Introduction
Though the traditional systems of medicine prefer plant origin drugs, but some metals and minerals are also used as drugs in certain cases, that may produce moderate to severe toxicity even at therapeutic dose level if used unjudious- ly. Therefore, because of likelihood of toxicity and pharmacokinetic inconvenience such drugs are detoxified by various means. Taklees (calci- nation) practiced in Unani medicine is one of the detoxification methods in which metals and minerals are incinerated at high temperature1 so as to reduce the elements of toxicity. In Unani medicine, the incinerated substance, which changes into oxide form, is known as Kushta, a Persian word, meaning killed2 and refers to a substance that has been killed (incinerated) at high temperature (usually >500°C). In this method, fire of cow dung cakes is used as the source of heat. Kushta is a well-established dosage form of Unani medicine and is supposed to be very effective in view of its small particle size facilitating rapid absorption and quick action compared with other dosage forms, which are coarsely powdered and slowly absorbed.3 But, there seems lack of scientific data on the meth- ods of preparation, standardization and toxicity of kushta.
In Unani medicine, toxicity studies are not being conducted in a well organized manner as is carried out in modern medicine, rather toxic- ity of a drug is denoted merely under the head- ing of Mazarrat (toxic effects) observed after giving a certain dose level directly on human being, which does always not give precise results and leaves behind a lot of variations in the dose of toxic drugs.
Apart from its use in varied fields, Arsenic tri- oxide is also used as therapeutic agent for cen- turies4 and is still in use in Unani medicine.3,5 Crude arsenic has extensively been studied for its toxicity,6-8 but very few scientific reports are available on its kushta form. In view of absence of sufficient data on chronic toxicity of Kushta Sammulfar (KSF) and being a new dosage form, we selected it for chronic toxicity study using standard parameters prescribed for chronic tox- icity of drugs. The data were analyzed by appro- priate statistical tests to observe any significant variation (P<0.05) in different groups to see the relative toxicity.
The research protocol was approved by Institutional Animals Ethics Committee (IAEC) of National Institute of Unani Medicine (NIUM), Bangalore, India (vide Reg. 953/C/06/CPCSEA), April 2011. A voucher specimen was submitted to the department of Ilmul Advia (Pharmacology), NIUM.
Materials and Methods
Materials
Arsenic trioxide (As2O3) was procured from Nice Pvt. Ltd., Kerala, India. Alum was purchased from local market of Bangalore.
Preparation of the test drug
KSF was prepared following the method described in National Formulary of Unani medi- cine.5 In the calcination process, Boota and Gil- e-Hikmat are assumed essential. Boota is a heat- proof clay bowl in which drug, which is to be cal- cined, is kept and the bowl is then covered with another bowl of similar dimension. Gil-e-Hikmat is the process of application of specific semi solid material made of moist clay and dry cotton wool pounded together till they are mixed well and applied around the bowl especially at the junction of the edges of the two bowls to make the material airtight. Arsenic trioxide and alum were taken in the ratio of 1, 2 and put in the Boota. A 222 cu feet pit was dug and the Boota was placed inside the heap of 2.5 kg of cow dung cakes placed in the pit. Over it, 2.5 kg of cow dung cakes were again placed so that the Boota would remain in the middle. The cakes were then ignited. The Boota was taken out of the pit after all the cakes were ignited and the fire cooled down and opened cautiously so as to sep- arate the Kushta easily. The Kushta was pound- ed manually, weighed and preserved in sterilized glass tubes with airtight lids.
Dose of the test drug
The effective dose (ED50) of KSF in humans has been described in Unani literature as 10-15 mg.5 The dose for Wistar rats was calculated by the conversion factor of 7 and was found to be 1.75 mg–1 kg.9
Experimental animals
Forty healthy Wistar rats of either sex; weigh- ing 150-250 g; 2-3 months of age were used. The animals were divided into four groups of ten ani- mals each.10 The animals were kept under stan- dard laboratory conditions. They were housed in polypropylene cages at 23-30°C and kept on 12 h light and dark cycle and had free access to feed and water.
