Integrated Assessment of Silver Nanoparticles on Plant Growth and Cytogenotoxicity Using Triticum and Allium Bioassays
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles Used
2.2. Experimental Variants
2.3. Wheat Exposure to the Test Solutions
2.3.1. Determination of Axial Organs Growth and Weight of Wheat Seedlings
2.3.2. Determination of the Content of Assimilatory Pigments, Polyphenols and Proline
2.4. The Assessment of Cell Viability and Cytogenotoxic Effects
2.4.1. The Evans Blue Assay
2.4.2. The Allium Assay
2.5. Statistical Analysis
3. Results
3.1. Nanoparticle Size Verification
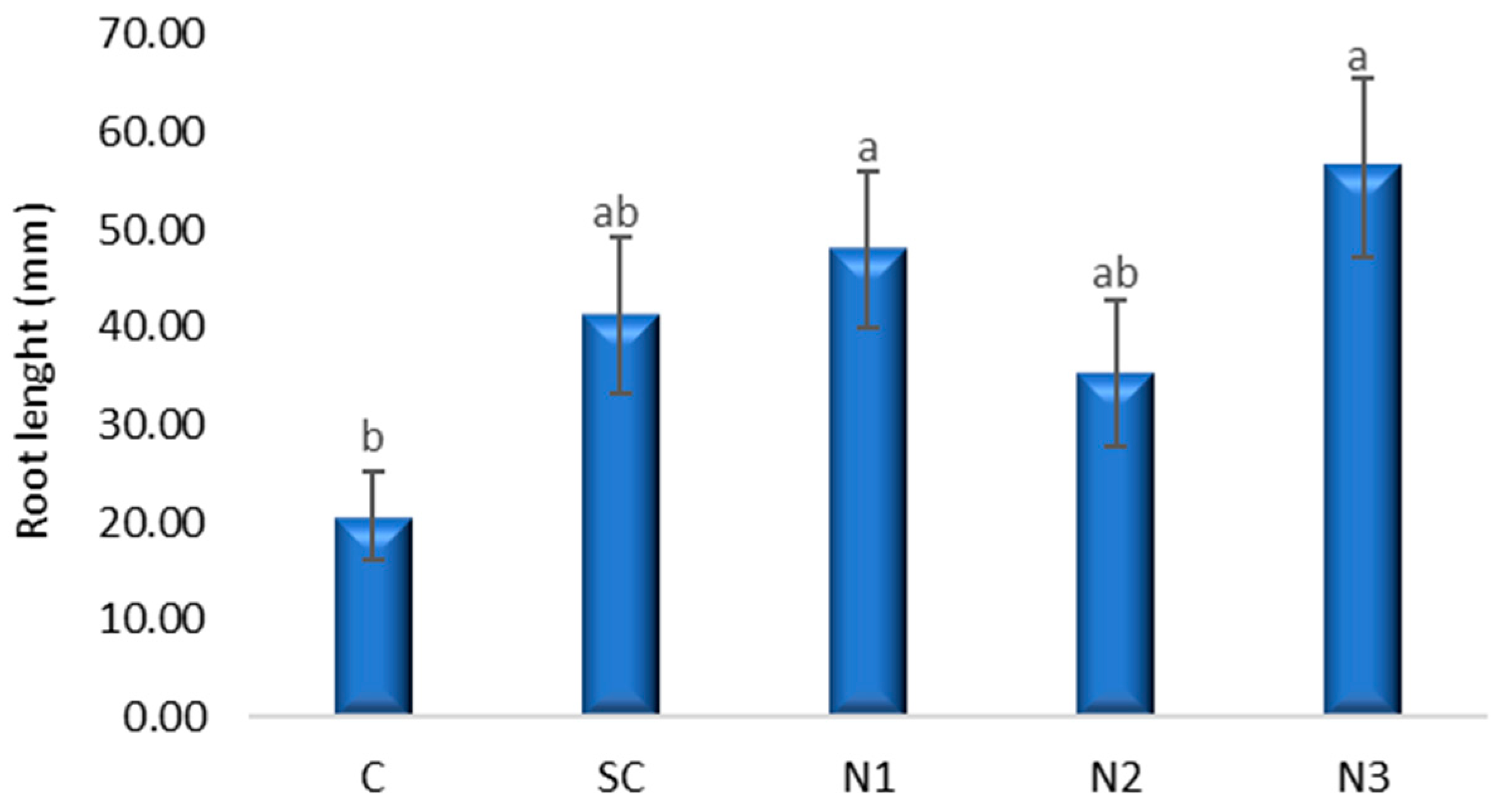
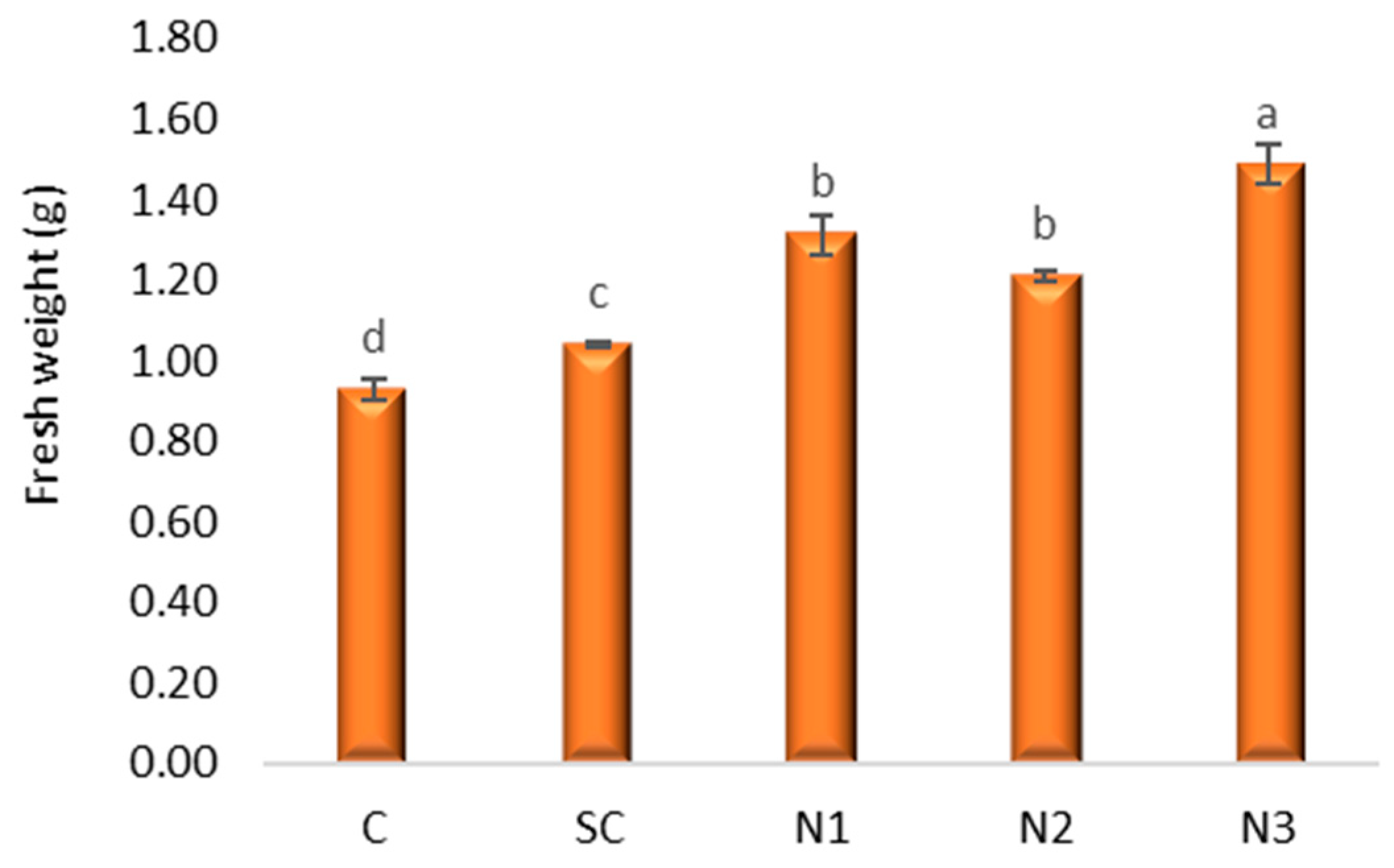
3.2. The Effects Induced by Silver Nanoparticles on Axial Organ Growth and Seedlings Weight of Triticum aestivum L.
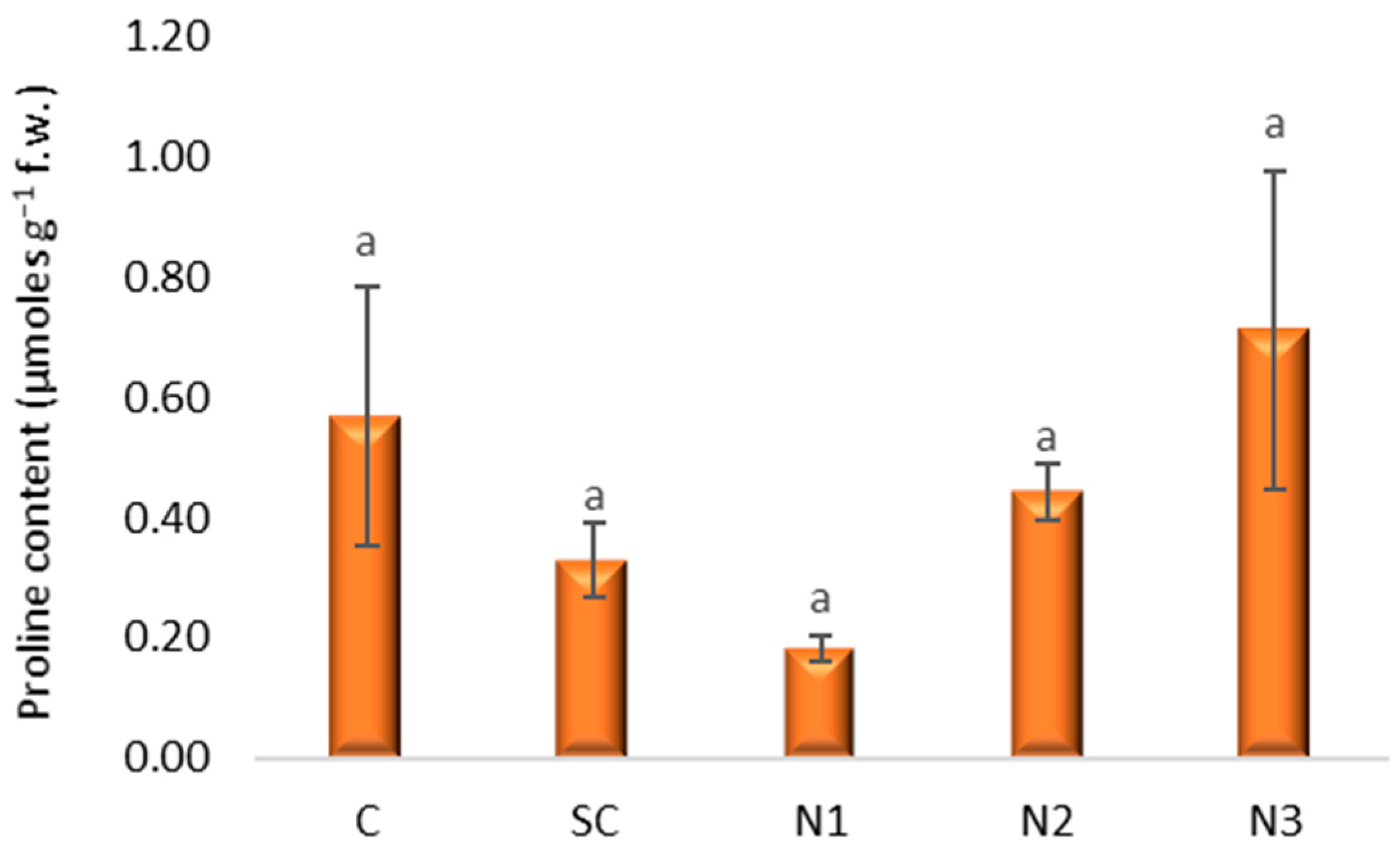
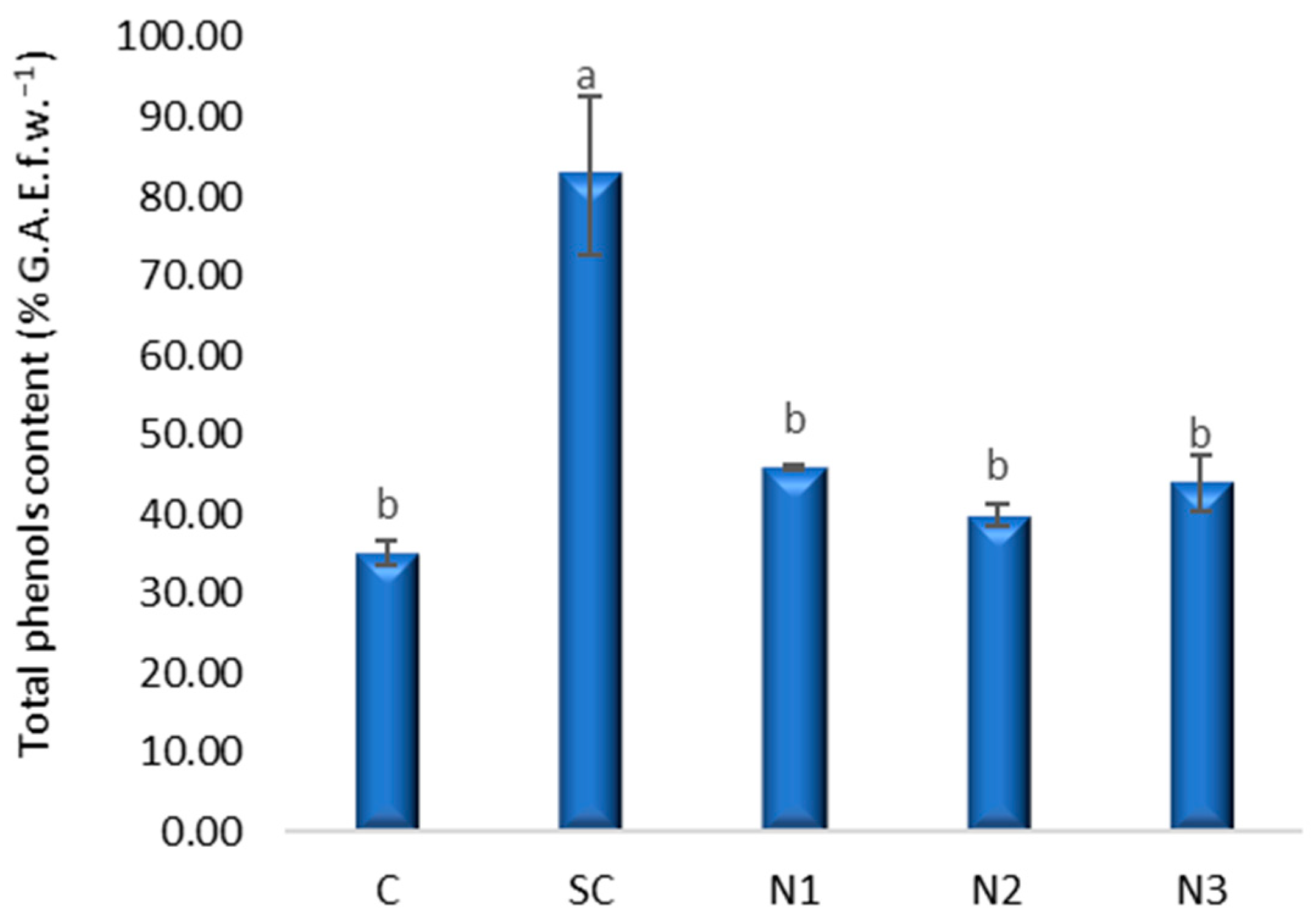
3.3. Effects of Silver Nanoparticles on Proline, Polyphenols, and Assimilatory Pigments Content in Wheat Leaves
3.3.1. Proline Content
3.3.2. Total Phenols Content
3.3.3. Assimilatory Pigments Content
3.3.4. Allium cepa L. Root Cells Viability
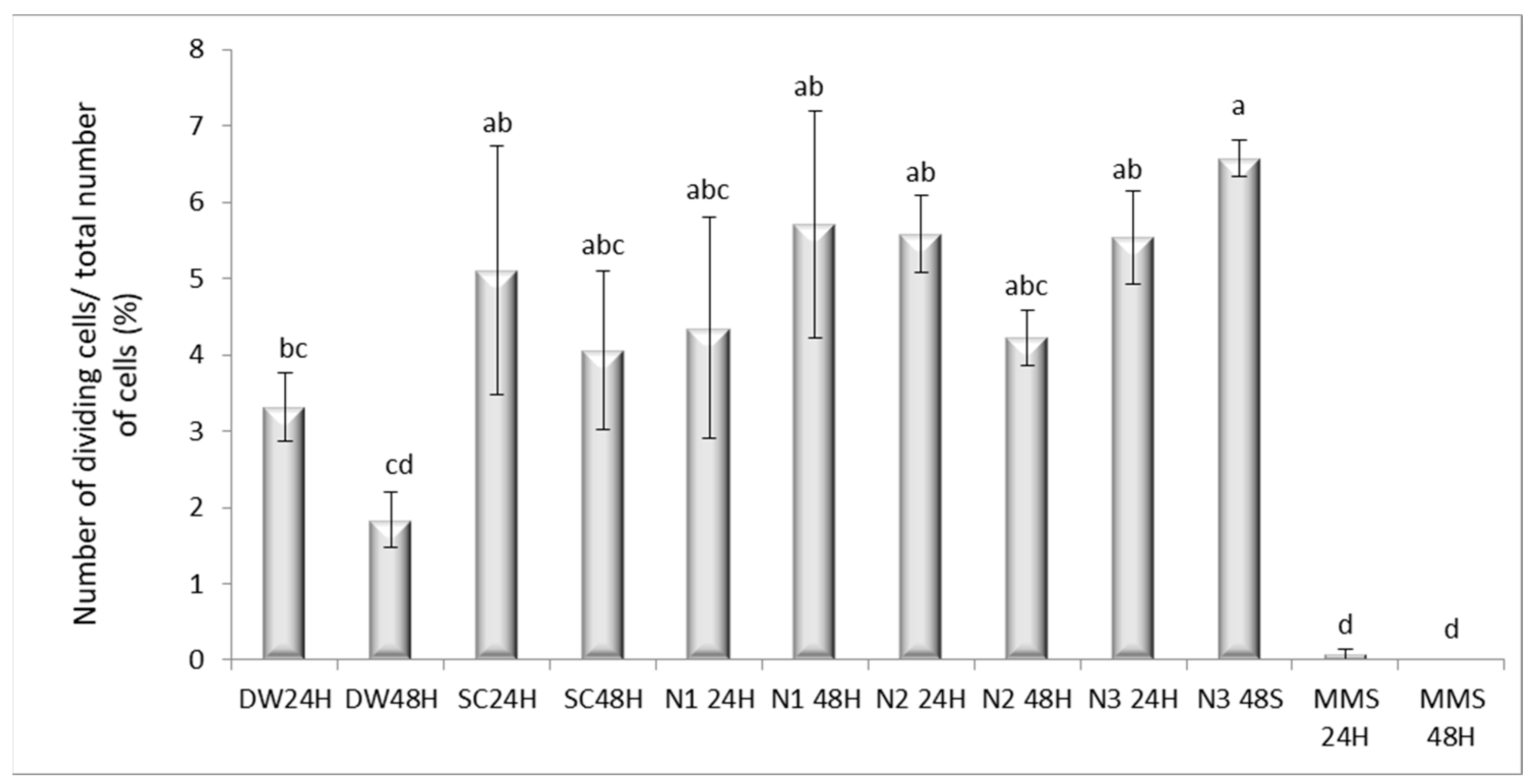
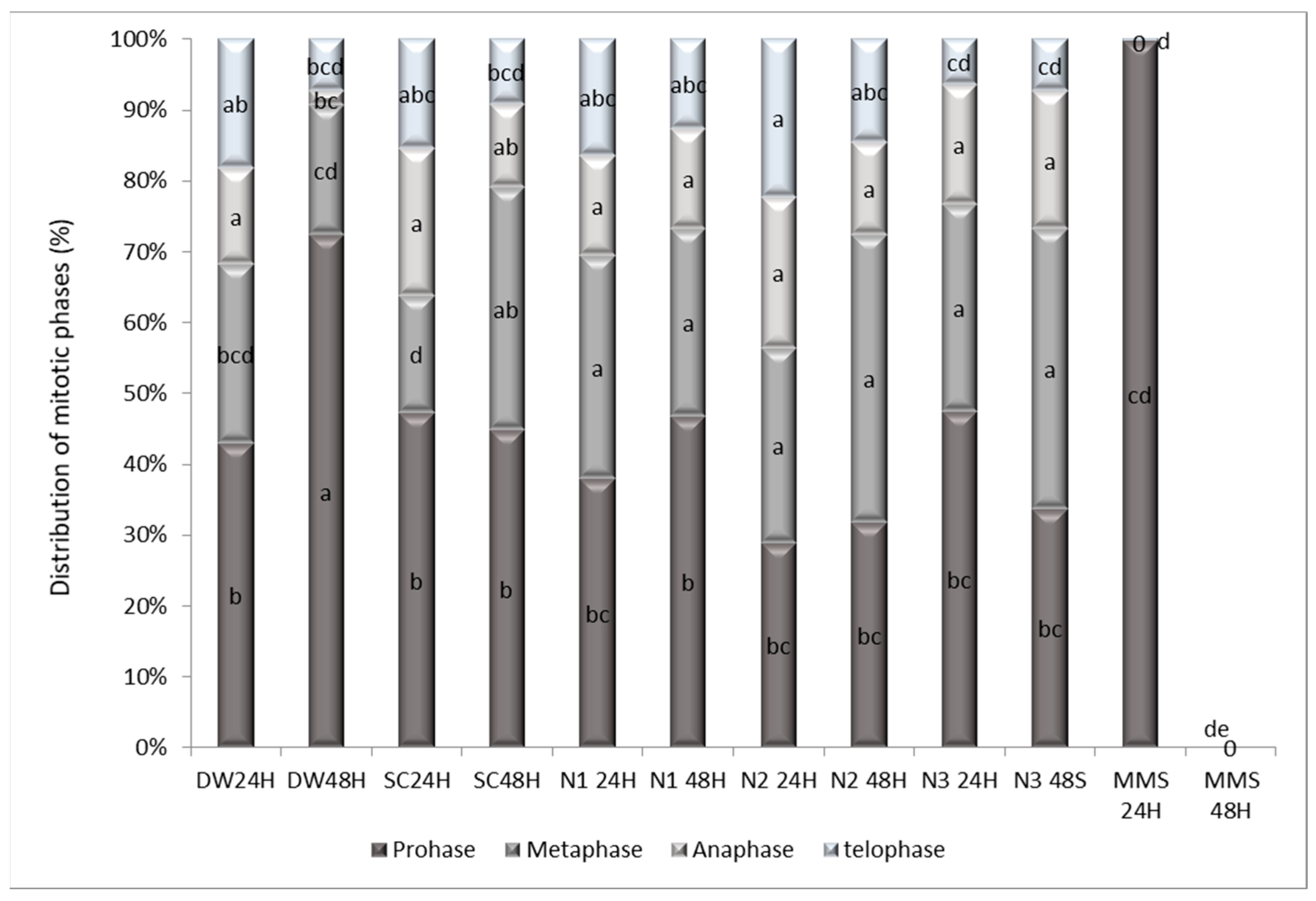
3.4. The Effects Induced by AgNPs on the Mitotic Index, Distribution of Mitotic Phases and Genetic Material in Allium cepa L.
3.4.1. Mitotic Index Variation and Distribution of Mitotic Phases
3.4.2. Chromosomal Aberrations Frequency
4. Discussion
4.1. The Effects Induced by AgNPs on Axial Organ Growth and Seedling Weight of Triticum aestivum L.
4.2. Biochemical Responses of Triticum aestivum L. Seedling Leaves to AgNP Exposure
4.3. Assessment of Cell Viability Alterations in Allium cepa L. Following AgNPs Exposure
4.4. Cytogenetic and Mitotic Responses of A. cepa Root Meristems to AgNPs Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| BFSTEM | bright field scanning transmission electron microscopy |
| d.w. | dry weight |
| f.w. | fresh weight |
| G.A.E. | gallic acid equivalents |
| MMS | methyl methane sulfonate |
| ROS | reactive oxygen species |
| SAC | spindle assembly checkpoint |
| SDS | sodium dodecyl sulfate |
| SE | standard deviation |
References
- Rönkkö, T.; Timonen, H. Overview of Sources and Characteristics of Nanoparticles in Urban Traffic-Influenced Areas. J. Alzheimer’s Dis. 2019, 72, 15–28. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Guzmán-Báez, G.A.; Trejo-Téllez, L.I.; Ramírez-Olvera, S.M.; Salinas-Ruíz, J.; Bello-Bello, J.J.; Alcántar-González, G.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Silver Nanoparticles Increase Nitrogen, Phosphorus, and Potassium Concentrations in Leaves and Stimulate Root Length and Number of Roots in Tomato Seedlings in a Hormetic Manner. Dose-Response 2021, 19, 15593258211044576. [Google Scholar] [CrossRef]
- Shaikhaldein, H.O.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Salih, A.M. Biosynthesis and Characterization of Silver Nanoparticles Using Ochradenus arabicus and Their Physiological Effect on Maerua oblongifolia Raised in Vitro. Sci. Rep. 2020, 10, 17569. [Google Scholar] [CrossRef] [PubMed]
