Virgin Coconut Oil and Its Lauric Acid, Between Anticancer Activity and Modulation of Chemotherapy Toxicity: A Review
Abstract
1. Introduction
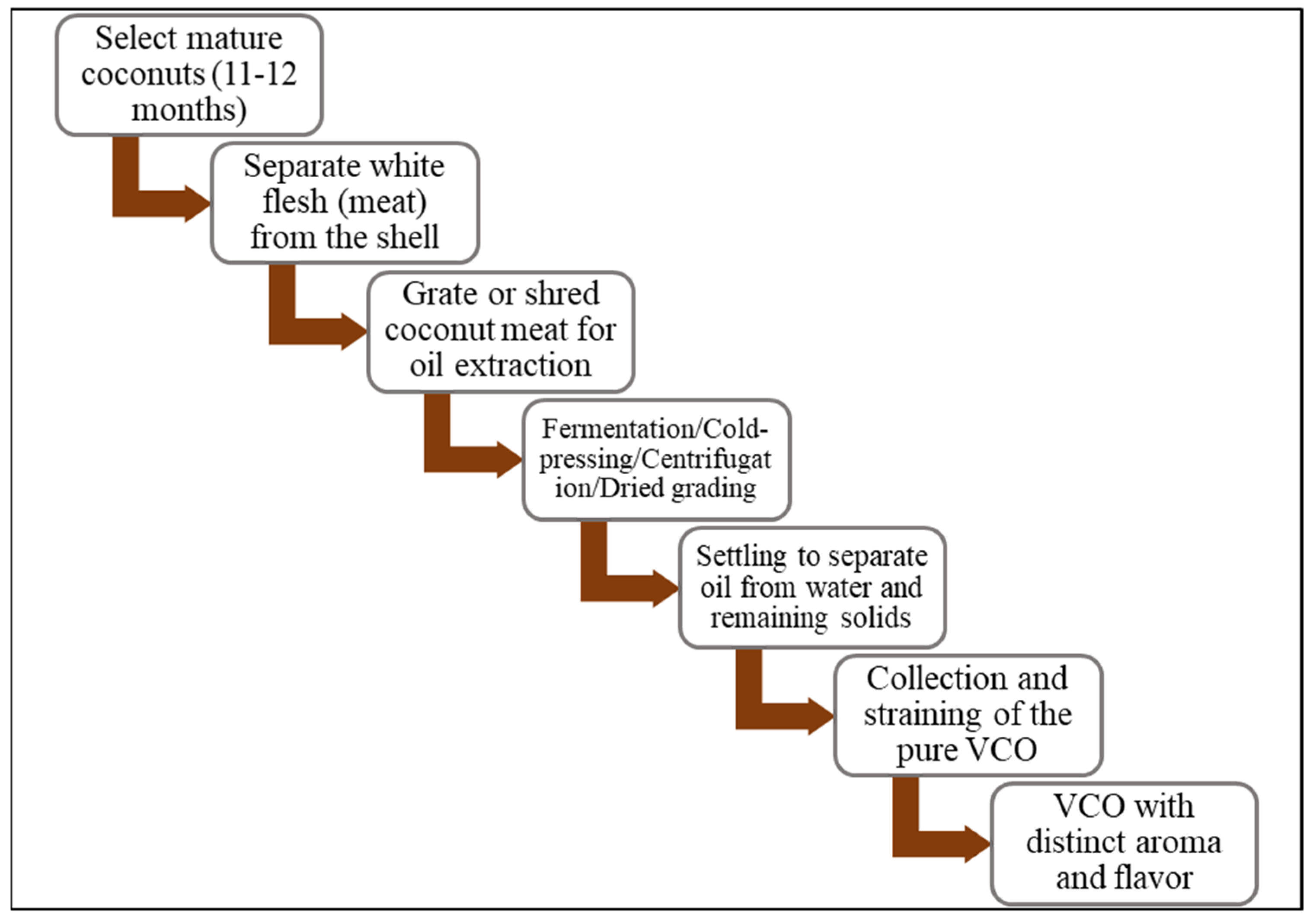