Experimental design
The study was carried out according to the methods of Gupta et al.,11 Ghosh12 and Klaassan.10 Three dose levels of test drug were given to three groups of rats. First group received five times ED50, second group 10 times ED50 and the third group 15 times ED50, for three months. Fourth group which served as control received normal saline (NS). After three months, blood was collected for studying hema- tological parameters viz. hemoglobin estima- tion, red blood cells (RBC) count, total lympho- cyte count (TLC), differential lymphocyte count (DLC), biochemical parameters viz. estimation of blood glucose, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT) and serum creatinine. Liver and kidney were preserved for histopathol- ogy.11 Parameters for observing signs of toxicity such as change in body weight, skin color, motor activities, food intake, weight of vital organs like liver and kidney and gross changes in them were considered.10,12 The test drug was given orally in the suspension of 5% gum acacia once in a day, in the morning. The groups and treatment regi- men used are:
- -
Group I (plain control): treated with NS as vehicle in the dose of 5 mL/kg;13
- -
Group II (KSF-I): received Kushta Sammulfar in the dose of 8.75 mg–1 kg (5 times of ED50);
- -
Group III (KSF-II): received Kushta Sammulfar in the dose of 17.50 mg–1 kg (10 times of ED50);
- -
Group IV (KSF-III): received Kushta Sammulfar in the dose of 26.25 mg–1 kg (15 times of ED50).11
General observations
Body weight of the animals was taken every 14 days.12 Food intake was measured, periodical- ly by giving known amount of diet to the ani- mals, daily. Early in the morning, the feed was reweighed and the amount consumed was calcu- lated by difference.14 General behavior, skin color, skin pigmentation, body hair loss, palpable mass, motility, tremor and convulsions15 were observed every 14 days.10
Hematology, biochemistry and histopathology
At the end of the experiment, all animals were killed after 12 h fasting by over dosing of thiopental sodium. Blood was collected at the end of the experiment through heart puncture for hematology and biochemistry. Liver and kid- ney were weighed, examined grossly and micro- scopically.
Hematology
Hemoglobin was estimated by Sahli’s method. RBC and white blood cells were counted by Improved Neubauer Haemocytometer. DLC was done by the method described by Chatterjee.16
Biochemistry
Various biochemical tests10 were done by auto analyzer (Star 21 Plus). Blood sugar was esti- mated by GOD-POD, end point method. SGOT was estimated by UV Kinetic (IFCC) method. SGPT was estimated by UV Kinetic (IFCC) method. Blood urea was estimated by Urease/ GLDH method. Serum creatinine was estimated by Picrate method.
Histopathology
Liver and kidney were preserved into 10% for- malin buffer overnight, dehydrated and finally embedded in paraffin through histokinet pro- cessing. Sections of 5-µm thickness were cut, stained with routine hematoxylin and eosin.17,18 Histopathological changes were seen under a binocular microscope.
Analysis of data
The data were expressed as mean±SEM and the values for the test and control groups were compared by using one-way analysis of variance (ANOVA) followed by different multiple compar- ison tests. The significance level was considered (P<0.05).
Results
General observation
The animals of all groups showed slight weight gain throughout the experiment, which was not significant statistically. Food intake was found significantly increased in KSF-I (P<0.05) and KSF-II (P<0.01). Before weighing the organs, gross examination did not show any changes in any group. The effect on kidney and liver weight was also found non significant sta- tistically. Relative liver weight in control, KSF-I, KSF-II and KSF-III was found to be 3.4±0.085, 3.7±0.086, 3.6±0.079, respectively. Relative kid- ney weight (RKW) in control, KSF-I, KSF-II and KSF-III, was found to be 0.54±0.014, 0.46±0.012, 0.42±0.017, 0.48±0.019%, respectively. The RKW was found significantly decreased in KSF-II (P<0.01). The low and moderate doses of KSF produced mild to moderate diarrhea, whereas the higher dose produced severe diarrhea. All animals were ultimately recovered from diar- rhea after 42 days of the experiment. Body hair loss was seen in the neck region in all the test groups from third observation of the experiment except KSF-I. The hair loss gradually increased throughout the experiment. In KSF-II mild hair loss was observed in 2 out of 10 animals. In KSF- III severe hair loss was seen in 5 out of 10 ani- mals. Motility of animals of test groups was found increased throughout the experiment as compared to control group.