- Latif, H.H.; Ghareib, M.; Tahon, M.A. Phytosynthesis of Silver Nanoparticles Using Leaf Extracts from Ocimum basilicum and Mangifira indica and Their Effect on Some Biochemical Attributes of Triticum aestivum. Gesunde Pflanz. 2017, 69, 39–46. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The Size-Dependent Effects of Silver Nanoparticles on Germination, Early Seedling Development and Polar Metabolite Profile of Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut–Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Gruyer, N.; Dorais, M.; Bastien, C.; Dassylva, N.; Triffault-Bouchet, G. Interaction between silver nanoparticles and plant growth. Acta Hortic. 2014, 1037, 795–800. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Nafady, N.A. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Rosmarinus officinalis and Its Effect on Tomato and Wheat Plants. J. Agric. Sci. 2015, 7, p277. [Google Scholar] [CrossRef]
- Shibli, R.; Mohusaien, R.; Abu-Zurayk, R.; Qudah, T.; Tahtamouni, R. Silver Nanoparticles (Ag NPs) Boost Mitigation Powers of Chenopodium Quinoa (Q6 Line) Grown under In Vitro Salt-Stressing Conditions. Water 2022, 14, 3099. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Zhang, Z.; Huang, Y.; Li, G.; Li, Y.; Deng, X.; Li, J. Short-Term Exposure to Silver Nano-Particles Alters the Physiology and Induces Stress-Related Gene Expression in Nelumbo Nucifera. Plant Physiol. Biochem. 2022, 177, 38–45. [Google Scholar] [CrossRef]
- Arridho, S.; Ilyas, S.; Damayanti, T.A.; Widajati, E.; Qadir, A. Impact of Seed Treatment with Azadirachta indica Based Silver Nanoparticles on the Early Growth and Resistance of Soybean Plant under Drought Stress. J. Ecol. Eng. 2025, 26, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Batool, F.; Ahmed, A.; Wagi, S.; Gondal, H.Y.; Maqsood, F.; Abdelrahman, E.A.; Naeem, H.K.; Kanwal, S.; Mustaqeem, M.; et al. Green Synthesized Silver Nanoparticles: Characterization, Phytostimulatory Impacts, and Degradation Potential for Organic Pollutants. Biocatal. Agric. Biotechnol. 2024, 55, 102993. [Google Scholar] [CrossRef]
- Iori, V.; Muzzini, V.G.; Venditti, I.; Casentini, B.; Iannelli, M.A. Phytotoxic Impact of Bifunctionalized Silver Nanoparticles (AgNPs-Cit-L-Cys) and Silver Nitrate (AgNO3) on Chronically Exposed Callus Cultures of Populus nigra L. Environ. Sci. Pollut. Res. 2023, 30, 116175–116185. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, P.; Kulpa, D.; Drozd, R.; Przewodowski, W.; Przewodowska, A. Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures. Molecules 2020, 25, 5511. [Google Scholar] [CrossRef] [PubMed]
- Nurlaelah, E.; Handayani, W.; Yuniati, R.; Sazali, E.S. The Effect of Biogenic Silver Nanoparticles on the Germination and Phenophase of Soybean (Glycine max (L.) Merr.) Var. Anjasmoro. Plant Nano Biol. 2025, 13, 100170. [Google Scholar] [CrossRef]
