Lead Poisoning in the Americas: Sources, Regulations, Health Impacts, and Molecular Mechanisms
Abstract
1. Introduction
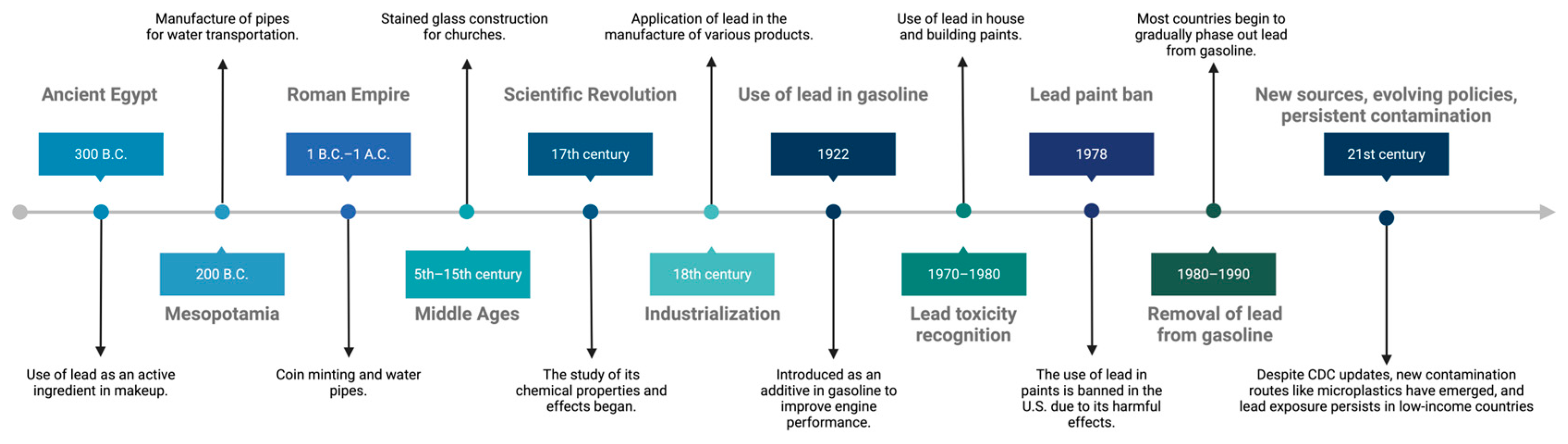
2. Historical Context
3. Regulations and Strategies for Lead Poisoning Prevention
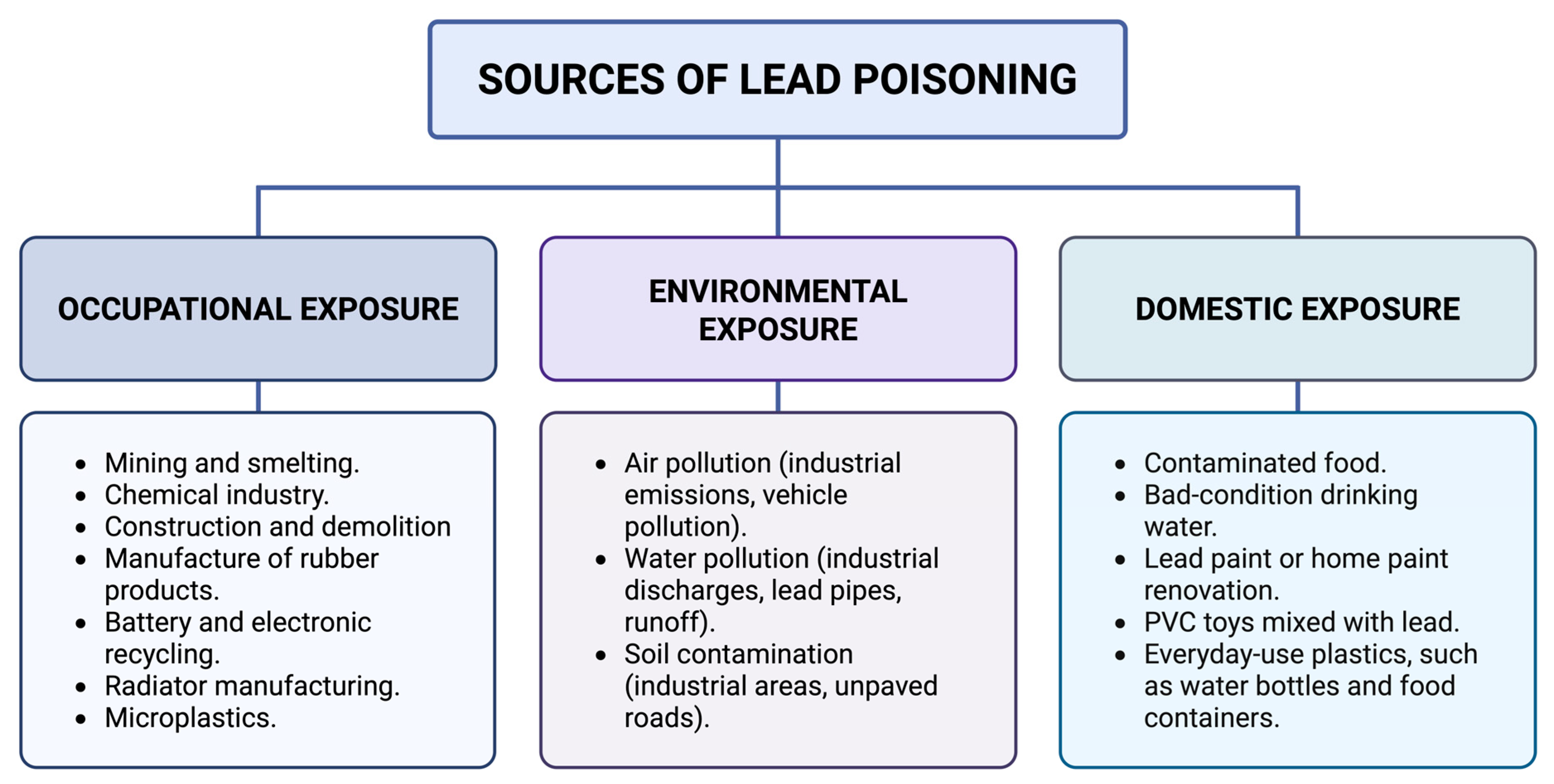
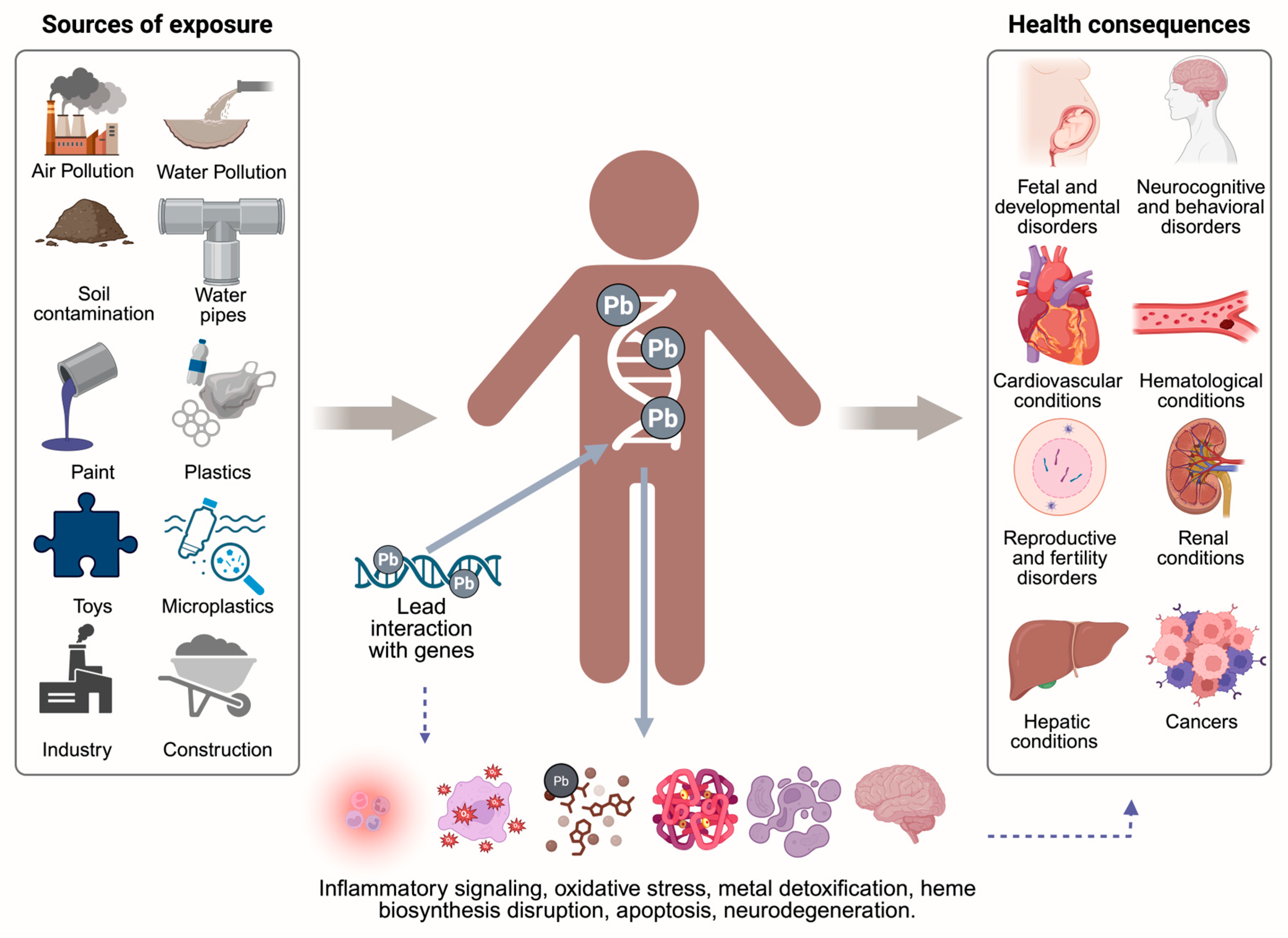
4. Diversity of Lead Poisoning Sources
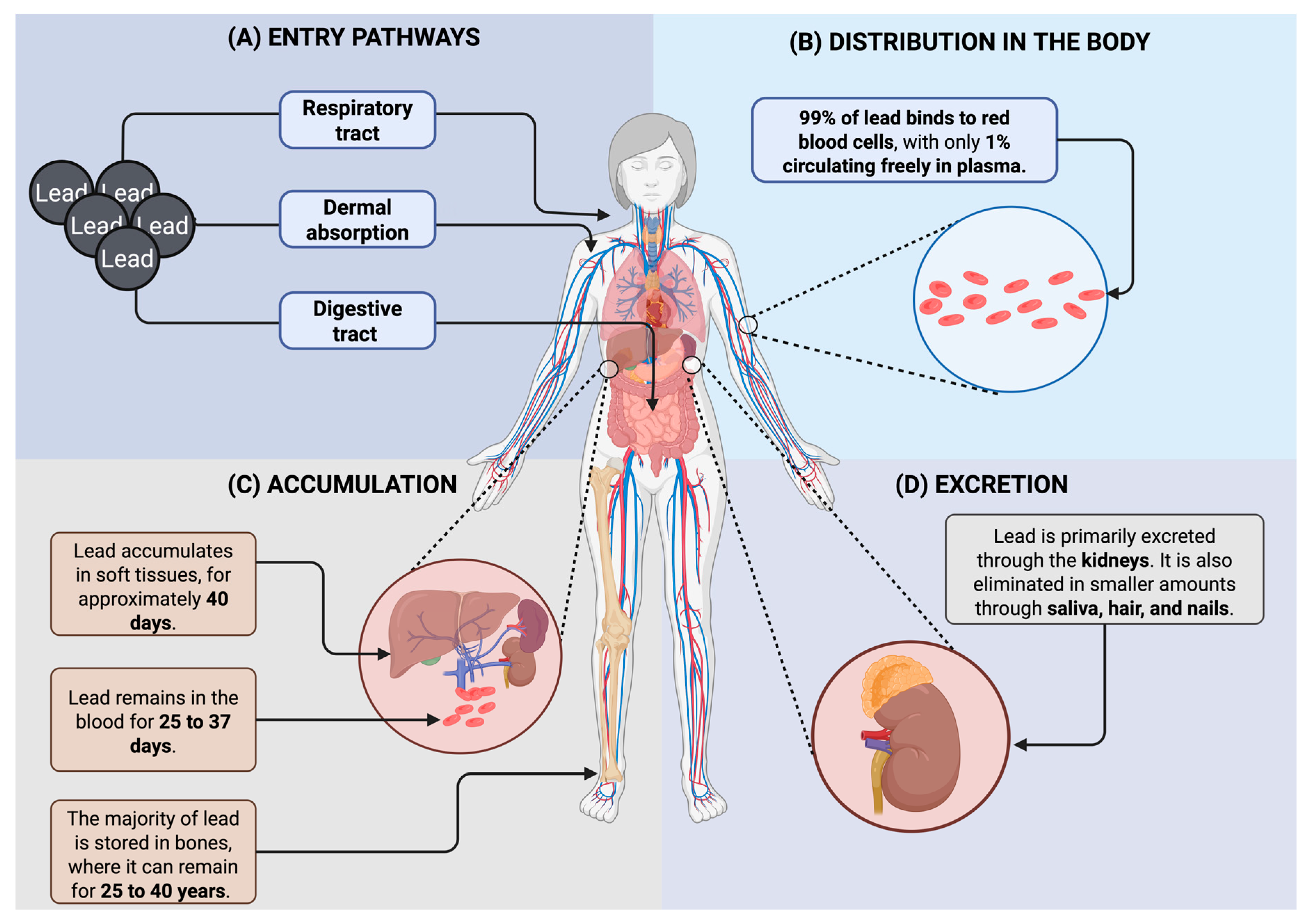
5. Lead Toxicokinetics and Mechanisms
6. Health Impacts of Lead Poisoning on Pregnant Women, Children and Adults
6.1. Impact of Lead Exposure on Maternal and Fetal Health During Pregnancy
6.2. Effects of Lead Poisoning in Children
6.3. Lead Poisoning in Adults
7. Molecular Mechanisms of Lead Toxicity
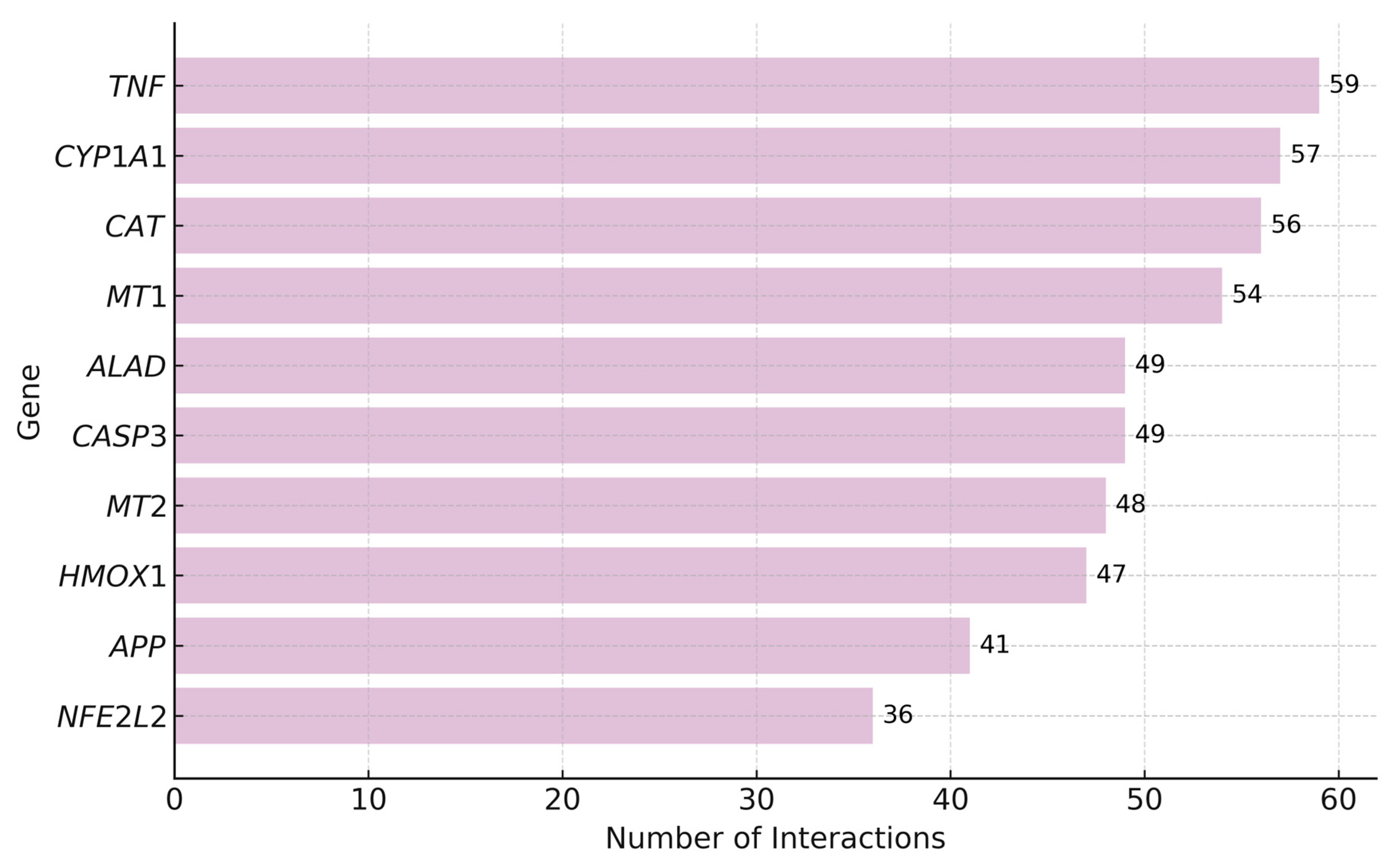
7.1. Top Genes Interacting with Lead
7.2. Pathologies Associated with Lead Exposure
7.3. Significant Pathways Enriched in Genes Interacting with Lead
8. Economic and Nutritional Factors in Lead Poisoning
9. Blood Lead Levels in Lead Poisoning
10. Key Findings and Conceptual Summary of Lead Toxicity
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Njati, S.Y.; Maguta, M.M. Lead-Based Paints and Children’s PVC Toys Are Potential Sources of Domestic Lead Poisoning—A