The Antiproliferative Activity of Tatridin A Against Prostate Cancer Cells Is Lost in Acid Medium by Transformation to Desacetyl-β-Cyclopyrethrosin
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Structure Elucidation of Tatridin A and Desacetyl-β-Cyclopyrethrosin
2.2. X-Ray Single-Crystal Structure
2.3. Cell Culture
2.4. Real-Time Cell Death Assay
2.5. Selection of the Standard Working Concentration
2.6. Clonogenic Assays
2.7. Reactive Oxygen Species Measurement
2.8. Mitochondrial Membrane Potential Analysis
2.9. Analysis of NF-κB Activation
2.10. Western Blot Analysis
3. Results
3.1. Structure Elucidation of Tatridin A and Desacetyl-β-Cyclopyrethrosin
3.2. Mechanistic Proposal for the Isomerization of Tatridin A to Desacetyl-β-Cyclopyrethrosin
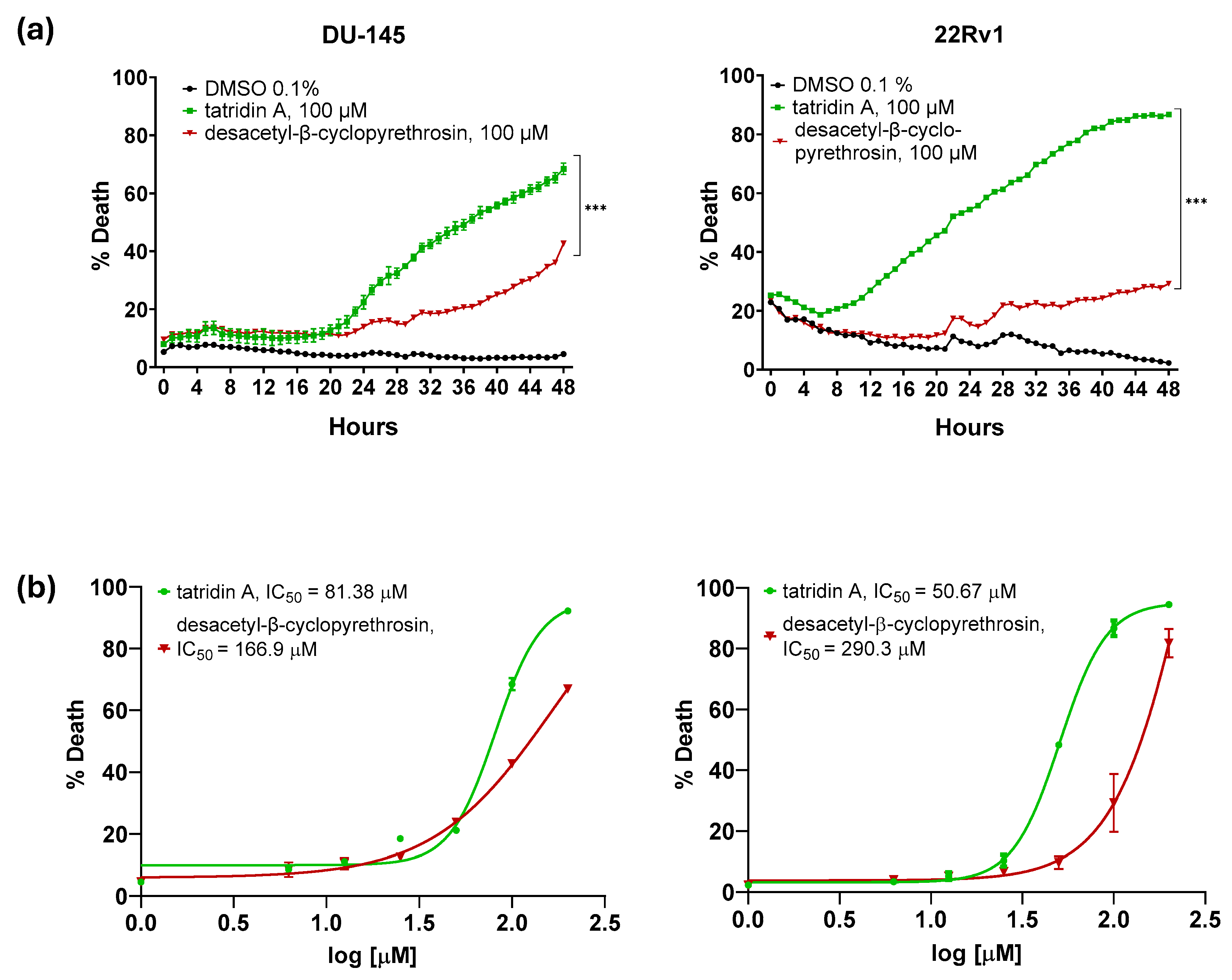
3.3. Tatridin A Shows Cytotoxic Effects Against DU-145 and 22Rv1 Cell Lines
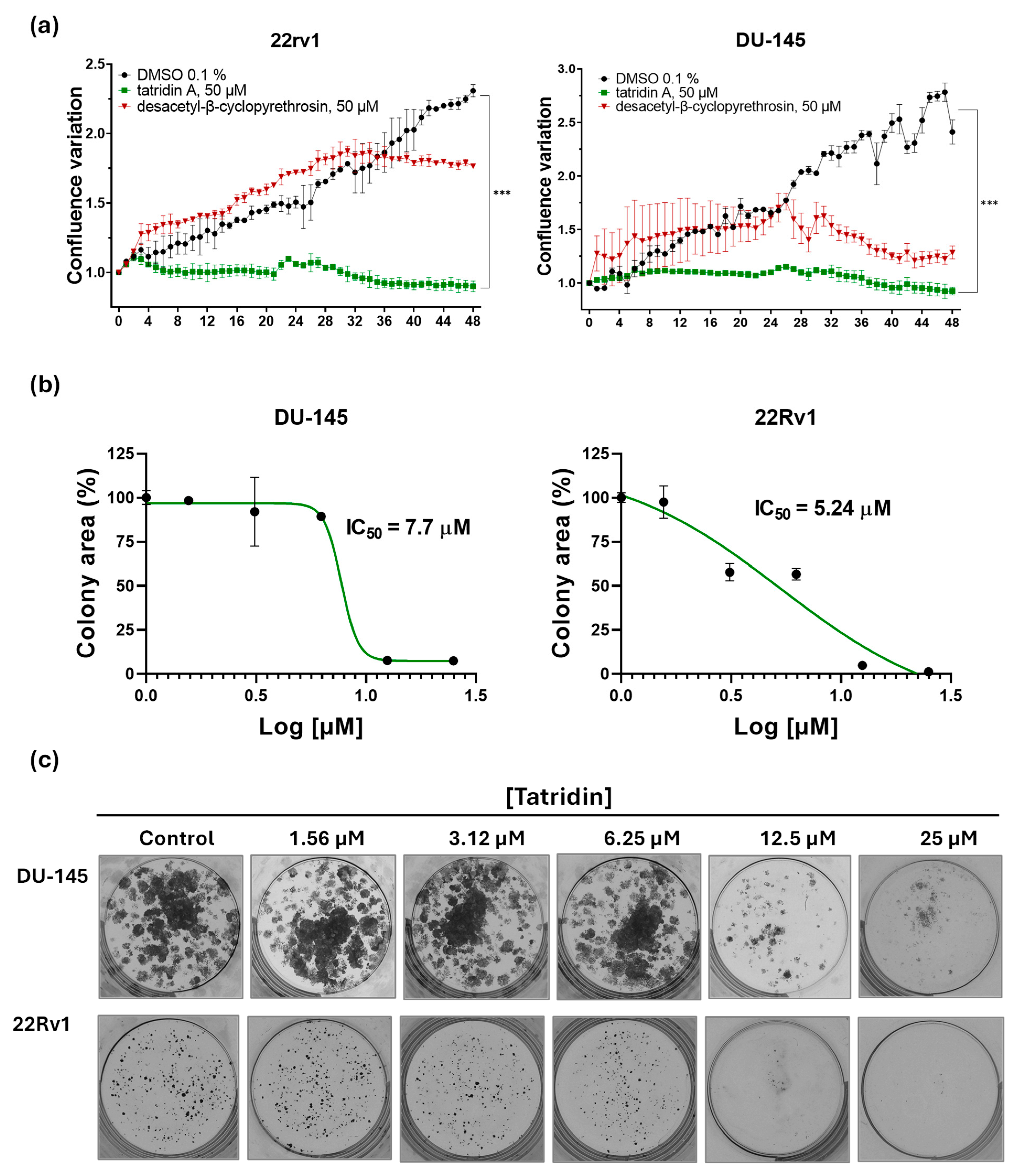
3.4. Tatridin A Reduces the Proliferative Activity of DU-145 Cells and 22Rv1 Cells
3.5. Tatridin A Treatment Increase Cellular Reactive Oxygen Species Production and Decreases Mitochondrial Membrane Potential
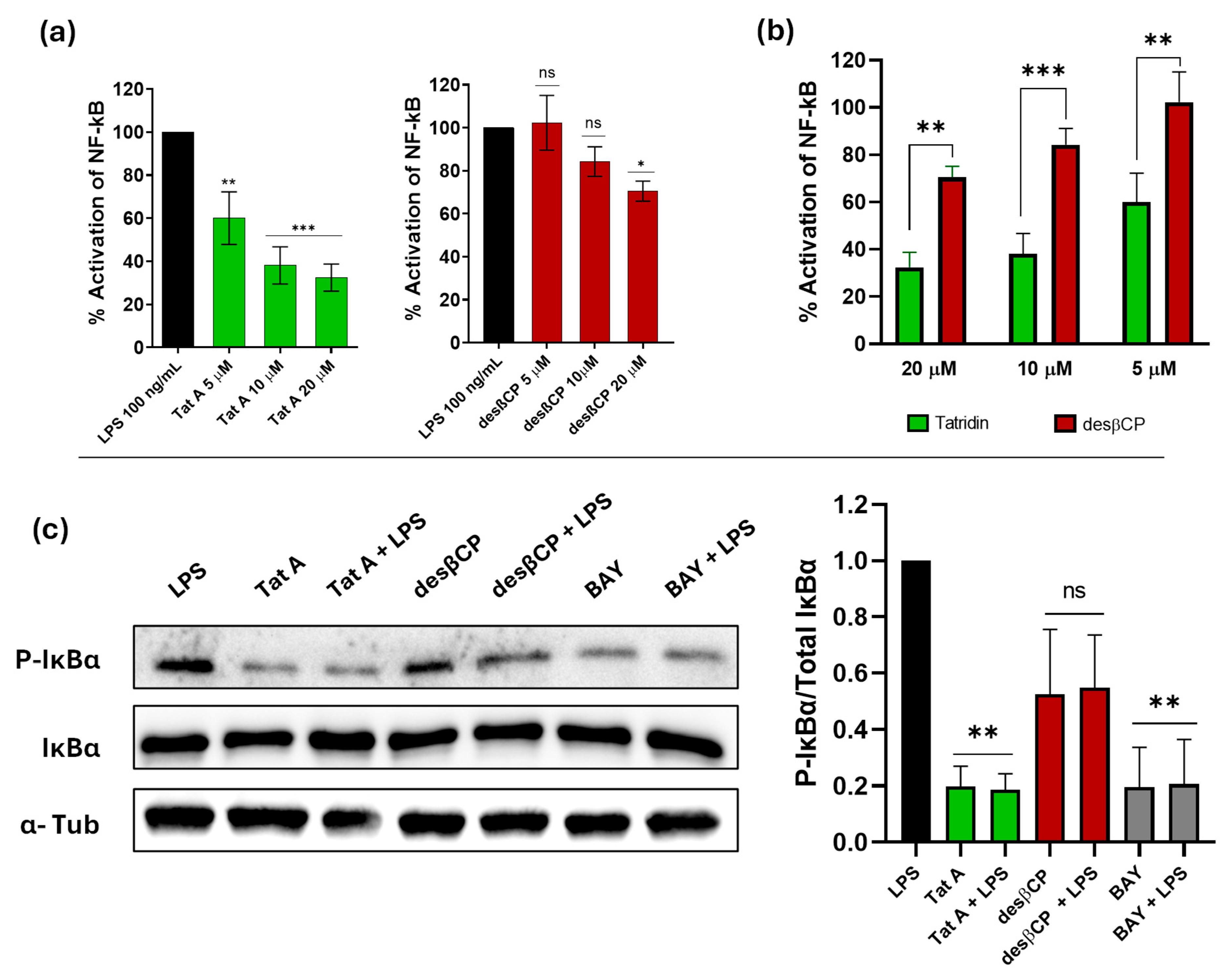
3.6. Tatridin A Reduces the Activity of the NF-κB Pathway More Efficiently than Eudesmane in the THP-1 Reporter Cell Line
3.7. Tatridin A Inhibits IκBα Phosphorylation Akin to Other Classical NF-κB Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Androgen receptor |
| CD2Cl2 | Deuterated dichloromethane |
| CH2Cl2 | Dichloromethane |
| DMSO | Dimethyl sulfoxide |
| EtOAc | Ethyl acetate |
| FBS | Fetal bovine serum |
| H2DCFDA | Dichlorodihydrofluorescein diacetate |
| IC50 | Half maximal inhibitory concentration |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear transcription factor kappa B |
| NMR | Nuclear magnetic resonance |
| ORTEP | Oak Ridge Thermal Ellipsoid Plot |
| PC | Prostate cancer |
| ROS | Reactive oxygen species |
| SEAP | Secreted embryonic alkaline phosphatase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TMRM | Tetramethylrhodamine methyl ester |
References
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Basílio, J.; Hochreiter, B.; Hoesel, B.; Sheshori, E.; Mussbacher, M.; Hanel, R.; Schmid, J.A. Antagonistic Functions of Androgen Receptor and NF-κB in Prostate Cancer-Experimental and Computational Analyses. Cancers 2022, 14, 6164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Altuwaijri, S.; Deng, F.; Chen, L.; Lal, P.; Bhanot, U.K.; Korets, R.; Wenske, S.; Lilja, H.G.; Chang, C.; et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am. J. Pathol. 2009, 175, 489–499. [Google Scholar] [CrossRef]
- Mayora, A.; Arvelo, F. Prostate cancer and apoptosis. Investig. Clin. 2011, 52, 376–396. [Google Scholar]
- Domingo-Domenech, J.; Mellado, B.; Ferrer, B.; Truan, D.; Codony-Servat, J.; Sauleda, S.; Alcover, J.; Campo, E.; Gascon, P.; Rovira, A.; et al. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br. J. Cancer 2005, 93, 1285–1294. [Google Scholar] [CrossRef]