- Jităreanu, A.; Caba, I.-C.; Trifan, A.; Pădureanu, S.; Agoroaei, L. Triticum Aestivum Assay—A Useful Tool for Environmental Monitoring and Toxicity Assessment. Not. Bot. Horti Agrob. 2019, 47, 1005–1018. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Adhinarayanreddy, V.; Vemanna, R.S.; Sreeman, S.; Makarla, U. Quantification of Membrane Damage/Cell Death Using Evan’s Blue Staining Technique. Bio-Protocol 2017, 7, e2519. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium Test as a Standard in Environmental Monitoring. Hereditas 2008, 102, 99–112. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, Accumulation and Transport of Silver Nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive Probabilistic Modelling of Environmental Emissions of Engineered Nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Drăghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and Radiation-Assisted Methods for Obtaining Metal Nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll Mutations in Barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ortan, A.; Fierascu, I.; Ungureanu, C.; Fierascu, R.C.; Avramescu, S.M.; Dumitrescu, O.; Dinu-Pirvu, C.E. Innovative Phytosynthesized Silver Nanoarchitectures with Enhanced Antifungal and Antioxidant Properties. Appl. Surf. Sci. 2015, 358, 540–548. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Tedesco, I.; Spagnuolo, C.; Russo, G.L.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver Nanoparticles: Aggregation Behavior in Biorelevant Conditions and Its Impact on Biological Activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef]
- Pociecha, E.; Gorczyca, A.; Dziurka, M.; Matras, E.; Oćwieja, M. Silver Nanoparticles and Silver Ions Differentially Affect the Phytohormone Balance and Yield in Wheat. Agriculture 2021, 11, 729. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant Response to Silver Nanoparticles: A Critical Review. Crit. Rev. Biotechnol. 2022, 42, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, T.; Genaidy, A.; Abdelraheem, W.; Dionysiou, D.; Andersen, C. The Effects of Metallic Engineered Nanoparticles upon Plant Systems: An Analytic Examination of Scientific Evidence. Sci. Total Environ. 2017, 579, 93–106. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Colman, B.P.; Dale, A.L.; Truong, L.; Yang, X.Y.; Bone, A.J.; Brown, G.E.; Tanguay, R.L.; Di Giulio, R.T.; et al. Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity. Environ. Sci. Technol. 2013, 47, 13440–13448. [Google Scholar] [CrossRef]
- Feng, L.; Xu, N.; Qu, Q.; Zhang, Z.; Ke, M.; Lu, T.; Qian, H. Synergetic Toxicity of Silver Nanoparticle and Glyphosate on Wheat (Triticum aestivum L.). Sci. Total Environ. 2021, 797, 149200. [Google Scholar] [CrossRef]
- Asanova, A.A.; Yashin, S.E.; Trofimova, T.V.; Polonskiy, V.I. Application of Silver Nanoparticles to Improve Wheat Seedlings Growth. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 052041. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.; Wattoo, F.H.; Hussain, M.; Ejaz, M.; Saira, H. Assessment of AgNPs Exposure on Physiological and Biochemical Changes and Antioxidative Defence System in Wheat (Triticum aestivum L.) under Heat Stress. IET Nanobiotechnol. 2019, 13, 230–236. [Google Scholar] [CrossRef]
