Microcystin-LR Acute Exposure Does Not Alter In Vitro and In Vivo ATP, ADP and AMP Hydrolysis in Adult Zebrafish (Danio rerio) Brain Membranes
Abstract
:Introduction
Materials and Methods
Animals
Reagents
In vivo exposure
In vitro exposure
Membrane preparation
Nucleotide hydrolysis assay
Protein determination
Statistical analysis
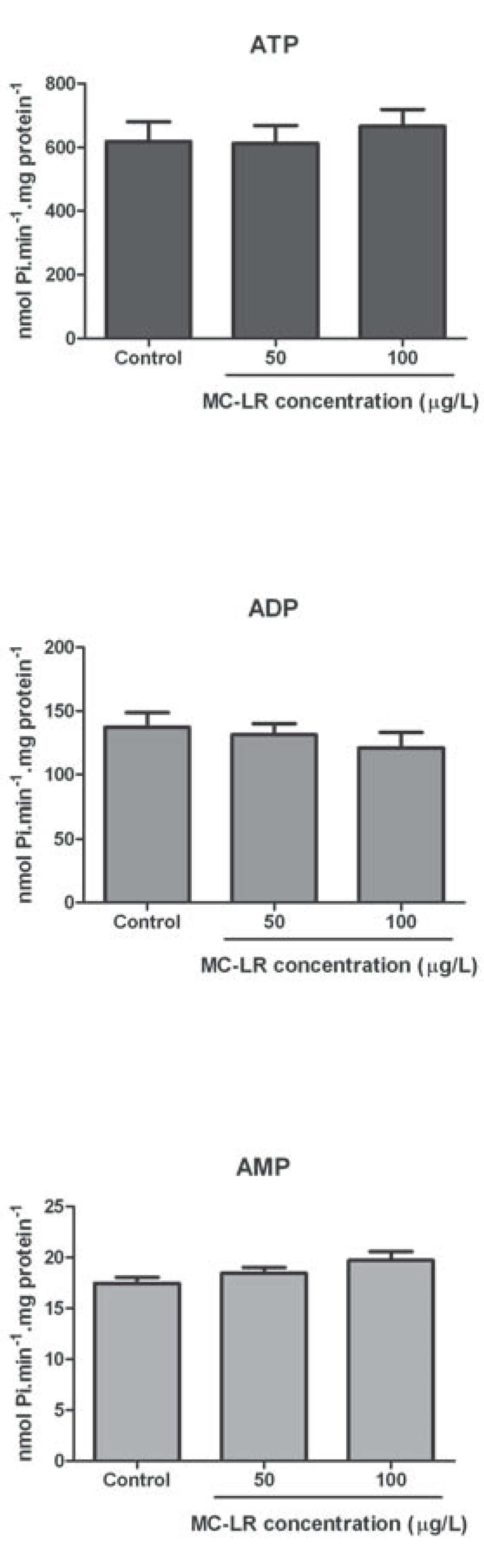
Results
Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ Toxicol Chem 2006, 25, 72–86. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Lin, L.; Hong, H. Protein profiles in zebrafish (Danio rerio) brains exposed to chronic microcystin-LR. Chemosphere 2010, 81, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Sun, Y.; Fu, W.; Guo, Z.; Xu, L. Microcystin-LR induces cytoskeleton system reorganization through hyperphosphorylation of tau and HSP27 via PP2A inhibition and subsequent activation of the p38 MAPK signaling pathway in neuroendocrine (PC12) cells. Toxicology 2011, 290, 218–229. [Google Scholar] [CrossRef]
- Kist, L.W.; Rosemberg, D.B.; Pereira, T.C.; de Azevedo, M.B.; Richetti, S.K.; de Castro Leão, J.; et al. Microcystin-LR acute exposure increases AChE activity via transcriptional ache activation in zebrafish (Danio rerio) brain. Comp Biochem Physiol C Toxicol Pharmacol 2012, 155, 247–252. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res 2001, 52, 44–56. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol Ver 1998, 50, 413–492. [Google Scholar]
- Goepfert, C.; Imai, M.; Brouard, S.; Csizmadia, E.; Kaczmare, K.; Robson, S. CD39 modulates endothelial cell activation and apoptosis. Mol Med 2000, 6, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, S.F.; Burgstahler, A.D.; Nathanson, J.A. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA 1996, 93, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, B.; Song, L.; Nie, P. Gene expression profiles in liver of zebrafish treated with microcystin-LR. Environ Toxicol Pharmacol 2008, 26, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liang, H.; Zhou, W.; Zhang, X. Apoptotic responses of zebrafish (Danio rerio) after exposure with microcystin-LR under different ambient temperatures. J Appl Toxicol, Epub ahead of print. 2012. [Google Scholar]
- Kist, L.W.; Piato, A.L.; da Rosa, J.G.; Koakoski, G.; Barcellos, L.J.; Yunes, J.S.; et al. Acute Exposure to microcystin-producing cyanobacterium microcystis aeruginosa alters adult zebrafish (Danio rerio) swimming performance parameters. J Toxicol 2011, 2011, 280304. [Google Scholar] [CrossRef] [PubMed]