- Gannon, P.O.; Lessard, L.; Stevens, L.M.; Forest, V.; Bégin, L.R.; Minner, S.; Tennstedt, P.; Schlomm, T.; Mes-Masson, A.M.; Saad, F. Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. Eur. J. Cancer 2013, 49, 2441–2448. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.; Wang, S.; Boldogh, I.; Tian, B.; Brasier, A.R. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell. Signal. 2007, 19, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef]
- Thomas-Jardin, S.E.; Dahl, H.; Nawas, A.F.; Bautista, M.; Delk, N.A. NF-κB signaling promotes castration-resistant prostate cancer initiation and progression. Pharmacol. Ther. 2020, 211, 107538. [Google Scholar] [CrossRef] [PubMed]
- Lessard, L.; Mes-Masson, A.M.; Lamarre, L.; Wall, L.; Lattouf, J.B.; Saad, F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003, 91, 417–420. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.Y.; Cho, J.S.; Son, S.M.; Choi, S.S.; Yun, Y.P.; Yoo, H.S.; Yoon, D.Y.; Oh, K.W.; Han, S.B.; et al. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kappaB. Eur. J. Pharmacol. 2010, 631, 1–9. [Google Scholar] [CrossRef]
- Suhail, M.; Tarique, M.; Muhammad, N.; Naz, H.; Hafeez, A.; Zughaibi, T.A.; Kamal, M.A.; Rehan, M. A Critical Transcription Factor NF-κB as a Cancer Therapeutic Target and its Inhibitors as Cancer Treatment Options. Curr. Med. Chem. 2021, 28, 4117–4132. [Google Scholar] [CrossRef]
- Verzella, D.; Fischietti, M.; Capece, D.; Vecchiotti, D.; Del Vecchio, F.; Cicciarelli, G.; Mastroiaco, V.; Tessitore, A.; Alesse, E.; Zazzeroni, F. Targeting the NF-κB pathway in prostate cancer: A promising therapeutic approach? Curr. Drug Targets 2016, 17, 311–320. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Nunes Dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Structure-activity relationships of sesquiterpene lactones. Stud. Nat. Prod. Chem. 2006, 33, 309–392. [Google Scholar]
- An, S.; Chun, J.; Lee, J.; Kim, Y.S.; Noh, M.; Ko, H. Unraveling Stereochemical Structure-Activity Relationships of Sesquiterpene Lactones for Inhibitory Effects on STAT3 Activation. Biomol. Ther. 2024, 32, 627–634. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Azarken, R. Síntesis Biomimética de Lactonas Sesquiterpénicas Aisladas de Umbelíferas. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 2008. [Google Scholar]
- Ruiz-Reyes, E.; Suarez, M. Lactonas sesquiterpénicas. Divers. Estructural Sus Act. Biol. 2015, 46, 9–24. [Google Scholar]
- Bombaça, A.C.S.; Dossow, D.V.; Barbosa, J.M.C.; Paz, C.; Burgos, V.; Menna-Barreto, R.F.S. TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosomacruzi. Molecules 2018, 23, 2800. [Google Scholar] [CrossRef]
- Paz, C.; Ortiz, L.; Schilde, U. Crystal structure of erioflorin isolated from Podanthus mitiqui (L.). Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Quintana, J.; Eiroa, J.L.; López, M.; Triana, J.; Bermejo, J.; Estévez, F. Potent induction of apoptosis by germacranolide sesquiterpene lactones on human myeloid leukemia cells. Eur. J. Pharmacol. 2003, 482, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Voli, A.; Mozzicafreddo, M.; Pollastro, F.; Tosco, A.; Monti, M.C. Targeting phosphoglycerate kinases by tatridin A, a natural sesquiterpenoid endowed with anti-cancer activity, using a proteomic platform. Front. Mol. Biosci. 2023, 10, 1212541. [Google Scholar] [CrossRef]