Hematology
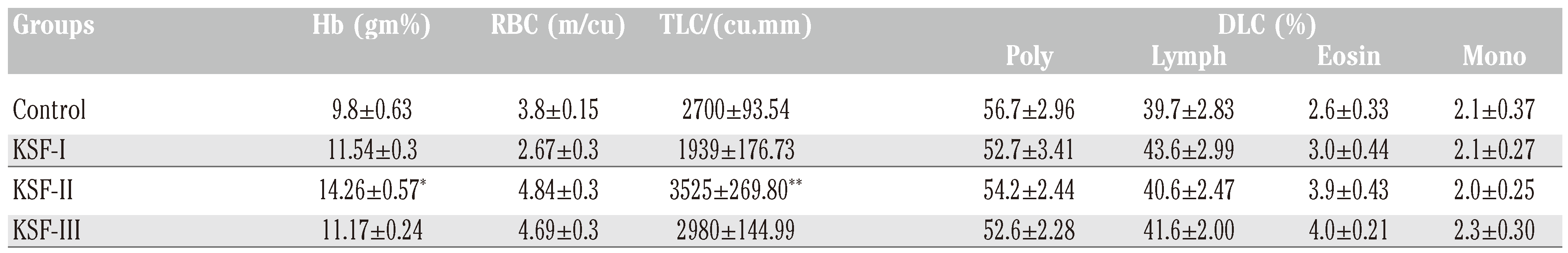
Significant increase in TLC was found in KSF- II (P<0.05) as compared to control group. The hemoglobin level increased significantly in KSF- II (P<0.001) as compared to control group (
Table 1).
Biochemistry
Blood sugar level increased significantly in all the test groups (P<0.001) except KSF-I. SGOT increased significantly in KSF-III (P<0.01). SGPT significantly increased in KSF-III (P<0.05) as