- Siebel, A.M.; Rico, E.P.; Capiotti, K.M.; Piato, A.L.; Cusinato, C.T.; Franco, T.M.; et al. In vitro effects of antiepileptic drugs on acetylcholinesterase and ectonucleotidase activities in zebrafish (Danio rerio) brain. Toxicol In Vitro 2010, 24, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Murphy, P.A.; Kirkham, D.; Henley, J.M. Interaction of guanine nucleotides with [3H]kainate and 6-[3H]cyano-7-nitroquinoxaline-2,3-dione binding in goldfish brain. J Neurochem 1993, 61, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Delfert, D.; Junger, K.D. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 1986, 157, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72, 218–254. [Google Scholar] [CrossRef]
- Burnstock, G. Cotransmission. Curr Opin Pharmacol 2004, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Ribeiro, J.A. ATP as a presynaptic modulator. Life Sci 2000, 68, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Senger, M.R.; Arizi, M.d.B.; Bernardi, G.F.; Dias, R.D.; et al. Methanol alters ecto-nucleotidases and acetylcholinesterase in zebrafish brain. Neurotoxicol Teratol 2006, 28, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Ethanol alters acetyl-cholinesterase activity and gene expression in zebrafish brain. Toxicol Lett 2007, 174, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Senger, M.R.; de Bem Arizi, M.; Dias, R.D.; Souto, A.A.; et al. Ethanol and acetaldehyde alter NTPDase and 5’-nucleotidase from zebrafish brain membranes. Neurochem Int 2008, 52, 290–296. [Google Scholar] [CrossRef] [PubMed]

© Copyright L.W. Kist et al., 2012 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Kist, L.W.; Fritsch, R.S.; Yunes, J.S.; Bonan, C.D.; Bogo, M.R. Microcystin-LR Acute Exposure Does Not Alter In Vitro and In Vivo ATP, ADP and AMP Hydrolysis in Adult Zebrafish (Danio rerio) Brain Membranes. J. Xenobiot. 2012, 2, e9. https://doi.org/10.4081/xeno.2012.e9
Kist LW, Fritsch RS, Yunes JS, Bonan CD, Bogo MR. Microcystin-LR Acute Exposure Does Not Alter In Vitro and In Vivo ATP, ADP and AMP Hydrolysis in Adult Zebrafish (Danio rerio) Brain Membranes. Journal of Xenobiotics. 2012; 2(1):e9. https://doi.org/10.4081/xeno.2012.e9
Chicago/Turabian StyleKist, Luiza Wilges, Rachel Seemann Fritsch, João Sarkis Yunes, Carla Denise Bonan, and Maurício Reis Bogo. 2012. "Microcystin-LR Acute Exposure Does Not Alter In Vitro and In Vivo ATP, ADP and AMP Hydrolysis in Adult Zebrafish (Danio rerio) Brain Membranes" Journal of Xenobiotics 2, no. 1: e9. https://doi.org/10.4081/xeno.2012.e9
APA StyleKist, L. W., Fritsch, R. S., Yunes, J. S., Bonan, C. D., & Bogo, M. R. (2012). Microcystin-LR Acute Exposure Does Not Alter In Vitro and In Vivo ATP, ADP and AMP Hydrolysis in Adult Zebrafish (Danio rerio) Brain Membranes. Journal of Xenobiotics, 2(1), e9. https://doi.org/10.4081/xeno.2012.e9