Review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Low Blood Lead Levels Impair Intellectual and Hematological Function in Children from Cartagena, Caribbean Coast of Colombia. J. Trace Elem. Med. Biol. 2017, 44, 233–240. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Ansari, J.A.; Chaurasia, P.; Ahmad, M.K.; Kunwar, S.; McClean, S.; Yogarajah, P. A Study of Maternal and Umbilical Cord Blood Lead Levels in Pregnant Women. Indian J. Clin. Biochem. 2023, 38, 94–101. [Google Scholar] [CrossRef]
- Mohsenipour, R.; Aflatoonian, M.; Alimadadi, H.; Rahmani, P.; Esmaeili, N.; Yazdi, M.; Abbasi, F.; Solgi, F.; Sharifi, F.; Vafaii, N.; et al. Lead Poisoning as a Differential Diagnosis in Pediatric Patients with Chronic Abdominal Pain: A Case–Control Study in Tehran-Iran. BMC Gastroenterol. 2024, 24, 344. [Google Scholar] [CrossRef]
- Rădulescu, A.; Lundgren, S. A Pharmacokinetic Model of Lead Absorption and Calcium Competitive Dynamics. Sci. Rep. 2019, 9, 14225. [Google Scholar] [CrossRef]
- Armijos, R.X.; Weigel, M.M.; Obeng-Gyasi, E.; Racines-Orbe, M. Elevated Blood Lead and Metal/Metalloid Levels and Environmental Exposure Sources in Urban Ecuadorian School-Age Children and Mothers. Int. J. Hyg. Environ. Health 2021, 235, 113770. [Google Scholar] [CrossRef] [PubMed]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Walter, K. What Is Lead Poisoning? JAMA 2023, 329, 1040. [Google Scholar] [CrossRef] [PubMed]