- CrysAlisPRO, Oxford Diffraction/Agilent Technologies UK Ltd., Yarnton. England. Available online: https://scholar.google.com/scholar?q=Agilent%20%282011%29.%20CrysAlis%20PRO.%20Agilent%20Technologies%20UK%20Ltd%2C%20Yarnton%2C%20England (accessed on 28 September 2025).
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Russell, P.J.; Kingsley, E.A. Human Prostate Cancer Cell Lines. In Prostate Cancer Methods and Protocols; Russell, P.J., Jackson, P., Kingsley, E.A., Eds.; Springer: New York, NY, USA, 2003; pp. 21–39. [Google Scholar]
- Altuwaijri, S.; Lin, H.K.; Chuang, K.H.; Lin, W.J.; Yeh, S.; Hanchett, L.A.; Rahman, M.M.; Kang, H.Y.; Tsai, M.Y.; Zhang, Y.; et al. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res. 2003, 63, 7106–7112. [Google Scholar]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef]
- Grootjans, S.; Hassannia, B.; Delrue, I.; Goossens, V.; Wiernicki, B.; Dondelinger, Y.; Bertrand, M.J.; Krysko, D.V.; Vuylsteke, M.; Vandenabeele, P.; et al. A real-time fluorometric method for the simultaneous detection of cell death type and rate. Nat. Protoc. 2016, 11, 1444–1454. [Google Scholar] [CrossRef]
- Wilcock, D.J.; Badrock, A.P.; Wong, C.W.; Owen, R.; Guerin, M.; Southam, A.D.; Johnston, H.; Telfer, B.A.; Fullwood, P.; Watson, J.; et al. Oxidative stress from DGAT1 oncoprotein inhibition in melanoma suppresses tumor growth when ROS defenses are also breached. Cell Rep. 2022, 39, 110995. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Choudhry, P. High-Throughput Method for Automated Colony and Cell Counting by Digital Image Analysis Based on Edge Detection. PLoS ONE 2016, 11, e0148469. [Google Scholar] [CrossRef]
- Ajayi, B.E.; Oboh, B.; Minari, J.B.; Sexton, D.W.; Sarker, S.D.; Fatokun, A.A. Cola rostrata K. Schum. constituents induce cytotoxicity through reactive oxygen species generation and mitochondrial membrane depolarisation. Explor. Target. Anti-Tumor Ther. 2023, 4, 1328–1344. [Google Scholar] [CrossRef]
- Creed, S.; McKenzie, M. Measurement of Mitochondrial Membrane Potential with the Fluorescent Dye Tetramethylrhodamine Methyl Ester (TMRM). In Cancer Metabolism: Methods and Protocols; Haznadar, M., Ed.; Springer: New York, NY, USA, 2019; pp. 69–76. [Google Scholar]
- Cantini, N.; Schepetkin, I.A.; Danilenko, N.V.; Khlebnikov, A.I.; Crocetti, L.; Giovannoni, M.P.; Kirpotina, L.N.; Quinn, M.T. Pyridazinones and Structurally Related Derivatives with Anti-Inflammatory Activity. Molecules 2022, 27, 3749. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Bencze, G.; Cohen, P.; Tonks, N.K. The anti-inflammatory compound BAY-11-7082 is a potent inhibitor of protein tyrosine phosphatases. FEBS J. 2013, 280, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Strickson, S.; Campbell, D.G.; Emmerich, C.H.; Knebel, A.; Plater, L.; Ritorto, M.S.; Shpiro, N.; Cohen, P. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem. J. 2013, 451, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, F.; Bhadane, N.R. Badgerin, a new germacranolide from Artemisia arbuscula subspecies arbuscula. J. Org. Chem. 1972, 37, 274–277. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Bhadane, N.R. Sesquiterpene lactones of Artemisia arbuscula and A. tridentata. Phytochemistry 1973, 12, 857–862. [Google Scholar] [CrossRef]
- Sanz, J.F.; Marco, J.A. Nmr Studies of Tatridin A and Some Related Sesquiterpene Lactones from Tanacetum vulgare. J. Nat. Prod. 1991, 54, 591–596. [Google Scholar] [CrossRef]
- Saroglou, V.; Karioti, A.; Rancic, A.; Dimas, K.; Koukoulitsa, C.; Zervou, M.; Skaltsa, H. Sesquiterpene Lactones from Anthemis melanolepis and Their Antibacterial and Cytotoxic Activities. Prediction of Their Pharmacokinetic Profile. J. Nat. Prod. 2010, 73, 242–246. [Google Scholar] [CrossRef]
- Turdybekov, K.M.; Morozova, O.A.; Ivasenko, S.A.; Makhmutova, A.S.; Turdybekov, D.M.; Adekenov, S.M. Molecular Structure and Absolute Configuration of Tatridin A and B from Lepidolopha karatavica. Chem. Nat. Compd. 2021, 57, 83–87. [Google Scholar] [CrossRef]
- Craig, R.E.R.; Campbell, J.A.; Craig, A.C.; Campana, C.F.; Kelsey, R.G. Tatridin A from Artemisia arbuscula ssp. arbuscula: Crystal Structure of Tatridin A Diacetate and the Identification of Deacetyltulirinol. J. Nat. Prod. 1990, 53, 1585–1586. [Google Scholar] [CrossRef]
- Abduazimov, B.K.; Tashkhodzhaev, B.; Nasirov, S.; Sham’yanov, I.D.; Yagudaev, M.R.; Malikov, V.M.; Aminov, S.N. Structure of mucrolide. Chem. Nat. Compd. 1991, 27, 15–19. [Google Scholar] [CrossRef]
- Bohlmann, F.; Adler, A.; Jakupovic, J.; King, R.M.; Robinson, H. A dimeric germacranolide and other sesquiterpene lactones from Mikania species. Phytochemistry 1982, 21, 1349–1355. [Google Scholar] [CrossRef]
- Jakupovic, J.; Aal, M.A.; Eid, F.; Bohlmann, F.; El-Dahmy, S.; Sarg, T. Further glaucolides and other sesquiterpene lactones from Brocchia cinerea. Phytochemistry 1988, 27, 2219–2224. [Google Scholar] [CrossRef]
- Konstantinopoulou, M.; Karioti, A.; Skaltsas, S.; Skaltsa, H. Sesquiterpene Lactones from Anthemis altissima and Their Anti-Helicobacter pylori Activity. J. Nat. Prod. 2003, 66, 699–702. [Google Scholar] [CrossRef]
- Lazanaki, M.; Tsikalas, G.; Tsiftsoglou, O.S.; Katerinopoulos, H.; Hadjipavlou-Litina, D.; Lazari, D. Secondary Metabolites and Their Biological Evaluation from the Aerial Parts of Staehelina uniflosculosa Sibth. & Sm. (Asteraceae). Int. J. Mol. Sci. 2024, 25, 10586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Wang, N.; Shi, X.-L.; Wang, M.-M.; Zhu, Q.-M.; Chang, J.; Feng, Y.-L.; Zhang, J.; Qiu, F.; Sun, C.-P. Sesquiterpenoids from Inula britannica and their potential mechanism for immunomodulation. Phytochemistry 2025, 231, 114343. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S. Discussion on Decomposition of Chloroform. Yakugaku Zasshi 1966, 86, 1125–1132. [Google Scholar] [CrossRef]