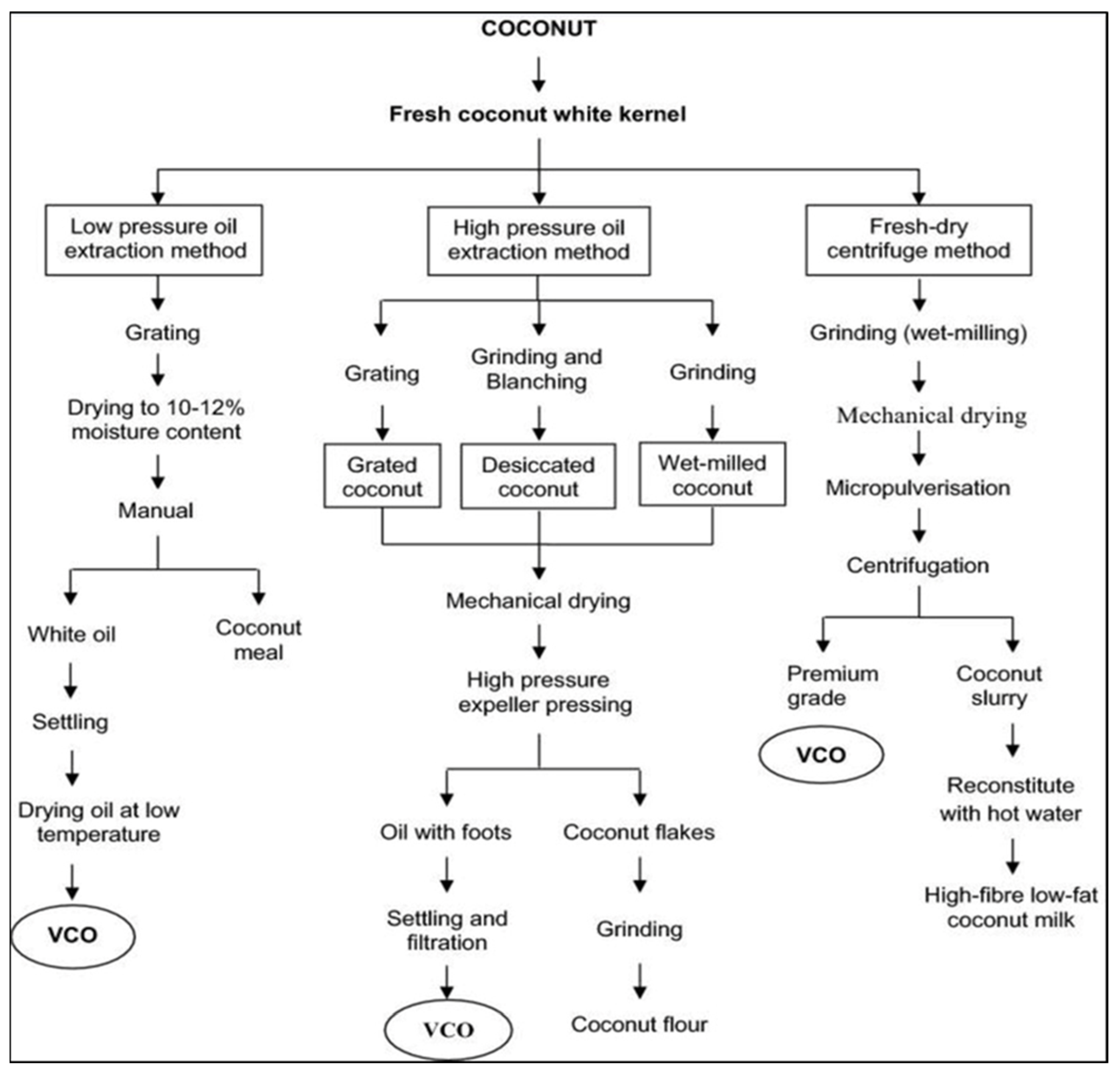
2. Extraction and Composition of VCO
2.1. Extraction of VCO (Wet Process)
2.2. Extraction of Copra Coconut Oil (Dry Process)