- La-Llave-León, O.; Salas Pacheco, J.M.; Estrada Martínez, S.; Esquivel Rodríguez, E.; Castellanos Juárez, F.X.; Sandoval Carrillo, A.; Lechuga Quiñones, A.M.; Vázquez Alanís, F.; García Vargas, G.; Méndez Hernández, E.M.; et al. The Relationship between Blood Lead Levels and Occupational Exposure in a Pregnant Population. BMC Public Health 2016, 16, 1231. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; González Esquivel, D.F.; Blanco Ayala, T.; Pineda, B.; Gómez Manzo, S.; Marcial Quino, J.; Carrillo Mora, P.; Pérez de la Cruz, V. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Hosseini, A.; Fayaz, A.; Hassanian-Moghaddam, H.; Zamani, N.; Hadeiy, S.K.; Gholami, N.; Dara, N.; Khatami, K.; Rohani, P.; Phillips, S. Blood Lead Concentrations among Pediatric Patients with Abdominal Pain: A Prospective Cross-Sectional Study. BMC Gastroenterol. 2021, 21, 493. [Google Scholar] [CrossRef]
- Lead Poisoning|Children’s Environmental Health Collaborative. Available online: https://ceh.unicef.org/spotlight-risk/lead-poisoning (accessed on 25 November 2024).