- Teipel, J.; Gottstein, V.; Hölzle, E.; Kaltenbach, K.; Lachenmeier, D.W.; Kuballa, T. An Easy and Reliable Method for the Mitigation of Deuterated Chloroform Decomposition to Stabilise Susceptible NMR Samples. Chemistry 2022, 4, 776–785. [Google Scholar] [CrossRef]
- Cardona, M.L.; Fernández, I.; García, B.; Pedro, J.R. Revision of the Structure of an Eudesmanolide Isolated from Lasiolaena santosii. J. Nat. Prod. 1990, 53, 1042–1045. [Google Scholar] [CrossRef]
- Jain, T.C.; McCloskey, J.E. A facile and stereospecific cyclization of costunolide. Tetrahedron Lett. 1969, 10, 2917–2919. [Google Scholar] [CrossRef]
- Jain, T.C.; McCloskey, J.E. Carbocyclization in natural products—I: Amberlite IR-120 cation exchange resin catalyzed cyclization of costunolide. Structure of β-cyclocostunolide. Tetrahedron 1975, 31, 2211–2214. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sánchez, J.F.; Barrón, A.; Ramírez, A. Biomimetic cyclizations of a germacranolide from Tanacetum annuum. Phytochemistry 1992, 31, 332–335. [Google Scholar] [CrossRef]
- Azarken, R.; Guerra, F.M.; Moreno-Dorado, F.J.; Jorge, Z.D.; Massanet, G.M. Substituent effects in the transannular cyclizations of germacranes. Synthesis of 6-epi-costunolide and five natural steiractinolides. Tetrahedron 2008, 64, 10896–10905. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Álvarez, M. Palladium II promoted rearrangement of germacranolides. Synthesis of (+)-stoebenolide and (+)-dehydromelitensin. Tetrahedron Lett. 1998, 39, 1401–1404. [Google Scholar] [CrossRef]
- de Pascual Teresa, J.; González, M.S.; Caballero, M.C.; Parra, T.; Bellido, I.S. Transannular cyclization of heliangolides. Tetrahedron Lett. 1987, 28, 821–824. [Google Scholar] [CrossRef]
- Justicia, J.; de Cienfuegos, L.A.; Estévez, R.E.; Paradas, M.; Lasanta, A.M.; Oller, J.L.; Rosales, A.; Cuerva, J.M.; Oltra, J.E. Ti-catalyzed transannular cyclization of epoxygermacrolides. Synthesis of antifungal (+)-tuberiferine and (+)-dehydrobrachylaenolide. Tetrahedron 2008, 64, 11938–11943. [Google Scholar] [CrossRef]
- Lu, T.; Fischer, N.H. Spectral Data of Chemical Modification Products of Costunolide. Spectrosc. Lett. 1996, 29, 437–448. [Google Scholar] [CrossRef]
- Merkhatuly, N.; Abeuova, S.B.; Omarova, A.T.; Aldabergenova, S.K.; Balmagambetova, L.T. Biomimetic cyclization of E,E-germacranolide (+)-hanphilline. Russ. J. Gen. Chem. 2015, 85, 2821–2822. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.M.; Raccuglia, R.A.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.-H.; Bruno, M. Acid Rearrangment of Epoxy-germacranolides and Absolute Configuration of 1β,10α-Epoxy-salonitenolide. Nat. Prod. Commun. 2010, 5, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; Sanz-Cervera, J.F.; García-Lliso, V.; Domingo, L.R.; Carda, M.; Rodríguez, S.; López-Ortiz, F.; Lex, J. Influence of conformational factors on acid-catalyzed cyclizations of germacranolides: Molecular structure of the cyclization products of gallicin and 8α-hydroxygallicin (shonachalin a). Liebigs Ann. 1995, 1837–1841. [Google Scholar] [CrossRef]