- Singh, Y.; Kaushal, S.; Sodhi, R.S. Biogenic Synthesis of Silver Nanoparticles Using Cyanobacterium Leptolyngbya Sp. WUC 59 Cell-Free Extract and Their Effects on Bacterial Growth and Seed Germination. Nanoscale Adv. 2020, 2, 3972–3982. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Saratale, R.G.; Shinde, S.; Syed, A.; Ameen, F.; Ghodake, G. Green Synthesis of Silver Nanoparticles Using Laminaria japonica Extract: Characterization and Seedling Growth Assessment. J. Clean. Prod. 2018, 172, 2910–2918. [Google Scholar] [CrossRef]
- Umair Raza, M.; Abasi, F.; Shahbaz, M.; Ehsan, M.; Seerat, W.; Akram, A.; Raja, N.I.; Mashwani, Z.u.-R.; Hassan, H.U.; Proćków, J. Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants. Molecules 2023, 28, 2044. [Google Scholar] [CrossRef]
- Atia, W. Silver nanoparticles and NPK fertilizer effects on the proline, peroxidase, and catalase enzymes in wheat. SABRAO J. Breed. Gen. 2024, 56, 2405–2415. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, S.; Zhao, W.; Wang, P.; Zhao, S.; Xu, Y.; Wang, D. Regulation Mechanism of Sodium Citrate on Fresh-Cut Yam Yellowing. Postharvest Biol. Technol. 2022, 191, 111965. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-de León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of Stevia and Citric Acid on the Stability of Phenolic Compounds and in Vitro Antioxidant and Antidiabetic Capacity of a Roselle (Hibiscus sabdariffa L.) Beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Gómez-Merino, F.C.; Pestryakov, A. Hormetic Response by Silver Nanoparticles on In Vitro Multiplication of Sugarcane (Saccharum Spp. Cv. Mex 69-290) Using a Temporary Immersion System. Dose-Response 2017, 15, 155932581774494. [Google Scholar] [CrossRef]
- Kruszka, D.; Sawikowska, A.; Kamalabai Selvakesavan, R.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver Nanoparticles Affect Phenolic and Phytoalexin Composition of Arabidopsis thaliana. Sci. Total Environ. 2020, 716, 135361. [Google Scholar] [CrossRef] [PubMed]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory Effect of Silver Nanoparticles on the Growth and Flowering of Potted Oriental Lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Cvjetko, P.; Balen, B.; Pavoković, D.; Šikić, S.; Peharec Štefanić, P.; Tkalec, M.; Biba, R. Silver Nanoparticles Affect Germination and Photosynthesis in Tobacco Seedlings. Acta Bot. Croat. 2021, 80, 1–11. [Google Scholar] [CrossRef]
- Tejada-Alvarado, J.J.; Meléndez-Mori, J.B.; Ayala-Tocto, R.Y.; Goñas, M.; Oliva, M. Influence of Silver Nanoparticles on Photosynthetic Pigment Content and Mineral Uptake in Pineapple Seedlings Grown In Vitro under Aluminum Stress. Agronomy 2023, 13, 1186. [Google Scholar] [CrossRef]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Shaw, A.K.; Roy, D.; Roy, S.; Saha, S.; Hossain, Z. Imbalance of Redox Homeostasis and Antioxidant Defense Status in Maize under Chromium (VI) Stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Fernandez-Da Silva, R.; Menendez-Yuffa, A. Viability in Protoplasts and Cell Suspensions of Coffea Arabica Cv. Catimor. Electron. J. Biotechnol. 