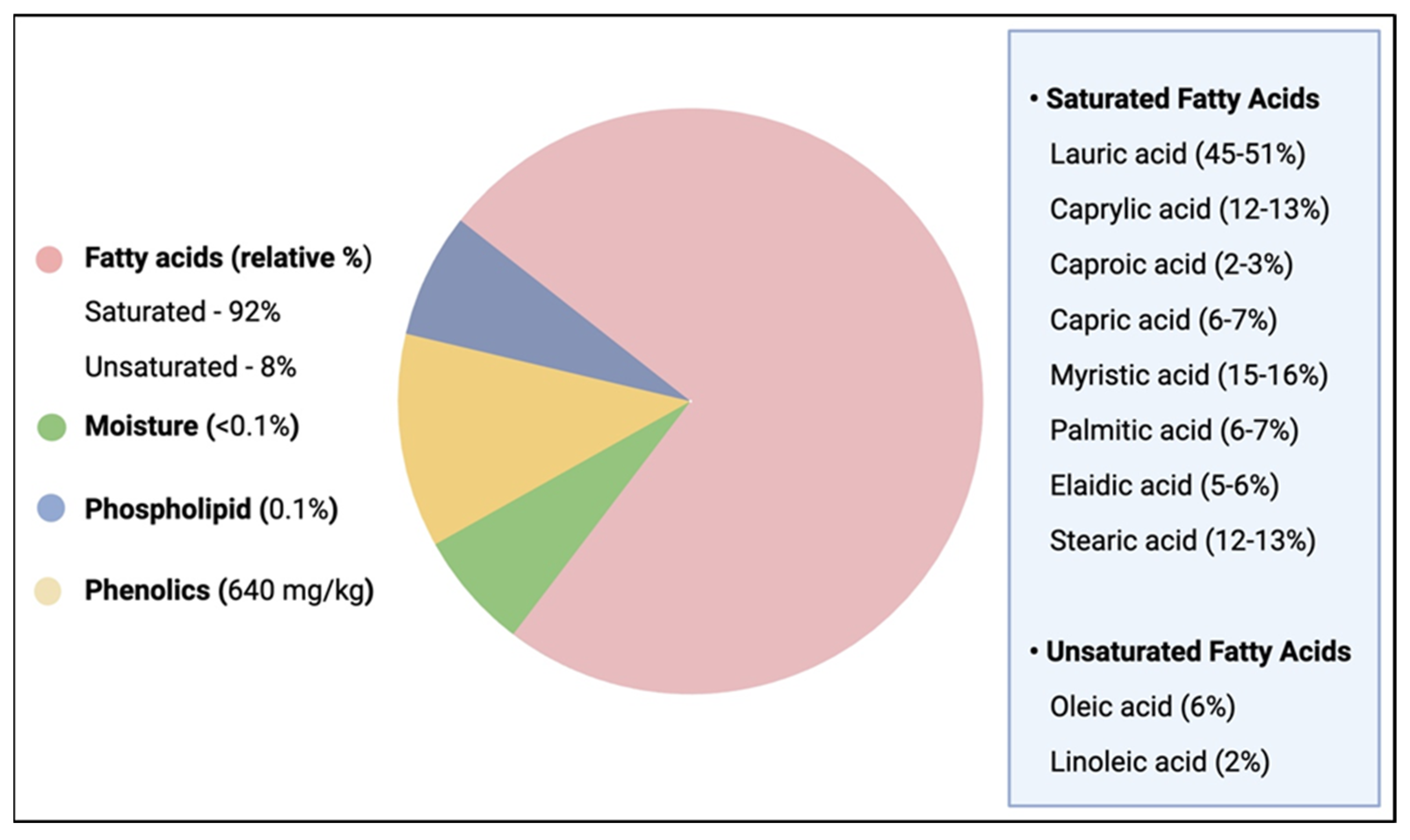
2.3. Composition of VCO
3. Health Benefits of VCO and LA
3.1. VCO Health Benefits
3.2. LA Health Benefits