- Hsu, J.L.; Pan, S.L.; Ho, Y.F.; Hwang, T.L.; Kung, F.L.; Guh, J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011, 185, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; González-Chavarría, I.; Burgos, V.; Cabrera-Pardo, J.R.; Schmidt, B.; Paz, C. Erioflorin and Erioflorin Acetate Induce Cell Death in Advanced Prostate Cancer Through ROS Increase and NF-κB Inhibition. J. Xenobiot. 2025, 15, 45. [Google Scholar] [CrossRef]
- Talhouk, R.S.; Nasr, B.; Fares, M.B.; Ajeeb, B.; Nahhas, R.; Al Aaraj, L.; Talhouk, S.N.; Ghaddar, T.H.; Saliba, N.A. Anti-Inflammatory and Cytostatic Activities of a Parthenolide-Like Sesquiterpene Lactone from Cota palaestina subsp. syriaca. Evid.-Based Complement. Altern. Med. 2015, 2015, 474597. [Google Scholar] [CrossRef]
- Zhang, T.; Si, J.G.; Zhang, Q.B.; Ding, G.; Zou, Z.M. New Highly Oxygenated Germacranolides from Carpesium divaricatum and their Cytotoxic Activity. Sci. Rep. 2016, 6, 27237. [Google Scholar] [CrossRef]
- Yan, C.; Long, Q.; Zhang, Y.D.; Babu, G.; Krishnapriya, M.V.; Qiu, J.F.; Song, J.R.; Rao, Q.; Yi, P.; Sun, M.; et al. Germacranolide sesquiterpenes from Carpesium cernuum and their anti-leukemia activity. Chin. J. Nat. Med. 2021, 19, 528–535. [Google Scholar] [CrossRef]
- Vasas, A.; Lajter, I.; Kúsz, N.; Király, S.B.; Kovács, T.; Kurtán, T.; Bózsity, N.; Nagy, N.; Schelz, Z.; Zupkó, I.; et al. Isolation, Structure Determination of Sesquiterpenes from Neurolaena lobata and Their Antiproliferative, Cell Cycle Arrest-Inducing and Anti-Invasive Properties against Human Cervical Tumor Cells. Pharmaceutics 2021, 13, 2088. [Google Scholar] [CrossRef]
- Xu, D.D.; Yan, Y.; Jiang, C.X.; Liang, J.J.; Li, H.F.; Wu, Q.X.; Zhu, Y. Sesquiterpenes and diterpenes with cytotoxic activities from the aerial parts of Carpesium humile. Fitoterapia 2018, 128, 50–56. [Google Scholar] [CrossRef]
- Bai, M.; Chen, J.J.; Xu, W.; Dong, S.H.; Liu, Q.B.; Yao, G.D.; Lin, B.; Huang, X.X.; Song, S.J. Germacranolides from Elephantopus scaber L. and their cytotoxic activities. Phytochemistry 2020, 178, 112479. [Google Scholar] [CrossRef] [PubMed]
- García-Manzano, M.F.; Joray, M.B.; Laiolo, J.; Palacios, S.M.; Carpinella, M.C. Cytotoxic Activity of Germacrane-Type Sesquiterpene Lactones from Dimerostemma aspilioides. J. Nat. Prod. 2020, 83, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say no to DMSO: Dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, S.; Bruno, M.; Raimondo, F.M.; Spadaro, V.; Varol, M.; Koparal, A.T.; Maggio, A. Cytotoxic effect of eudesmanolides isolated from flowers of Tanacetum vulgare ssp. siculum. Molecules 2012, 17, 8186–8195. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, W.; Gong, T.; Yang, Y.; Chen, R.Y.; Yu, D.Q. Two new eudesmanolides from Inula racemosa. J. Asian Nat. Prod. Res. 2010, 12, 788–792. [Google Scholar] [CrossRef]
- Chimplee, S.; Graidist, P.; Srisawat, T.; Sukrong, S.; Bissanum, R.; Kanokwiroon, K. Anti-breast cancer potential of frullanolide from Grangea maderaspatana plant by inducing apoptosis. Oncol. Lett. 2019, 17, 5283–5291. [Google Scholar] [CrossRef]
