chronic study was designed to assess the toxicity of PQ at long duration (3 months) and also to find the possibility of ameliorating the toxicity effects using antioxidant (vitamin C).
From the liver enzymes – SGOT, SGPT, ALP and GGT – results obtained, all had changes that were highly significant (P≤0.001) both within and between subgroups (
Table 2 and
Figure 1,
Figure 2,
Figure 3 and
Figure 4), and these changes were all dose and exposure-time dependent. The enzymes activity values of the subgroups that received PQ only were almost two to five folds increased compared to that of the subgroups that received vitamin C in addition to the PQ insult. SGOT values of vitamin C treated subgroups where significantly lower than those on PQ insult only (B
2<B
1, C
2<C
1, and D
2<D
1 from month 1 to month 3 (
Figure 1) except the control group A, in-which the vitamin C treated subgroup (A
2) had slightly elevated SGOT values throughout the three months than the subgroup without vitamin C and PQ (A
1) (A
1<A
2). These changes were also observed in the values of other enzymes – SGPT, ALP and GGT (
Figure 2,
Figure 3 and
Figure 4).
It has been shown that PQ toxicity initiates lipid peroxidation which causes toxic destruction of lipid membrane bilayers initiating release of membrane bound enzymes – SGPT, ALP and GGT – to the cytoplasm [
15,
16,
17,
18]. This explains the highly elevated values of these enzymes in PQ only treated subgroups (B
2, C
1 and D
1) as compared to the subgroups that received, in addition to PQ insult, vitamin C (B
2, C
2, and D
2) in
Figure 2,
Figure 3 and
Figure 4. Indicating that vitamin C, to a large extent, reduced the toxic insults [
6] and acted as a substrate for the antioxidant enzyme ascorbate peroxidase, a function that is particularly important in stress resistance [
19], thereby maintaining and repairing cellular integrity and function [
15,
16,
17] which led to the lowered values obtained in (B
2, C
2 and D
2) as seen in
Figure 2,
Figure 3 and
Figure 4.
Generally, there was an improvement in enzyme activity in the subgroups on vitamin C during the PQ toxic insult (B
2, C
2, and D
2) in
Figure 1,
Figure 2,
Figure 3 and
Figure 4. Though these values were still high when compared to the control subgroups (A
1 and A
2) and above the reference values for rat’s liver enzymes, it’s still a pointer to health improvement. These shows that if vitamin c treatment was continued for a longer period it could have totally repaired the liver cells and improve the health status of the animals.
Conclusions
The result of this study has demonstrated that vitamin c has the capacity to improve the health status of animals under toxic insult. Exposure of rats to PQ induced a highly elevated liver enzyme – SGOT, SGPT, ALP and GGT – activities that were dose and time dependent, which on subsequent administration of vitamin C a reduction in the activity levels were observed throughout the study, indicating the reliability of vitamin c as an adjunct to the treatment of a toxic insult.
Recommendation
Vitamin C, a potent antioxidant should be one of the first line treatments (emergency procedure) for toxic insult. Vitamin C administration in PQ toxicity should not be ignored, and it should be extended even after patient’s recovery for adequate intracellular repairs to be achieved.