2006, 9. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; Bo, A.; Sun, J.; Li, M. Sodium Citrate Inhibits the Proliferation of Human Gastric Adenocarcinoma Epithelia Cells. Oncol. Lett. 2018, 15, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, C.; Liu, W.; Zhan, W.; Chen, Y.; Lu, J.; Bao, Y.; Wang, S.; Yu, C.; Zheng, L.; et al. Sodium Citrate Targeting Ca2+/CAMKK2 Pathway Exhibits Anti-Tumor Activity through Inducing Apoptosis and Ferroptosis in Ovarian Cancer. J. Adv. Res. 2024, 65, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Hasanuzzaman, M.; Roychowdhury, R.; Sarraf, M.; Afzal, S.; Das, S.; Rastogi, A. Silver Nanoparticles in Plant Health: Physiological Response to Phytotoxicity and Oxidative Stress. Plant Physiol. Biochem. 2024, 209, 108538. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of Silver Nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and Genotoxic Effects of Zinc Oxide Nanoparticles on Root Cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Kannaujia, R.; Srivastava, C.M.; Prasad, V.; Singh, B.N.; Pandey, V. Phyllanthus Emblica Fruit Extract Stabilized Biogenic Silver Nanoparticles as a Growth Promoter of Wheat Varieties by Reducing ROS Toxicity. Plant Physiol. Biochem. 2019, 142, 460–471. [Google Scholar] [CrossRef]
- Rank, J.; Nielsen, M.H. Allium Cepa Anaphase–Telophase Root Tip Chromosome Aberration Assay on N-Methyl-N-Nitrosourea, Maleic Hydrazide, Sodium Azide, and Ethyl Methanesulfonate. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 390, 121–127. [Google Scholar] [CrossRef]
- Bonciu, E.I.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoglu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Papini, A.; et al. An Evaluation for the Standardization of the Allium cepa Test as Cytotoxicity and Genotoxicity Assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Zhukovskaya, N.V.; Bystrova, E.I.; Dubrovsky, J.G.; Ivanov, V.B. Global Analysis of an Exponential Model of Cell Proliferation for Estimation of Cell Cycle Duration in the Root Apical Meristem of Angiosperms. Ann. Bot. 2018, 122, 811–822. [Google Scholar] [CrossRef]
- Ristea, M.-E.; Zarnescu, O. Effects of Indigo Carmine on Growth, Cell Division, and Morphology of Allium cepa L. Root Tip. Toxics 2024, 12, 194. [Google Scholar] [CrossRef]
- Trela-Makowej, A.; Orzechowska, A.; Szymańska, R. Less Is More: The Hormetic Effect of Titanium Dioxide Nanoparticles on Plants. Sci. Total Environ. 2024, 910, 168669. [Google Scholar] [CrossRef]
- Chahardoli, A.; Sharifan, H.; Karimi, N.; Kakavand, S.N. Uptake, Translocation, Phytotoxicity, and Hormetic Effects of Titanium Dioxide Nanoparticles (TiO2NPs) in Nigella arvensis L. Sci. Total Environ. 2022, 806, 151222. [Google Scholar] [CrossRef]
- Samadi, N.; Yahyaabadi, S.; Rezayatmand, Z. Stress Effects of TiO2 and NP-TiO2 on Catalase Enzyme and Some Physiological Characteristics of Melissa officinalis L. Eur. J. Med. Plants 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [PubMed]