- Sun, Y.; St Clair, D.K.; Xu, Y.; Crooks, P.A.; St Clair, W.H. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010, 70, 2880–2890. [Google Scholar] [CrossRef]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The oxygen radicals involved in the toxicity induced by parthenolide in MDA-MB-231 cells. Oncol. Rep. 2014, 32, 167–172. [Google Scholar] [CrossRef]
- Jorge, J.; Neves, J.; Alves, R.; Geraldes, C.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B. Parthenolide Induces ROS-Mediated Apoptosis in Lymphoid Malignancies. Int. J. Mol. Sci. 2023, 24, 9167. [Google Scholar] [CrossRef]
- Chen, J.; Chen, B.; Zou, Z.; Li, W.; Zhang, Y.; Xie, J.; Liu, C. Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget 2017, 8, 107701–107715. [Google Scholar] [CrossRef]
- Zhang, S.; Ong, C.N.; Shen, H.M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208, 143–153. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ren, H.; Yan, Q.L.; Li, Y.L.; Liu, Q.; Yao, G.D.; Song, S.J. SCP-7, a germacrane-type sesquiterpene lactone derivative, induces ROS-mediated apoptosis in NSCLC cells in vitro and in vivo. Eur. J. Pharmacol. 2022, 925, 174989. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Iinuma, M.; Matsuura, N.; Yi, K.; Naoi, M.; Nakayama, T.; Nozawa, Y.; Akao, Y. A potent apoptosis-inducing activity of a sesquiterpene lactone, arucanolide, in HL60 cells: A crucial role of apoptosis-inducing factor. J. Pharmacol. Sci. 2005, 97, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, K.T.; Chi, S.G.; Park, J.H. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2001, 24, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Naumann, M. Beyond IkappaBs: Alternative regulation of NF-kappaB activity. FASEB J. 2007, 21, 2642–2654. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.H.; Tu, W.C.; Wang, D.W.; Lu, M.J.; Shao, Y. Costunolide Inhibits Chronic Kidney Disease Development by Attenuating IKKβ/NF-κB Pathway. Drug Des. Dev. Ther. 2024, 18, 2693–2712. [Google Scholar] [CrossRef] [PubMed]
- Saadane, A.; Masters, S.; DiDonato, J.; Li, J.; Berger, M. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am. J. Respir. Cell Mol. Biol. 2007, 36, 728–736. [Google Scholar] [CrossRef]
- Rauert-Wunderlich, H.; Siegmund, D.; Maier, E.; Giner, T.; Bargou, R.C.; Wajant, H.; Stühmer, T. The IKK inhibitor Bay 11-7082 induces cell death independent from inhibition of activation of NFκB transcription factors. PLoS ONE 2013, 8, e59292. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S.; Dean, K.; Franzoso, G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: Molecular basis and biological significance. Oncogene 2006, 25, 6731–6748. [Google Scholar] [CrossRef] [PubMed]

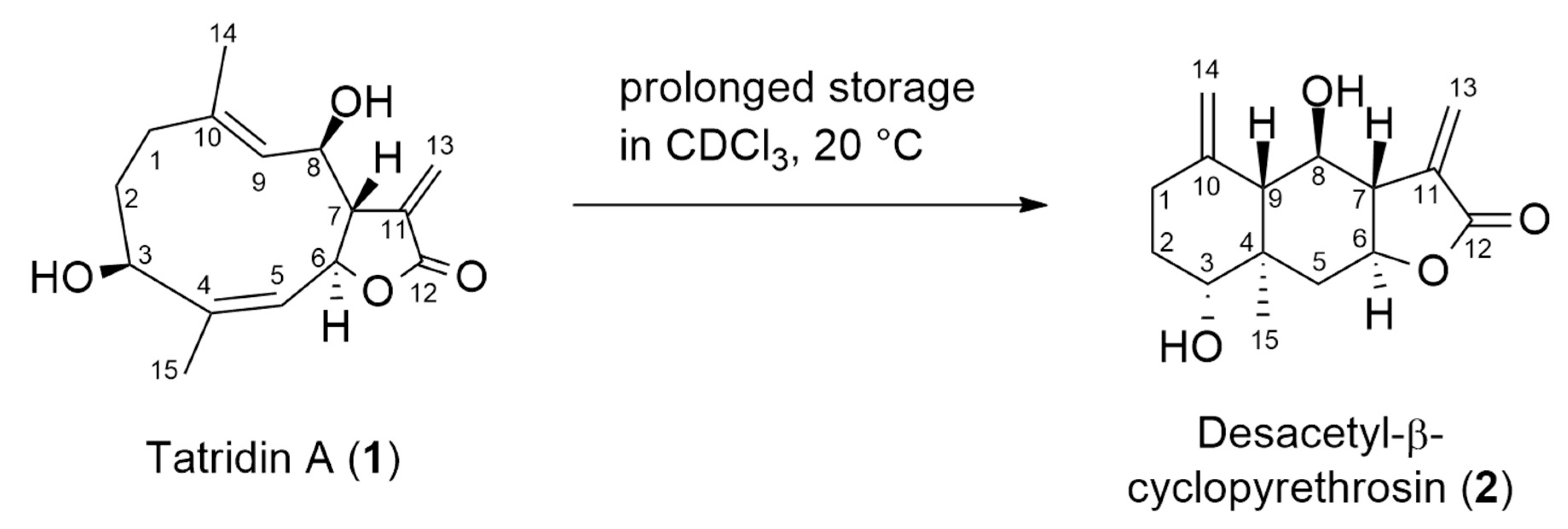



| Tatridin A (1) | Desacetyl-β-Cyclopyrethrosin (2) | ||||||
|---|---|---|---|---|---|---|---|
| Position | d(1H)/ppm | m (J (Hz)) | d(13C)/ppm | Position | d(1H)/ppm | m (J (Hz)) | d(13C)/ppm |
| 1 | 2.23 (α) | dd (11.8, 6.2) | 36.1 | 1 | 2.30 (α) | ddd (13.3, 5.3, 2.1) | 35.5 |