compared to control group. Blood urea increased significantly in KSF-II (P<0.05), KSF- III (P<0.05). Serum creatinine level was found significantly increased in KSF-I (P<0.001), KSF- III (P<0.001), as compared to control group. Detailed results are shown in
Table 2.
Histopathology
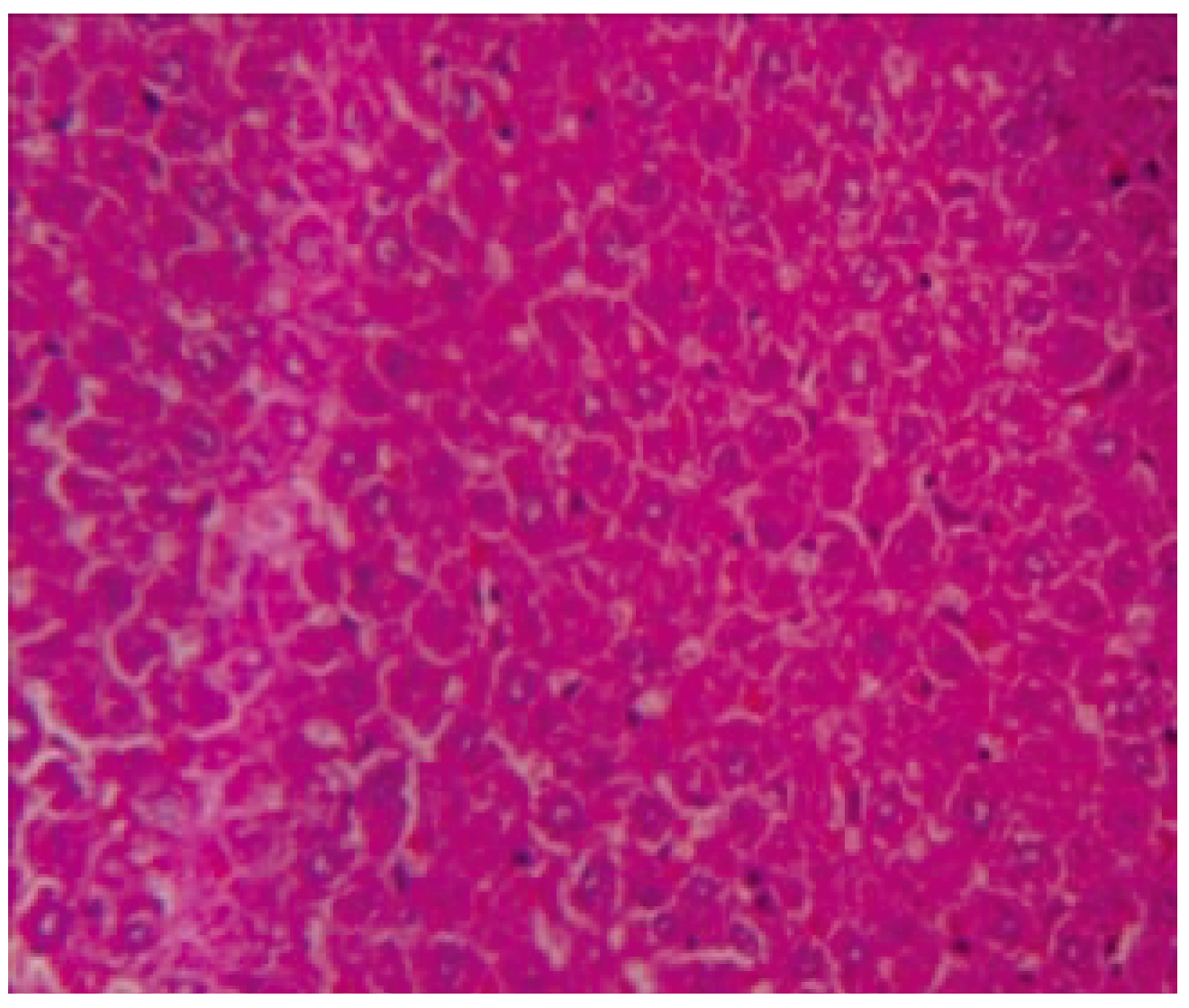
Histopathology of liver of control group showed normal structure (
Figure 1). No evidence of hepatitis was observed in the KSF-I (