- World Health Organization (WHO). Available online: https://www.who.int (accessed on 25 November 2024).[Green Version]
- Kordas, K.; Ravenscroft, J.; Cao, Y.; McLean, E.V. Lead Exposure in Low and Middle-Income Countries: Perspectives and Lessons on Patterns, Injustices, Economics, and Politics. Int. J. Environ. Res. Public Health 2018, 15, 2351. [Google Scholar] [CrossRef]
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead Toxicity Update. A Brief Review. Med. Sci. Monit. 2005, 11, RA329–RA336. [Google Scholar]
- Sampson, R.J. Legacies of Inequality, Legacy Lead Exposures, and Improving Population Well-Being. Proc. Natl. Acad. Sci. USA 2022, 119, e2202401119. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Todd, A.C. Lead Poisoning. West. J. Med. 1994, 161, 153–159. [Google Scholar] [PubMed]
- CDC Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/index.html (accessed on 25 November 2024).
- Angrand, R.C.; Collins, G.; Landrigan, P.J.; Thomas, V.M. Relation of Blood Lead Levels and Lead in Gasoline: An Updated Systematic Review. Environ. Health 2022, 21, 138. [Google Scholar] [CrossRef]
- Lee, J.; Hu, M. Effect of Environmental and Socioeconomic Factors on Increased Early Childhood Blood Lead Levels: A Case Study in Chicago. Int. J. Environ. Res. Public Health 2024, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; González-González, L.; Piña-Pozas, M.; Mérida-Ortega, Á.; Gamboa-Loira, B.; Blanco-Muñoz, J.; Torres-Sánchez, L.E.; Hurtado-Díaz, M.; Cortez-Lugo, M.; Guerra, G.; et al. State of Children Environmental Health Research in Latin America. Ann. Glob. Health 2018, 84, 204–211. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Mielke, H.W.; Filippelli, G.M. Assessing Unequal Airborne Exposure to Lead Associated With Race in the USA. GeoHealth 2023, 7, e2023GH000829. [Google Scholar] [CrossRef]
- CDC CDC Updates Blood Lead Reference Value. Available online: https://www.cdc.gov/lead-prevention/php/news-features/updates-blood-lead-reference-value.html (accessed on 8 July 2025).
- Massos, A.; Turner, A. Cadmium, Lead and Bromine in Beached Microplastics. Environ. Pollut. 2017, 227, 139–145. [Google Scholar] [CrossRef]
- Piai, K.d.A.; Olympio, K.P.K. Children’s Blood Lead Levels in Latin America and the Caribbean—Recommendations to Combat This Well-Known Persistent Public Health Problem. Curr. Opin. Environ. Sci. Health 2023, 32, 100454. [Google Scholar] [CrossRef]
- Ericson, B.; Hu, H.; Nash, E.; Ferraro, G.; Sinitsky, J.; Taylor, M.P. Blood Lead Levels in Low-Income and Middle-Income Countries: A Systematic Review. Lancet Planet. Health 2021, 5, e145–e153. [Google Scholar] [CrossRef] [PubMed]
- Pantic, I.; Tamayo-Ortiz, M.; Rosa-Parra, A.; Bautista-Arredondo, L.; Wright, R.O.; Peterson, K.E.; Schnaas, L.; Rothenberg, S.J.; Hu, H.; Téllez-Rojo, M.M. Children’s Blood Lead Concentrations from 1988 to 2015 in Mexico City: The Contribution of Lead in Air and Traditional Lead-Glazed Ceramics. Int. J. Environ. Res. Public Health 2018, 15, 2153. [Google Scholar] [CrossRef]
- Welton, M.; Rodriguez-Lainz, A.; Loza, O.; Brodine, S.; Fraga, M. Use of Lead-Glazed Ceramic Ware and Lead-Based Folk Remedies in a Rural Community of Baja California, Mexico. Glob. Health Promot. 2018, 25, 6–14. [Google Scholar] [CrossRef]
- Reuer, M.K.; Bower, N.W.; Koball, J.H.; Hinostroza, E.; De la Torre Marcas, M.E.; Surichaqui, J.A.H.; Echevarria, S. Lead, Arsenic, and Cadmium Contamination and Its Impact on Children′s Health in La Oroya, Peru. Int. Sch. Res. Not. 2012, 2012, 231458. [Google Scholar] [CrossRef]
- Neumann, P. Toxic Talk and Collective (In)Action in a Company Town: The Case of La Oroya, Peru. Soc. Probl. 2016, 63, 431–446. [Google Scholar] [CrossRef]
- Queirolo, E.I.; Ettinger, A.S.; Stoltzfus, R.J.; Kordas, K. Association of Anemia, Child and Family Characteristics With Elevated Blood Lead Concentrations in Preschool Children From Montevideo, Uruguay. Arch. Environ. Occup. Health 2010, 65, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, D. “We Are Not Marginals”: The Cultural Politics of Lead Poisoning in Montevideo, Uruguay. Lat. Am. Perspect. 2013, 40, 202–217. [Google Scholar] [CrossRef]
- de Almeida Lopes, A.C.B.; Navas-Acien, A.; Zamoiski, R.; Silbergeld, E.K.; Carvalho, M.d.F.H.; Buzzo, M.L.; Urbano, M.R.; Martins Junior, A.d.C.; Paoliello, M.M.B. Risk Factors for Lead Exposure in Adult Population in Southern Brazil. J. Toxicol. Environ. Health A 2015, 78, 92–108. [Google Scholar] [CrossRef]
- Barbosa, F.; Fillion, M.; Lemire, M.; Sousa Passos, C.J.; Lisboa Rodrigues, J.; Philibert, A.; Guimarães, J.-R.; Mergler, D. Elevated Blood Lead Levels in a Riverside Population in the Brazilian Amazon. Environ. Res. 2009, 109, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Caravanos, J.; Carrelli, J.; Dowling, R.; Pavilonis, B.; Ericson, B.; Fuller, R. Burden of Disease Resulting from Lead Exposure at Toxic Waste Sites in Argentina, Mexico and Uruguay. Environ. Health 2016, 15, 72. [Google Scholar] [CrossRef]
- Bousquet, A.G.; Eaves, L.A.; Haley, K.; Catalano, D.; Williams, G.B.; Hartwell, H.J.; Brennan, C.; Fry, R.C. Identifying and Responding to Lead in Drinking Water in a University Setting. Int. J. Environ. Res. Public Health 2024, 21, 561. [Google Scholar] [CrossRef]
- NHANES—National Health and Nutrition Examination Survey Homepage. Available online: https://www.cdc.gov/nchs/nhanes/index.html (accessed on 25 November 2024).