| 1.90 (β) | m | 2.06 (β) | m | ||||
| 2 | 1.95 (α) | m | 28.6 | 2 | 1.60 (α) | dddd (13.6, 13.0, 11.5, 5.2) | 32.4 |
| 1.70 (β) | dddd (13.8, 11.1, 6.3, 2.4) | 1.82 (β) | dddd (12.6, 5.2, 5.2, 2.1) | ||||
| 3 | 4.42 | ddd (11.1, 4.5, 4.3) | 66.7 | 3 | 3.56 | ddd (11.4, 5.0, 5.0) | 78.4 |
| 4 | - | - | 143.6 | 4 | - | - | 43.6 |
| 5 | 5.27 | d (10.4) | 127.1 | 5 | 2.46 (α) | dd (11.6, 3.7) | 41.3 |
| 1.53 (β) | dd (11.9, 11.9) | ||||||
| 6 | 4.63 | t (9.5) | 75.1 | 6 | 4.04 | ddd (12.3, 11.4, 3.8) | 77.6 |
| 7 | 2.78 | tt (8.9, 3.3) | 53.3 | 7 | 2.60 | dddd (11.3, 10.0, 3.1, 3.1) | 55.5 |
| 8 | 4.51 | ddd (10.7, 9.1, 4.5) | 71.2 | 8 | 4.13 | ddd (10.0, 10.0, 6.0) | 67.9 |
| 9 | 4.96 | d (10.6) | 132.0 | 9 | 2.01 | d (10.0) | 57.8 |
| 10 | - | - | 133.8 | 10 | - | - | 145.2 |
| 11 | - | - | 140.4 | 11 | - | - | 140.1 |
| 12 | - | - | 170.4 | 12 | - | - | 170.7 |
| 13 | 6.16 (α) | dd (3.2, 1.7) | 121.7 | 13 | 5.93 (α) | dd (3.0, 1.3) | 118.7 |
| 6.07 (β) | dd (3.5, 1.7) | 5.98 (β) | dd (3.2, 1.3) | ||||
| 14 | 1.77 | 3H, s (br) | 15.6 | 14 | 4.98 (α) | q (1.4) | 109.3 |
| 4.83 (β) | q (1.4) | ||||||
| 15 | 1.79 | 3H, d (1.5) | 17.1 | 15 | 0.84 | 3H, s | 14.2 |
| C3-OH | 3.92 | d (3.9) | - | C3-OH | 3.87 | d (5.2) | - |
| C8-OH | 4.23 | d (4.5) | - | C8-OH | 3.74 | d (6.0) | - |
| Cell Line | Compound | IC50 (Sytox, µM) | IC50 (Net Viability, µM) | IC50 (Clonogenic, µM) |
|---|---|---|---|---|
| DU-145 | Tatridin A | 81.38 ± 2.7 | 53.66 ± 1.8 | 7.70 ± 1.4 |
| DU-145 | DesβCP | 166.9 ± 3.2 | 101.20 ± 2.9 | ND |
| 22Rv1 | Tatridin A | 50.67 ± 1.9 | 35.38 ± 2.1 | 5.24 ± 0.95 |
| 22Rv1 | DesβCP | 290.3 ± 8.3 | 80.98 ± 3.7 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, C.; Pérez, R.; Céspedes-Méndez, C.; Burgos, V.; Baggio, R.; Suárez, S.; Schmidt, B.; Paz, C. The Antiproliferative Activity of Tatridin A Against Prostate Cancer Cells Is Lost in Acid Medium by Transformation to Desacetyl-β-Cyclopyrethrosin. J. Xenobiot. 2025, 15, 161. https://doi.org/10.3390/jox15050161
Villegas C, Pérez R, Céspedes-Méndez C, Burgos V, Baggio R, Suárez S, Schmidt B, Paz C. The Antiproliferative Activity of Tatridin A Against Prostate Cancer Cells Is Lost in Acid Medium by Transformation to Desacetyl-β-Cyclopyrethrosin. Journal of Xenobiotics. 2025; 15(5):161. https://doi.org/10.3390/jox15050161
Chicago/Turabian StyleVillegas, Cecilia, Rebeca Pérez, Camilo Céspedes-Méndez, Viviana Burgos, Ricardo Baggio, Sebastián Suárez, Bernd Schmidt, and Cristian Paz. 2025. "The Antiproliferative Activity of Tatridin A Against Prostate Cancer Cells Is Lost in Acid Medium by Transformation to Desacetyl-β-Cyclopyrethrosin" Journal of Xenobiotics 15, no. 5: 161. https://doi.org/10.3390/jox15050161
APA StyleVillegas, C., Pérez, R., Céspedes-Méndez, C., Burgos, V., Baggio, R., Suárez, S., Schmidt, B., & Paz, C. (2025). The Antiproliferative Activity of Tatridin A Against Prostate Cancer Cells Is Lost in Acid Medium by Transformation to Desacetyl-β-Cyclopyrethrosin. Journal of Xenobiotics, 15(5), 161. https://doi.org/10.3390/jox15050161