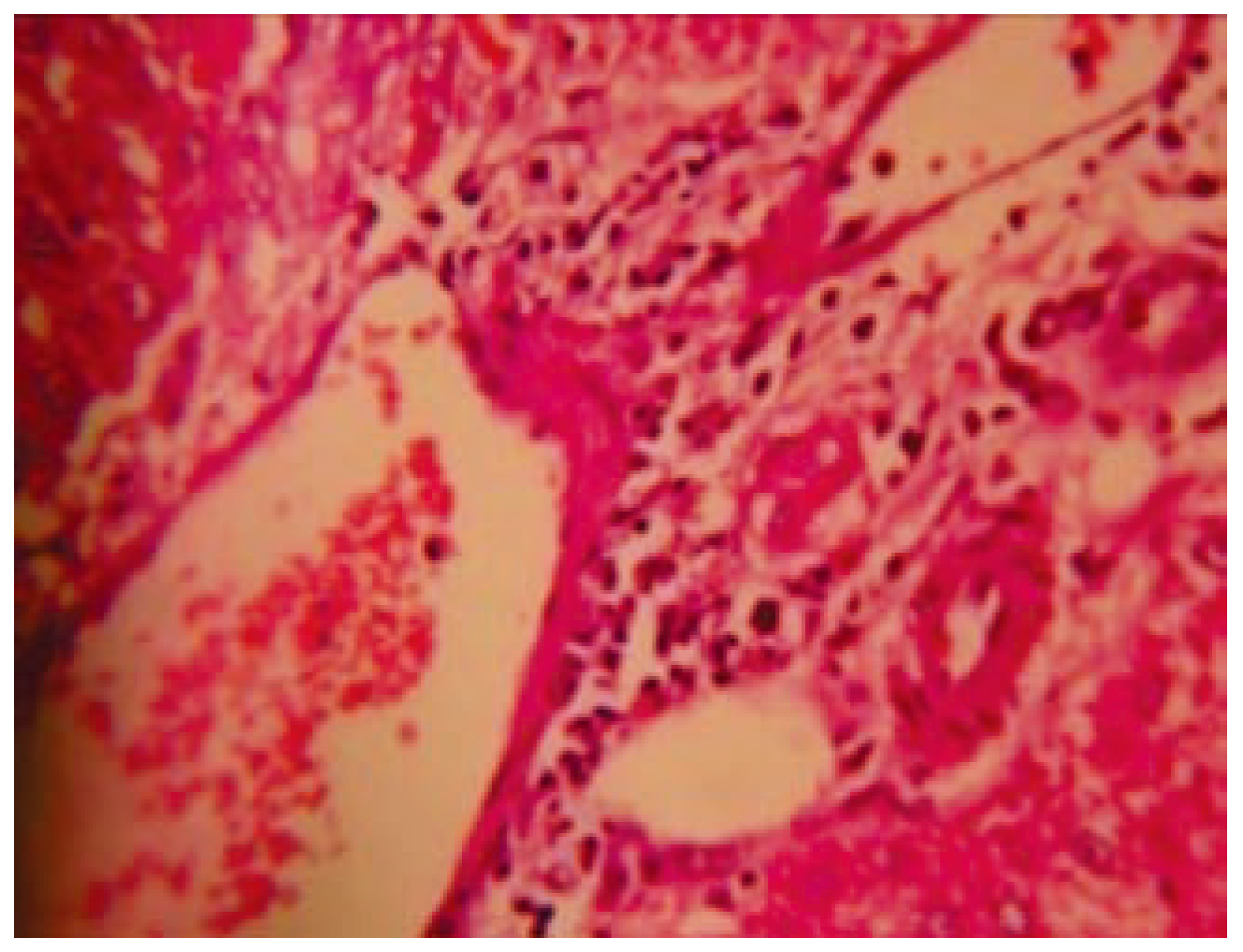
Figure 2), however, mild congestion was observed in cen- tral vein, hepatic vein and portal triad. Few hepa- tocytes were swelled up slightly. Few inflammato- ry cells were seen in portal triad area. Mild to moderate congestion was observed in central vein, hepatic vein and portal triad, in KSF-II and KSF-III. Capsule in KSF-II was found normal but thickened in KSF-III (
Figure 3 and
Figure 4).