- Olympio, K.P.K.; Gonçalves, C.G.; Salles, F.J.; Ferreira, A.P.S.d.S.; Soares, A.S.; Buzalaf, M.A.R.; Cardoso, M.R.A.; Bechara, E.J.H. What Are the Blood Lead Levels of Children Living in Latin America and the Caribbean? Environ. Int. 2017, 101, 46–58. [Google Scholar] [CrossRef]
- Neuwirth, L.S. Resurgent Lead Poisoning and Renewed Public Attention towards Environmental Social Justice Issues: A Review of Current Efforts and Call to Revitalize Primary and Secondary Lead Poisoning Prevention for Pregnant Women, Lactating Mothers, and Children within the U.S. Int. J. Occup. Environ. Health 2018, 24, 86–100. [Google Scholar] [CrossRef]
- Pereira, E.C.; Piai, K.d.A.; Salles, F.J.; Silva, A.S.d.; Olympio, K.P.K. A Comprehensive Analysis of Children’s Blood Lead Levels in Latin America and the Caribbean over the Last Eight Years: Progress and Recommendations. Sci. Total Environ. 2024, 928, 172372. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe-Pawlowski, L.L.; Miles, S.Q.; Courtney, J.G.; Materna, B.; Charlton, V. Effect of Magnitude and Timing of Maternal Pregnancy Blood Lead (Pb) Levels on Birth Outcomes. J. Perinatol. 2006, 26, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, H.H.; Chen, J.W.; Gu, K.D.; Zhang, Y.Z.; Zhu, Y.X.; Zhou, Y.K.; Ye, L.X. Adverse Health Effects of Lead Exposure on Children and Exploration to Internal Lead Indicator. Sci. Total Environ. 2009, 407, 5986–5992. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.B.; Spelta, L.E.W.; Sobalvarro, J.V.M.; Podestá, M.H.M.C.; Garcia, R.C.T.; dos Reis, T.M.; Torres, L.H. Gestational Lead Exposure and Its Effects on Fetal/Infant Development—A Systematic Review. Reprod. Toxicol. 2023, 117, 108342. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of Lead: A Review with Recent Updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Chen, S.; Abdulla, A.; Yan, H.; Mi, Q.; Ding, X.; He, J.; Yan, C. Proteome Signatures of Joint Toxicity to Arsenic (As) and Lead (Pb) in Human Brain Organoids with Optic Vesicles. Environ. Res. 2024, 243, 117875. [Google Scholar] [CrossRef]
- Gailey, S.; Sadler, R.C.; Harris, A.; Jenuwine, S.; Dannis, J.; Jones, N.M. Racial Differences in Residential Mobility after the Flint Water Crisis: A Survival Analysis. Soc. Sci. Med. 2025, 376, 117812. [Google Scholar] [CrossRef]
- Gibson, J.M.; Desclos, A.; Harrington, J.; McElmurry, S.P.; Mulhern, R. Effect of Community Water Service on Lead in Drinking Water in an Environmental Justice Community. Environ. Sci. Technol. 2024, 58, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Maneeprakorn, W.; Tumcharern, G.; Bamrungsap, S.; Chansaenpak, K.; Segkhoonthod, K.; Rattanabut, C.; Karn-orachai, K.; Ngamaroonchote, A.; Sangkaew, P.; Wongsuwan, P.; et al. Addressing Water Contamination and Associated Health Issues through Community-Based Interventions: A Case Study in Khon Kaen Province. Int. J. Environ. Res. Public Health 2024, 21, 729. [Google Scholar] [CrossRef] [PubMed]
- Leap, S.R.; Soled, D.R.; Sampath, V.; Nadeau, K.C. Effects of Extreme Weather on Health in Underserved Communities. Ann. Allergy Asthma Immunol. 2024, 133, 20–27. [Google Scholar] [CrossRef]
- Putsoane, T.; Bhanye, J.I.; Matamanda, A. Chapter 11—Extreme Weather Events and Health Inequalities: Exploring Vulnerability and Resilience in Marginalized Communities. In Developments in Environmental Science; Sivaramakrishnan, L., Dahiya, B., Sharma, M., Mookherjee, S., Karmakar, R., Eds.; Urban Health; Elsevier: Amsterdam, The Netherlands, 2024; Volume 15, pp. 225–248. [Google Scholar]
- Cantoral, A.; Betanzos-Robledo, L.; Collado-López, S.; García-Martínez, B.A.; Lamadrid-Figueroa, H.; Mariscal-Moreno, R.M.; Díaz-Ruiz, A.; Ríos, C.; Téllez-Rojo, M.M. Lead Levels in the Most Consumed Mexican Foods: First Monitoring Effort. Toxics 2024, 12, 318. [Google Scholar] [CrossRef]
- Córdoba-Gamboa, L.; Vázquez-Salas, R.A.; Romero-Martínez, M.; Cantoral, A.; Riojas-Rodríguez, H.; Bautista-Arredondo, S.; Bautista-Arredondo, L.F.; de Castro, F.; Tamayo-Ortiz, M.; Téllez-Rojo, M.M. Lead Exposure Can Affect Early Childhood Development and Could Be Aggravated by Stunted Growth: Perspectives from Mexico. Int. J. Environ. Res. Public Health 2023, 20, 5174. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Wang, B.; Ma, J.; Fan, D.; Sun, C.; He, B.; Wei, F.; Jiang, G. Levels and Source Apportionment of Children’s Lead Exposure: Could Urinary Lead Be Used to Identify the Levels and Sources of Children’s Lead Pollution? Environ. Pollut. 2015, 199, 18–25. [Google Scholar] [CrossRef]
- Shotyk, W.; Krachler, M. Lead in Bottled Waters: Contamination from Glass and Comparison with Pristine Groundwater. Environ. Sci. Technol. 2007, 41, 3508–3513. [Google Scholar] [CrossRef]
- Wang, T.; Kim, J.; Whelton, A.J. Management of Plastic Bottle and Filter Waste during the Large-Scale Flint Michigan Lead Contaminated Drinking Water Incident. Resour. Conserv. Recycl. 2019, 140, 115–124. [Google Scholar] [CrossRef]
- Kumar, A.; Pastore, P. Lead and Cadmium in Soft Plastic Toys. Curr. Sci. 2007, 93, 818–822. [Google Scholar]