- Hadjesfandiari, N.; Parambath, A. Stealth Coatings for Nanoparticles. In Engineering of Biomaterials for Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–361. ISBN 978-0-08-101750-0. [Google Scholar]
- Beemster, G.T.S.; Baskin, T.I. Analysis of Cell Division and Elongation Underlying the Developmental Acceleration of Root Growth in Arabidopsis thaliana 1. Plant Physiol. 1998, 116, 1515–1526. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T.S. Cell Cycle Modulation in the Response of the Primary Root of Arabidopsis to Salt Stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, Ş. Genotoxicity of Five Food Preservatives Tested on Root Tips of Allium cepa L. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 626, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zhang, F. Cell Cycle Regulation in the Plant Response to Stress. Front. Plant Sci. 2020, 10, 1765. [Google Scholar] [CrossRef]
- Scherer, M.D.; Sposito, J.C.V.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and Genotoxic Effects of Silver Nanoparticles on Meristematic Cells of Allium Cepa Roots: A Close Analysis of Particle Size Dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Waterman, D.P.; Caban-Penix, S.; Memisoglu, G.; Eapen, V.V.; Haber, J.E. Prolonged Cell Cycle Arrest in Response to DNA Damage in Yeast Requires the Maintenance of DNA Damage Signaling and the Spindle Assembly Checkpoint. Elife 2024, 13, RP94334. [Google Scholar] [CrossRef]
- Hubbs, A.; Porter, D.W.; Mercer, R.; Castranova, V.; Sargent, L.; Sriram, K. Nanoparticulates. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1373–1419. ISBN 978-0-12-415759-0. [Google Scholar]
- Komaki, S.; Schnittger, A. The Spindle Assembly Checkpoint in Arabidopsis Is Rapidly Shut Off during Severe Stress. Dev. Cell 2017, 43, 172–185.e5. [Google Scholar] [CrossRef]
- Nera, B.; Huang, H.-S.; Lai, T.; Xu, L. Elevated Levels of TRF2 Induce Telomeric Ultrafine Anaphase Bridges and Rapid Telomere Deletions. Nat. Commun. 2015, 6, 10132. [Google Scholar] [CrossRef]
- Fernández-Casañas, M.; Chan, K.-L. The Unresolved Problem of DNA Bridging. Genes 2018, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Duta-Cornescu, G.; Dugala, M.L.; Constantin, N.; Pojoga, M.-D.; Simon-Gruita, A. Evaluation of Clastogenic and Aneugenic Action of Two Bio-Insecticides Using Allium Bioassay. J. Xenobiot. 2025, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Chromosome Missegregation in Human Cells Arises through Specific Types of Kinetochore–Microtubule Attachment Errors. Proc. Natl. Acad. Sci. USA 2011, 108, 17974–17978. [Google Scholar] [CrossRef] [PubMed]
- Siri, S.O.; Martino, J.; Gottifredi, V. Structural Chromosome Instability: Types, Origins, Consequences, and Therapeutic Opportunities. Cancers 2021, 13, 3056. [Google Scholar] [CrossRef]
- Moore, R.C.; Bender, M.A. Time Sequence of Events Leading to Chromosomal Aberration Formation. Environ. Mol. Mutagen. 1993, 22, 208–213. [Google Scholar] [CrossRef]





 —cell with chromosomal aberrations, 400×. The original images of the chromosomal aberrations are provided in the Supplementary Material (Figures S3–S8).