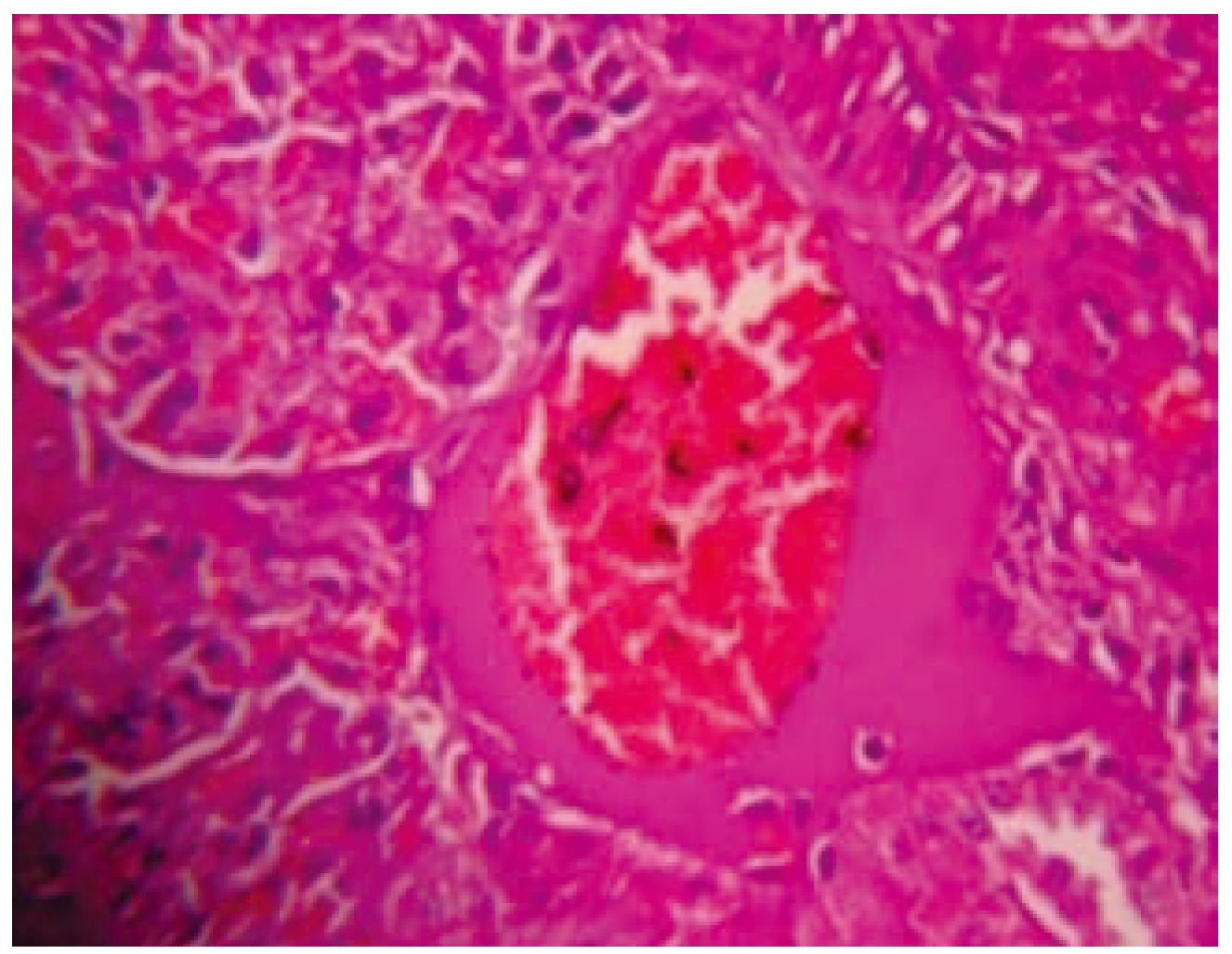
Normal capsule and normal glomeruli were observed in KSF-I. The tubules were lined by intact lining epithelial cells. Mild vascular con- gestion was observed. Mild interstitial edema and occasional inflammatory cells were seen in interstitial spaces. In the KSF-II studies showed normal capsule and normal glomeruli. The tubules were damaged occasionally. Moderate vascular congestion, mild interstitial edema and occasional inflammatory cells were seen in inter- stitial spaces of kidney. In the KSF-III section showed normal capsule, while moderate degree of glomerular congestion was seen. Mild to mod- erate tubular damage was observed. Severe degree of vascular congestion, mild interstitial edema and occasional inflammatory cells were seen in interstitial spaces (
Figure 5,
Figure 6,
Figure 7 and
Figure 8).