- Zeng, X.; Liu, D.; Wu, Y.; Zhang, L.; Chen, R.; Li, R.; Gu, W.; Zhang, L.; Liu, C.; Sun, Q. Heavy Metal Risk of Disposable Food Containers on Human Health. Ecotoxicol. Environ. Saf. 2023, 255, 114797. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Sosa, A.; Bares, C.; Battocletti, A.; Moll, M.J.; Pose, D.; Laborde, A.; González, H.; Feola, G. E-Waste Informal Recycling: An Emerging Source of Lead Exposure in South America. Ann. Glob. Health 2016, 82, 197–201. [Google Scholar] [CrossRef]
- Cittadino, A.; Ocello, N.; Majul, M.V.; Ajhuacho, R.; Dietrich, P.; Igarzabal, M.A. Heavy Metal Pollution and Health Risk Assessment of Soils from Open Dumps in the Metropolitan Area of Buenos Aires, Argentina. Environ. Monit. Assess. 2020, 192, 291. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.M.; Tavares, T.M.; Lins, L. Soil Contamination by a Lead Smelter in Brazil in the View of the Local Residents. Int. J. Environ. Res. Public Health 2018, 15, 2166. [Google Scholar] [CrossRef]
- Anticona, C.; Bergdahl, I.A.; Lundh, T.; Alegre, Y.; Sebastian, M.S. Lead Exposure in Indigenous Communities of the Amazon Basin, Peru. Int. J. Hyg. Environ. Health 2011, 215, 59–63. [Google Scholar] [CrossRef]
- Tume, P.; Barrueto, K.; Olguin, M.; Torres, J.; Cifuentes, J.; Ferraro, F.X.; Roca, N.; Bech, J.; Cornejo, O. The Influence of the Industrial Area on the Pollution Outside Its Borders: A Case Study from Quintero and Puchuncavi Districts, Chile. Environ. Geochem. Health 2020, 42, 2557–2572. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Samms-Vaughan, M.; Dickerson, A.S.; Loveland, K.A.; Ardjomand-Hessabi, M.; Bressler, J.; Shakespeare-Pellington, S.; Grove, M.L.; Boerwinkle, E. Factors Associated with Blood Lead Concentrations of Children in Jamaica. J. Environ. Sci. Health Part A 2015, 50, 529–539. [Google Scholar]
- Abduro Ogo, H.; Tang, N.; Li, X.; Gao, X.; Xing, W. Combined Toxicity of Microplastic and Lead on Submerged Macrophytes. Chemosphere 2022, 295, 133956. [Google Scholar] [CrossRef]
- Li, S.; Cao, L.; Liu, Q.; Sui, S.; Bian, J.; Zhao, X.; Gao, Y. Enhancing Pb Adsorption on Crushed Microplastics: Insights into the Environmental Remediation. Water 2024, 16, 3541. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H. Relationship between Lead Concentration in Maternal and Umbilical Cord Blood and Some Neonatal Outcomes in Primiparous Opium-Dependent Mothers in Zahedan, Southeast of Iran in 2022. BMC Pregnancy Childbirth 2023, 23, 753. [Google Scholar] [CrossRef] [PubMed]
- Anil, L.; Ma, Z.; Nambiar, A.; Watkins, S.M. Blood Lead Level Testing and Retesting Among Newly Arriving Refugee Children, Pennsylvania, 2015–2019. Am. J. Public Health 2022, 112, S706–S714. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Tan, J.; Tan, J.; Liu, C.; Yang, P.; Xian, Y.; Wang, Q. Exposure of Reproductive-Aged Women to Multiple Metals and Its Associations with Unexplained Recurrent Miscarriage. Toxics 2023, 11, 830. [Google Scholar] [CrossRef]
- Dickerson, A.S.; Schmidt, R.J. Invited Perspective: Protect and Serve—The Potential Role of Folate in Lead Risk Reduction. Environ. Health Perspect. 2024, 132, 101302. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, R.I.; Craina, M.; Dahma, G.; Popescu, A.V.; Erimescu, A.G.; Citu, I.; Dobrescu, A.; Horhat, F.G.; Vulcanescu, D.D.; Gorun, F.; et al. Heavy Metal Ion Concentration in the Amniotic Fluid of Preterm and Term Pregnancies from Two Cities with Different Industrial Output. Exp. Ther. Med. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Reuben, A.; Schaefer, J.D.; Moffitt, T.E.; Broadbent, J.; Harrington, H.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Caspi, A. Association of Childhood Lead Exposure With Adult Personality Traits and Lifelong Mental Health. JAMA Psychiatry 2019, 76, 418–425. [Google Scholar] [CrossRef]
- LeBrón, A.M.W.; Torres, I.R.; Valencia, E.; Dominguez, M.L.; Garcia-Sanchez, D.G.; Logue, M.D.; Wu, J. The State of Public Health Lead Policies: Implications for Urban Health Inequities and Recommendations for Health Equity. Int. J. Environ. Res. Public Health 2019, 16, 1064. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.T.; Betts, S.; Kan, E.C.; McConnell, R.; Lanphear, B.P.; Sowell, E.R. Association of Lead-Exposure Risk and Family Income with Childhood Brain Outcomes. Nat. Med. 2020, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.G.; Specht, A.; Punshon, T.; Jackson, B.P.; Bidlack, F.B.; Bakalar, C.A.; Mukherjee, R.; Davis, M.; Steadman, D.W.; Weisskopf, M.G. Lead Exposure across the Life Course and Age at Death. Sci. Total Environ. 2024, 927, 171975. [Google Scholar] [CrossRef]
- McFarland, M.J.; Hauer, M.E.; Reuben, A. Half of US Population Exposed to Adverse Lead Levels in Early Childhood. Proc. Natl. Acad. Sci. USA 2022, 119, e2118631119. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th Anniversary: Update 2025. Nucleic Acids Res. 2024, 53, D1328–D1334. [Google Scholar] [CrossRef]
- Kataba, A.; Yohannes, Y.B.; Nakata, H.; Yabe, J.; Toyomaki, H.; Muzandu, K.; Zyambo, G.; Ikenaka, Y.; Choongo, K.; Ishizuka, M.; et al. Association between Chronic Environmental Lead (Pb) Exposure and Cytokines in Males and Females of Reproductive Age from Kabwe, Zambia. Int. J. Environ. Res. Public Health 2023, 20, 5596. [Google Scholar] [CrossRef] [PubMed]
- Bemelmans, M.H.A.; Tits, L.J.H.v.; Buurman, W.A. Tumor Necrosis Factor: Function, Release and Clearance. Crit. Rev. Immunol. 2017, 37, 249–259. [Google Scholar] [CrossRef]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef]