—cell with chromosomal aberrations, 400×. The original images of the chromosomal aberrations are provided in the Supplementary Material (Figures S3–S8).
 —cell with chromosomal aberrations, 400×. The original images of the chromosomal aberrations are provided in the Supplementary Material (Figures S3–S8).
—cell with chromosomal aberrations, 400×. The original images of the chromosomal aberrations are provided in the Supplementary Material (Figures S3–S8).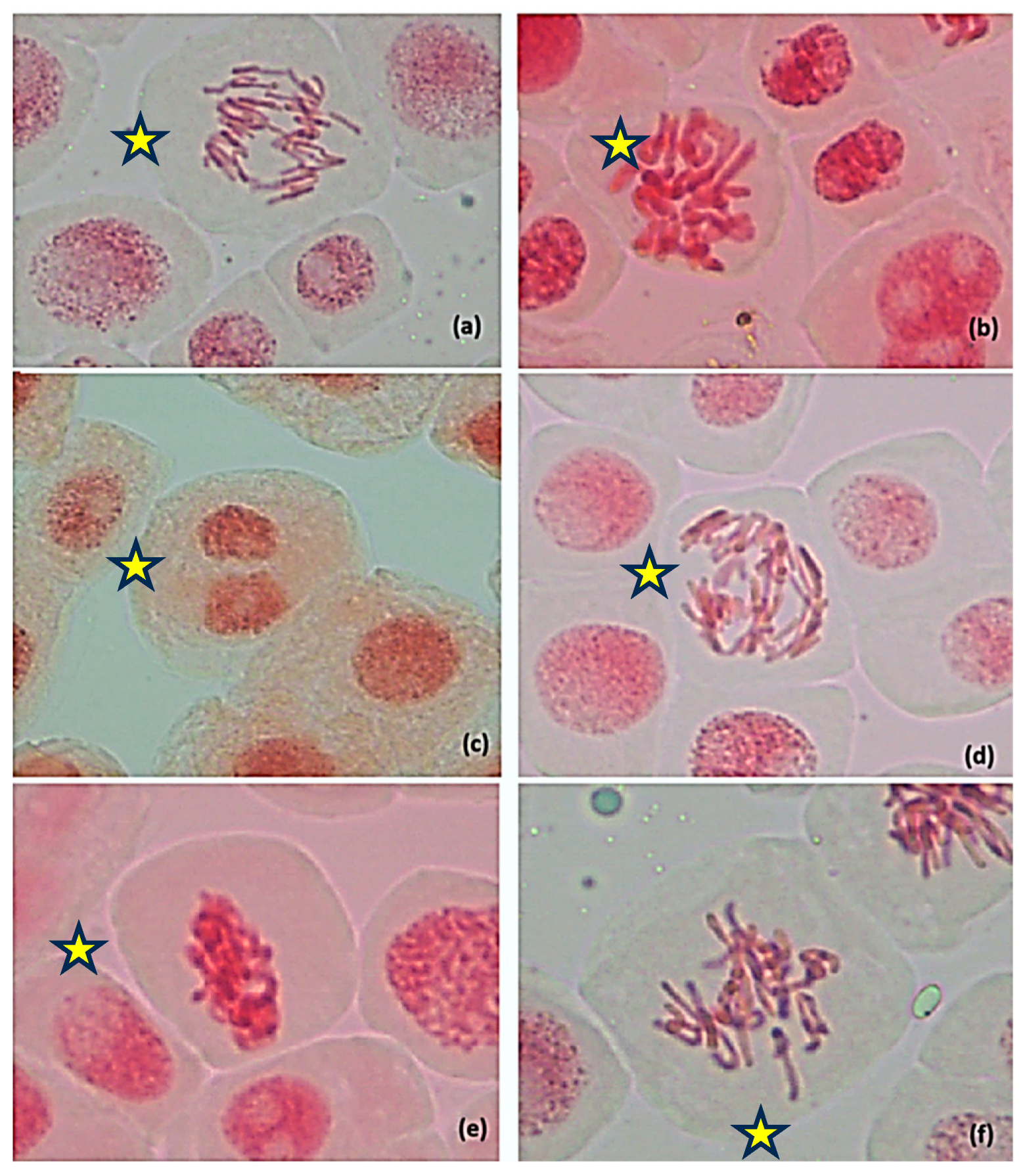
| Nr. Crt. | Variant Code | Content | Dilution |
|---|---|---|---|
| 1 | S | Negative control: distilled water | - |
| 2 | SC | Solvent control: Sodium citrate 2 mM | - |
| 3 | N1 | AgNPs dispersed in sodium citrate 2 mM | 10−1 (2 µg mL−1) |
| 4 | N2 | AgNPs dispersed in sodium citrate 2 mM | 10−2 (0.2 µg mL−1) |
| 5 | N3 | AgNPs dispersed in sodium citrate 2 mM | 10−3 (0.02 µg mL−1) |
| Experimental Variant | Binucleated Cells | Lagging Chromosomes | C-Mitosis | Sticky Chromosomes | Anaphase Bridges | Multipolar Anaphases | Other Aberrations | Total Aberrations |
|---|---|---|---|---|---|---|---|---|
| DW24H | 0.00 | 0.00 | 0.00 | 0.00 | 22.22 ± 14.7 ab | 0.00 | 0.00 | 2.74 ± 1.71 cd |
| DW48H | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SC24H | 0.03 ± 0.0 a | 3.74 ± 3.7 a | 0.00 | 0.1 ± 0.06 a | 22.22 ± 11.11 ab | 0.00 | 1.85 ± 1.85 a | 10.34 ± 3.25 abc |
| SC48H | 0.00 | 0.00 | 0.55 ± 0.35 a | 0.17 ± 0.17 a | 8.33 ± 8.33 b | 0.00 | 2.78 ± 2.78 a | 18.26 ± 7.66 a |
| N1 24H | 0.00 | 0.00 | 0.03 ± 0.03 b | 0.06 ± 0.06 a | 11.11 ± 7.35 b | 0.00 | 0.00 | 4.96 ± 0.92 bd |
| N1 48H | 0.04 ± 003 a | 0.07 ± 0.07 a | 0.06 ± 0.06 b | 0.00 | 11.43 ± 5.95 b | 0.00 | 6.06 ± 6.06 a | 5.6 ± 1.87 bcd |
| N2 24H | 0.00 | 0.00 | 0.07 ± 0.07 b | 0.00 | 46.52 ± 7.66 a | 5.59 ± 2.82 a | 0.00 | 12.16 ± 3.05 ab |
| N2 48H | 0.00 | 0.00 | 0.03 ± 0.03 b | 0.00 | 16.66 ± 16.67 b | 0.00 | 0.00 | 3.26 ± 2.18 bcd |
| N3 24H | 0.00 | 0.00 | 0.00 | 0.00 | 14.99 ± 14.68 b | 0.00 | 0.00 | 3.1 ± 0.57 bcd |
| N3 48S | 0.00 | 0.00 | 0.00 | 0.1 ± 0.06 a | 17.19 ± 5.19 b | 0.00 | 1.67 ± 1.67 a | 5.05 ± 0.41 bcd |
| MMS 24H | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MMS 48H | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Experimental Variants | Mitotic Index | Prophase Index | Metaphase Index | Anaphase Index | Telophase Index | Binucleated Cells | Lagging Chromosomes | C-Mitosis | Stickies | Anaphase Bridges | Multipolar Anaphases | Other Aberrations | Total Aberrations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitotic index | 1 | 0.314 | 0.531 ** | 0.661 ** | 0.377 ** | 0.21 | 0.442 ** | 0.038 | 0.29 | 0.465 | 0.222 | 0.122 | 0.337 ** |
| Prophase index | 0.314 | 1 | 0.21 | 0.056 | 0.173 | 0.228 | 0.217 | 0.269 | −0.034 | 0.033 | 0.2 | 0.032 | 0.275 |
| Metaphase index | 0.531 ** | 0.21 | 1 | 0.502 ** | 0.397 * | −0.147 | −0.042 | 0.144 | 0.164 | 0.317 | 0.058 | 0.077 | 0.275 |
| Anaphase index | 0.661 ** | 0.056 | 0.502 ** | 1 | 0.458 ** | 0.233 | 0.062 | −0.168 | 0.273 | 0.59 ** | 0.206 | 0.205 | 0.324 |