Discussion
No data are available on chronic toxicity of kushta Sammulfar to compare our findings; hence results of earlier studies carried out on crude arsenic may differ from our findings, even though we have tried to compare our findings with the studies carried out on crude arsenic as in calx too there remain some elemental parts. In a study on chronic toxicity of crude arsenic carried out in rats at dose 1.7 mg/kg (12 h) for 90 days, no change in the body and organ weight was recorded.19 In our study, too, no significant changes were observed. But increase in relative weight of liver of KSF-I suggested hypertrophy of the hepatic cells probably because liver tends to accumulate arsenic with repeated exposures over periods of months or years as shown by some studies. Some studies also revealed that arsenic causes hepatomegaly in 77% of patients after chronic exposure.4 Chen et al. (2004) stud- ied the effects of arsenic in mice liver and con- cluded that chronic oral inorganic arsenic expo- sure caused cellular hypertrophy and steatosis.20 Similarity between the findings of our study and previous studies, is suggestive of some degree of toxicity over prolong use of arsenic irrespective of its form. Significant reduction in RKW in KSF- II (P<0.05) indicated mild renal atrophy, which may be due to injurious effect of the test drug as inorganic arsenic accumulates in the kidney after repeated exposure and it is a major route for the excretion of arsenic.21 Presence of ele- mental arsenic in kushta may have caused this atrophy. The average daily food intake signifi- cantly increased in KSF-I (P<0.05) and KSF-II (P<0.01) in the beginning of the experiment, which may be due to hyperglycemia, observed in our study. Arsenic is more likely than other heavy metals to produce a dramatic gastro enteric picture after ingestion. In acute arsenic poisoning, bloody rice water diarrhea is often observed.22,23 Acute high exposure as well as longer term lower dose exposures of arsenic can produce diarrhea.24-26 Though, diarrhea was observed in our study in all the test groupsbut no bloody diarrhea at any dose level in any group was observed. This may be suggestive of mild to moderate gastroenteritis produced by calx form, which is less harmful than crude arsenic. Skin is a major target organ of arsenic toxicity. Many skin lesions such as hyper pigmentation, hypo pigmentation, hyperkeratosis and parakeratosis are usually seen in humans after chronic expo- sure to arsenic.27,28 Interestingly, the hallmark dermal lesions associated with human oral exposures to arsenic have not been found to occur in experimental animals. We too did not find dermal lesions in the animals in our study. Body hair loss after ingestion of arsenic is an indicator of toxicity. Hair loss was seen in the neck region in all groups except KSF-I from the third observation, which was gradually increased throughout the study. Excessive hair loss was seen in KSF-III. Some studies suggest- ed that even small dose of arsenic for prolong use can cause hair loss.24,29 Our findings sug- gested that arsenic can cause hair loss in any form, if used for long. In our study, motility was increased in all the test groups which validate the claim of Unani medicine that Kushta Sammulfar possesses Muharrik-e-Asab (nerve stimulant effect),30,31 however this claim is not supported by any scientific report.
Hematology is also good parameter for the assessment of toxicity. In our study, RBC count slightly increased in KSF-II and KSF-III. It may be due to Muqauwi-e-Dam (hemopoietic) effect of Kushta Sammulfar.32 Hemoglobin level increased slightly in KSF-I, and III and markedly in KSF-II (P<0.001), which may be due to increase in RBC count. However, studies have shown to produces anemia after chronic expo- sure of arsenic,20,23,29 but it may be because of kushta form of arsenic which contains less amount of elemental arsenic not causing mas- sive destruction of RBCs. Our findings are in consonance with the reports of Unani physi- cians.32 TLC count slightly decreased in KSF-I, while remarkably increased in KSF-II (P<0.01) as compared to control group found that the TLC count decreased when mice were given higher dose of arsenic. It may be due to apoptotic effect of arsenic on plasma cells.33 In DLC, polymorph count was slightly decreased in KSF-I, II, III. Granulocytopenia is a common effect of arsenic poisoning and is reported as resultant of acute, intermediate and chronic oral exposures. This effect might be due to cytotoxic effect of arsenic on the polymorph cells.20 The lymphocyte count was slightly increased in all the test groups as compared to control. This effect might be due to counteract poisonous effect of Kushta Sammulfar. Several studies related to animals as well as humans have shown that long term arsenic exposure can cause diabetes melli- tus.4,23,33-36 In our study, blood sugar level increased significantly (P<0.001) in all test groups except KSF-I. Hyperglycemias may be due to islet cells toxicity because arsenic admin- istration is reported to cause severe damage of islet cells.33 In male Wistar rats, exposed to 1.7 ppm of arsenite orally for 90 days, the pancreas pathology included the absence of insulin immunolabel in the Beta cells and a discontinu- ous peripheral pattern of glucagon.35 A study indicated that sub chronic exposure to As2O3 induces oxidative stress and oxidative damage in pancreas.36 Our findings are similar to previ- ous studies.