- Valentino, M.; Rapisarda, V.; Santarelli, L.; Bracci, M.; Scorcelletti, M.; Di Lorenzo, L.; Cassano, F.; Soleo, L. Effect of Lead on the Levels of Some Immunoregulatory Cytokines in Occupationally Exposed Workers. Hum. Exp. Toxicol. 2007, 26, 551–556. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider Roles in Cancer Progression and Prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef]
- Liu, H.; Shi, L.; Giesy, J.P.; Yu, H. Polychlorinated Diphenyl Sulfides Can Induce ROS and Genotoxicity via the AhR-CYP1A1 Pathway. Chemosphere 2019, 223, 165–170. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; Elbekai, R.H.; El-Kadi, A.O. Regulation of CYP1A1 by Heavy Metals and Consequences for Drug Metabolism. Expert Opin. Drug Metab. Toxicol. 2009, 5, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Korashy, H.M.; El-Kadi, A.O.S. The Role of Redox-Sensitive Transcription Factors NF-κB and AP-1 in the Modulation of the Cyp1a1 Gene by Mercury, Lead, and Copper. Free Radic. Biol. Med. 2008, 44, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Žák, A. Human Catalase, Its Polymorphisms, Regulation and Changes of Its Activity in Different Diseases. Folia Biol. 2014, 60, 153–167. [Google Scholar] [CrossRef]
- Kasperczyk, S.; Birkner, E.; Kasperczyk, A.; Zalejska-Fiolka, J. Activity of Superoxide Dismutase and Catalase in People Protractedly Exposed to Lead Compounds. Ann. Agric. Environ. Med. 2004, 11, 291–296. [Google Scholar]
- Corpas, F.J.; Barroso, J.B. Lead-Induced Stress, Which Triggers the Production of Nitric Oxide (NO) and Superoxide Anion (O2·-) in Arabidopsis Peroxisomes, Affects Catalase Activity. Nitric Oxide 2017, 68, 103–110. [Google Scholar] [CrossRef]
- Farmand, F.; Ehdaie, A.; Roberts, C.K.; Sindhu, R.K. Lead-Induced Dysregulation of Superoxide Dismutases, Catalase, Glutathione Peroxidase, and Guanylate Cyclase. Environ. Res. 2005, 98, 33–39. [Google Scholar] [CrossRef]
- Vašák, M. Advances in Metallothionein Structure and Functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef]
- Fernandes, K.C.M.; Martins, A.C., Jr.; Oliveira, A.Á.S.d.; Antunes, L.M.G.; Cólus, I.M.d.S.; Barbosa, F., Jr.; Barcelos, G.R.M. Polymorphism of Metallothionein 2A Modifies Lead Body Burden in Workers Chronically Exposed to the Metal. Public Health Genomics 2015, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yin, Z.; Yuan, G.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Liang, X.; et al. Quantification of Metallothionein on the Liver and Kidney of Rats by Subchronic Lead and Cadmium in Combination. Environ. Toxicol. Pharmacol. 2013, 36, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Qader, A.; Rehman, K.; Akash, M.S.H. Genetic Susceptibility of δ-ALAD Associated with Lead (Pb) Intoxication: Sources of Exposure, Preventive Measures, and Treatment Interventions. Environ. Sci. Pollut. Res. 2021, 28, 44818–44832. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, Y.B.; Nakayama, S.M.M.; Yabe, J.; Nakata, H.; Toyomaki, H.; Kataba, A.; Muzandu, K.; Ikenaka, Y.; Choongo, K.; Ishizuka, M. Blood Lead Levels and Aberrant DNA Methylation of the ALAD and P16 Gene Promoters in Children Exposed to Environmental-Lead. Environ. Res. 2020, 188, 109759. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, Function, and Biotechnological Aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Bihaqi, S.W.; Alansi, B.; Masoud, A.M.; Mushtaq, F.; Subaiea, G.M.; Zawia, N.H. Influence of Early Life Lead (Pb) Exposure on α-Synuclein, GSK-3β and Caspase-3 Mediated Tauopathy: Implications on Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 1114–1122. [Google Scholar] [CrossRef]
- Inesta-Vaquera, F.; Navasumrit, P.; Henderson, C.J.; Frangova, T.G.; Honda, T.; Dinkova-Kostova, A.T.; Ruchirawat, M.; Wolf, C.R. Application of the in Vivo Oxidative Stress Reporter Hmox1 as Mechanistic Biomarker of Arsenic Toxicity. Environ. Pollut. 2021, 270, 116053. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Cho, Y.; Bae, H.-G.; Okun, E.; Arumugam, T.V.; Jo, D.-G. Physiology and Pharmacology of Amyloid Precursor Protein. Pharmacol. Ther. 2022, 235, 108122. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, L.J.M.; Murumulla, L.; Bindu Lasya, C.; Krishna Prasad, D.; Challa, S. Exposure of Combination of Environmental Pollutant, Lead (Pb) and β-Amyloid Peptides Causes Mitochondrial Dysfunction and Oxidative Stress in Human Neuronal Cells. J. Bioenerg. Biomembr. 2023, 55, 79–89. [Google Scholar] [CrossRef]
- Garg, P.; Srivastava, N.; Srivastava, P. Comparative Investigation to Analyse the Critical Role of NFE2L2 Gene in Heavy Metal Induced Toxicity through in Silico Approaches. Environ. Health Eng. Manag. J. 2022, 9, 33–40. [Google Scholar] [CrossRef]