| Telophase index | 0.377 * | 0.173 | 0.397 * | 0.458 ** | 1 | −0.035 | −0.032 | 0.093 | 0.29 | 0.297 | 0.296 | −0.046 | 0.133 |
| Binucleated cells | 0.21 | 0.228 | −0.147 | 0.233 | -0.035 | 1 | 0.639 ** | −0.071 | −0.09 | 0.17 | −0.059 | 0.073 | 0.195 |
| Lagging chromosomes | 0.442 ** | 0.217 | −0.042 | 0.062 | −0.032 | 0.639 ** | 1 | −0.068 | −0.105 | 0.137 | −0.109 | 0.057 | 0.063 |
| C-mitosis | 0.038 | 0.269 | 0.144 | −0.168 | 0.093 | −0.071 | −0.068 | 1 | −0.104 | −0.129 | −0.035 | 0.433 ** | 0.801 ** |
| Stickies | 0.29 | −0.034 | 0.164 | 0.273 | 0.29 | −0.09 | −0.105 | −0.104 | 1 | −0.156 | −0.09 | −0.012 | 0.188 |
| Anaphase bridges | 0.465 ** | 0.033 | 0.317 | 0.59 ** | 0.297 | 0.17 | 0.137 | −0.129 | −0.156 | 1 | 0.472 ** | 0.118 | 0.364 ** |
| Multipolar anaphases | 0.222 | 0.2 | 0.058 | 0.206 | 0.296 | −0.059 | −0.109 | −0.035 | −0.09 | 0.472 ** | 1 | -0.073 | 0.258 |
| Other aberrations | 0.122 | 0.032 | 0.077 | 0.205 | −0.046 | 0.073 | 0.057 | 0.433 ** | −0.012 | 0.118 | −0.073 | 1 | 0.391 * |
| Total aberrations | 0.337 * | 0.275 | 0.275 | 0.324 | 0.133 | 0.195 | 0.063 | 0.801 ** | 0.188 | 0.364 * | 0.258 | 0.391 * | 1 |
| Evaluated Parameter | Experimental Variants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SC | N1 2 µg mL−1 | N2 0.2 µg mL−1 | N3 0.02 µg mL−1 | ||||||
| Significant Changes | ||||||||||
| + | − | + | − | + | − | + | − | + | − | |
| Root length | + | + | ||||||||
| Shoot length | + | |||||||||
| Fresh weight | + | + | + | + | ||||||
| Dry weight | + | + | + | |||||||
| Proline | ||||||||||
| Polyphenols | + | |||||||||
| Assimilatory pigments | ||||||||||
| Cell viability | − | |||||||||
| Mitotic index | + | |||||||||
| Total chromosomal aberrations | − | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisculungeanu, S.E.; Soare, L.C.; Luțu, O.A.; Păunescu, A.; Cîrstea, G.; Negrea, A.D.; Dobrescu, C.M.; Ionescu, N.A. Integrated Assessment of Silver Nanoparticles on Plant Growth and Cytogenotoxicity Using Triticum and Allium Bioassays. J. Xenobiot. 2025, 15, 147. https://doi.org/10.3390/jox15050147
Pisculungeanu SE, Soare LC, Luțu OA, Păunescu A, Cîrstea G, Negrea AD, Dobrescu CM, Ionescu NA. Integrated Assessment of Silver Nanoparticles on Plant Growth and Cytogenotoxicity Using Triticum and Allium Bioassays. Journal of Xenobiotics. 2025; 15(5):147. https://doi.org/10.3390/jox15050147
Chicago/Turabian StylePisculungeanu, Simona Elena, Liliana Cristina Soare, Oana Alexandra Luțu, Alina Păunescu, Georgiana Cîrstea, Aurelian Denis Negrea, Codruța Mihaela Dobrescu, and Nicoleta Anca Ionescu (Șuțan). 2025. "Integrated Assessment of Silver Nanoparticles on Plant Growth and Cytogenotoxicity Using Triticum and Allium Bioassays" Journal of Xenobiotics 15, no. 5: 147. https://doi.org/10.3390/jox15050147
APA StylePisculungeanu, S. E., Soare, L. C., Luțu, O. A., Păunescu, A., Cîrstea, G., Negrea, A. D., Dobrescu, C. M., & Ionescu, N. A. (2025). Integrated Assessment of Silver Nanoparticles on Plant Growth and Cytogenotoxicity Using Triticum and Allium Bioassays. Journal of Xenobiotics, 15(5), 147. https://doi.org/10.3390/jox15050147