Arsenic is known to produce disturbance in liver function; therefore, SGOT and SGPT may be reliable determinants of liver parenchymal injury. Increase in plasma SGOT and SGPT may be mainly due to the leakage of these enzymes from the liver cytosol into the blood stream.36 In our study, SGOT increased significantly in KSF- III (P<0.01), SGPT was also increased signifi- cantly in KSF-III (P<0.05). These findings reveal that Kushta Sammulfar also causes hepatotoxicity, if used for long. In low dose of KSF, SGOT and SGPT did not increase indicating that kushta is not injurious for liver in low dose. However, fur- ther follow up is suggested for 120 days or more to rule out the chronic use of these arsenic for- mulations and effect on various organs evaluat- ed using analytical techniques for estimation of free arsenic and arsenic trioxide.
Since, arsenic and its various preparations are reported to cause renal damage;19,37 there- fore, renal function test may be a good pointer of arsenic poisoning. Production of oxygen free radicals by arsenic induces tubular necrosis, which in turn increases tubular permeability resulting in diffusion and back leak of the fil- trate across the tubular basement membrane back into the interstitium and circulation lead- ing to an apparent decrease in glomerular filtra- tion rate. Under these circumstances, back leak of filtrate results in decreased excretion and increased retention of nitrogenous waste i.e. urea in serum.37 In our study, blood urea was sig- nificantly increased in KSF-II (P<0.05), III (P<0.05). It may be calculated that Kushta Sammulfar in moderate to high dose is toxic to the kidney. Serum creatinine was found signifi- cantly increased in KSF-I (P<0.001), KSF-III (P<0.001), showing almost all dose level toxic to the kidney.
Histopathological study of liver clearly demon- strated that the KSF produced toxicity to the liver as chronic hepatitis. These features were dose dependent as in low dose of KSF caused mild congestion in central vein, hepatic vein and por- tal triad, which may be due to prolonged use, no liver hyperplasia, adenomas, or cancer lesions were found. Similarly, the histopathological study of kidney demonstrated that the KSF pro- duced toxicity to the renal tissues and produced the features of chronic nephritis with graded doses. Several clinical studies have suggested that arsenic causes severe hepatic and renal damage even prolong use can cause cancer of those organs.38 In the 1980s, several studies reported on the carcinogenicity of inorganic arsenic, in hamster after intratracheal instilla- tion,39 in rats’ urinary bladder.40 Some studies have suggested that an animal model is still needed for completely proving and understand- ing the carcinogenic effect of arsenic. However, there have been several positive reports on the carcinogenicity of arsenic in animal models.39 The mild to moderate toxic effects of calx form of arsenic may be due to presence of elemental arsenic in oxide form as in our previous study we found 6.388±0.711 ppm elemental arsenic in KSF estimated by atomic absorption spectropho- tometery.41
Conclusions
KSF produced dose dependent toxicity. KSF-1, i.e. low dose of KSF did not produce remarkable toxic effects. Mild to moderate toxicity was observed in KSF-II and KSF- III. Though, liver and kidney tissues were not markedly damaged, but in view of risk benefit ratio (more risk than benefit) and low safety margin, use of KSF par- ticularly prolong use in human should be warned as self medication must be avoided, as it was found moderately toxic in rats. The study also validated the claim of Unani physicians regard- ing the use of KSF in low dose and a gap of cer- tain period for the next use.