- Gujral, P.; Orozco-Alonso, E.; Saliba, J.; Yan, X.; Blank, V. The NFE2L2 (NRF2) Transcription Factor Controls Genes Involved in the Oxidative Stress Response and Inflammation in Myometrial Cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2025, 1872, 119985. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Xiao, W.; Yan, N. Unravelling the Role of NFE2L1 in Stress Responses and Related Diseases. Redox Biol. 2023, 65, 102819. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, H.; Song, L.; Cen, M.; Wu, J. Association of Urinary Heavy Metals Co-Exposure and Adult Depression: Modification of Physical Activity. NeuroToxicology 2023, 95, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.S.; Lamadrid-Figueroa, H.; Téllez-Rojo, M.M.; Mercado-García, A.; Peterson, K.E.; Schwartz, J.; Hu, H.; Hernández-Avila, M. Effect of Calcium Supplementation on Blood Lead Levels in Pregnancy: A Randomized Placebo-Controlled Trial. Environ. Health Perspect. 2009, 117, 26–31. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Muthayya, S.; Moretti, D.; Kurpad, A.; Hurrell, R.F. Iron Fortification Reduces Blood Lead Levels in Children in Bangalore, India. Pediatrics 2006, 117, 2014–2021. [Google Scholar] [CrossRef]
- Bautista-Arredondo, L.F.; Trejo-Valdivia, B.; Estrada-Sánchez, D.; Tamayo-Ortiz, M.; Cantoral, A.; Figueroa, J.L.; Romero-Martínez, M.; Gómez-Acosta, L.M.; Cuevas-Nasu, L.; Tellez-Rojo, M.M. Intoxicación infantil por plomo en México: Otras fuentes de exposición más allá del barro vidriado (Ensanut 2022). Salud Pública México 2023, 65, s197–s203. [Google Scholar] [CrossRef] [PubMed]
- Counter, S.A.; Buchanan, L.H.; Ortega, F. Blood Lead Levels in Andean Infants and Young Children in Ecuador: An International Comparison. J. Toxicol. Environ. Health A 2015, 78, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Teye, S.O.; Yanosky, J.D.; Cuffee, Y.; Weng, X.; Luquis, R.; Farace, E.; Wang, L. Exploring Persistent Racial/Ethnic Disparities in Lead Exposure among American Children Aged 1–5 Years: Results from NHANES 1999–2016. Int. Arch. Occup. Environ. Health 2021, 94, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, W.; Zhong, Y.; Jiang, X.; Mei, H.; Chang, Y.; Wu, D.; Dou, J.; Vasquez, E.; Shi, X.; et al. Effects of Chronic Low-Level Lead (Pb) Exposure on Cognitive Function and Hippocampal Neuronal Ferroptosis: An Integrative Approach Using Bioinformatics Analysis, Machine Learning, and Experimental Validation. Sci. Total Environ. 2024, 917, 170317. [Google Scholar] [CrossRef]
| Category | Effects on Pregnant Woman | Effects on Fetus or Newborn | Reference(s) |
|---|---|---|---|
| General Exposure |
|
| [3,9,69] |
| Toxicokinetics and Mechanisms |
|
| [9,68,69] |
| Maternal Symptoms |
| - | [9] |
| Pregnancy Complications |
| - | [3] |
| Neurotoxicity and Fetal Development | - |
| [9,68,69] |
| Perinatal Outcomes | - |
| [66,68,69] |
| Environmental Risk Factors |
| - | [9] |
| Maternal–Cord Blood Correlation | - |
| [9] |
| Disease | Genes | Inference Score |
|---|---|---|
| Liver Cirrhosis, Experimental | 198 | 176.43 |
| Prostatic Neoplasms | 174 | 150.46 |
| Breast Neoplasms | 158 | 106.5 |
| Carcinoma, Hepatocellular | 153 | 128.36 |
| Chemical and Drug-Induced Liver Injury | 120 | 11.42 |
| Lung Neoplasms | 93 | 59.95 |
| Stomach Neoplasms | 92 | 80.07 |
| Autistic Disorder | 89 | 98.15 |
| Colorectal Neoplasms | 89 | 82.63 |
| Hypertension | 82 | 16.85 |
| Autism Spectrum Disorder | 78 | 66.49 |
| Disease Progression | 77 | 90.5 |
| Disease Models, Animal | 72 | 43.52 |
| Neoplasm Metastasis | 70 | 46.09 |
| Neoplasm Invasiveness | 69 | 45.73 |
| Carcinoma | 66 | 65.18 |
| Obesity | 66 | 51.11 |
| Pathway | Corrected p-Value | Annotated Genes |
|---|---|---|
| Metabolism | 2.53 × 10−187 | 489 |
| Immune System | 9.66 × 10−180 | 474 |
| Gene Expression | 9.79 × 10−145 | 399 |
| Signal Transduction | 2.20 × 10−141 | 473 |
| Protein Metabolism | 1.77 × 10−129 | 357 |
| Innate Immune System | 8.33 × 10−121 | 309 |
| Disease | 4.07 × 10−104 | 235 |
| Developmental Biology | 1.21 × 10−99 | 257 |
| Hemostasis | 5.93 × 10−96 | 196 |
| Metabolic Pathways | 1.94 × 10−83 | 258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Mendoza, B.M.; Garibaldi-Ríos, A.F.; Rizo De La Torre, L.D.C.; Puebla-Pérez, A.M.; Figuera, L.E.; Zúñiga-González, G.M.; Gómez-Meda, B.C.; Gutiérrez-Hurtado, I.A.; Scott-López, E.H.; Vázquez-González, V.; et al. Lead Poisoning in the Americas: Sources, Regulations, Health Impacts, and Molecular Mechanisms. J. Xenobiot. 2025, 15, 134. https://doi.org/10.3390/jox15040134
Torres-Mendoza BM, Garibaldi-Ríos AF, Rizo De La Torre LDC, Puebla-Pérez AM, Figuera LE, Zúñiga-González GM, Gómez-Meda BC, Gutiérrez-Hurtado IA, Scott-López EH, Vázquez-González V, et al. Lead Poisoning in the Americas: Sources, Regulations, Health Impacts, and Molecular Mechanisms. Journal of Xenobiotics. 2025; 15(4):134. https://doi.org/10.3390/jox15040134
Chicago/Turabian StyleTorres-Mendoza, Blanca Miriam, Asbiel Felipe Garibaldi-Ríos, Lourdes Del Carmen Rizo De La Torre, Ana María Puebla-Pérez, Luis E. Figuera, Guillermo Moisés Zúñiga-González, Belinda Claudia Gómez-Meda, Itzae Adonai Gutiérrez-Hurtado, Elvia Harumi Scott-López, Verónica Vázquez-González, and et al. 2025. "Lead Poisoning in the Americas: Sources, Regulations, Health Impacts, and Molecular Mechanisms" Journal of Xenobiotics 15, no. 4: 134. https://doi.org/10.3390/jox15040134
APA StyleTorres-Mendoza, B. M., Garibaldi-Ríos, A. F., Rizo De La Torre, L. D. C., Puebla-Pérez, A. M., Figuera, L. E., Zúñiga-González, G. M., Gómez-Meda, B. C., Gutiérrez-Hurtado, I. A., Scott-López, E. H., Vázquez-González, V., Gazcón-Rivas, C. P., & Gallegos-Arreola, M. P. (2025). Lead Poisoning in the Americas: Sources, Regulations, Health Impacts, and Molecular Mechanisms. Journal of Xenobiotics, 15(4), 134. https://doi.org/10.3390/jox15040134