4. Anticancer Effects of VCO and LA
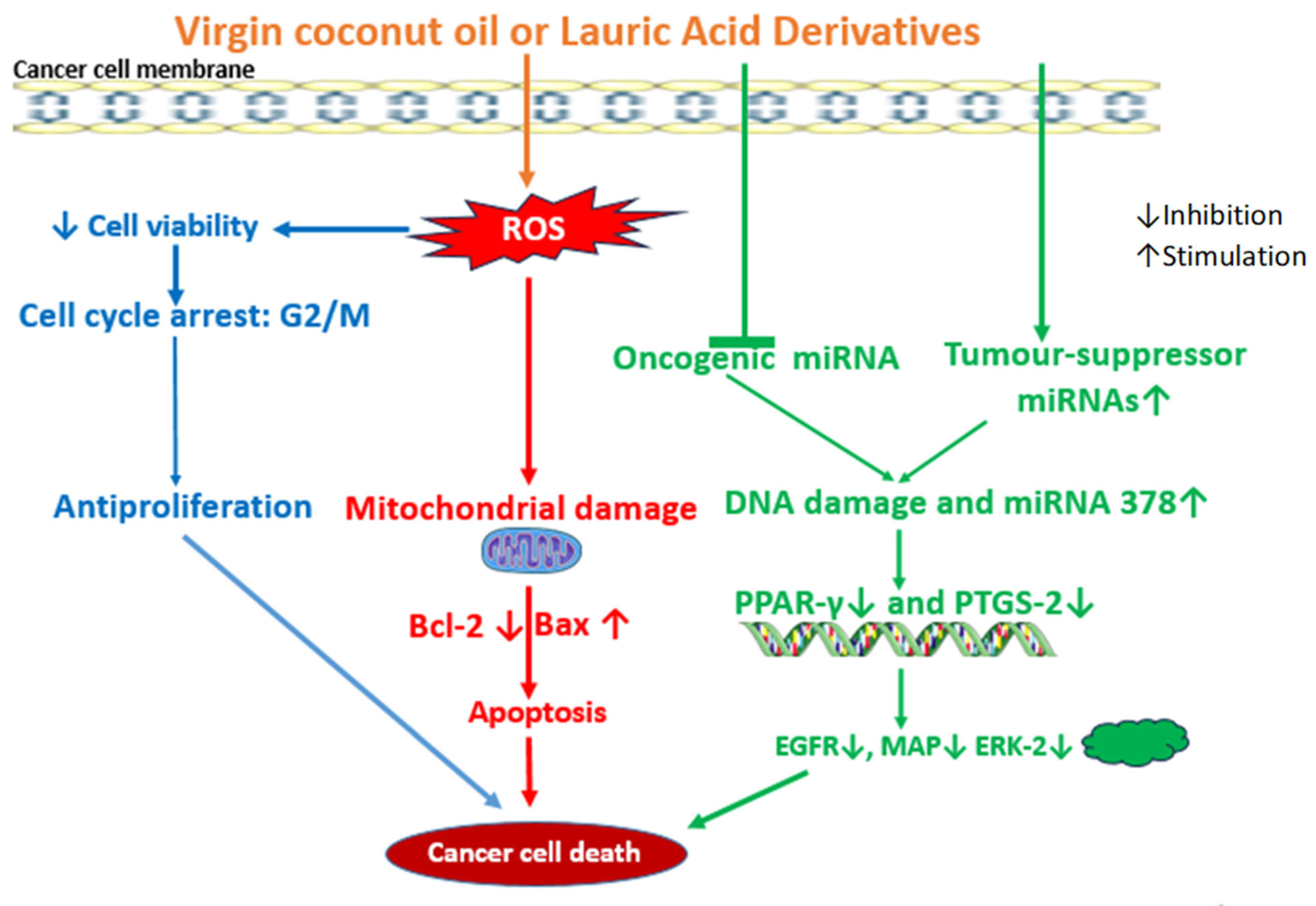
4.1. Anticancer Effects of VCO
4.2. Anticancer Effect of LA
LA-Based Nanocarriers for Therapeutic Delivery
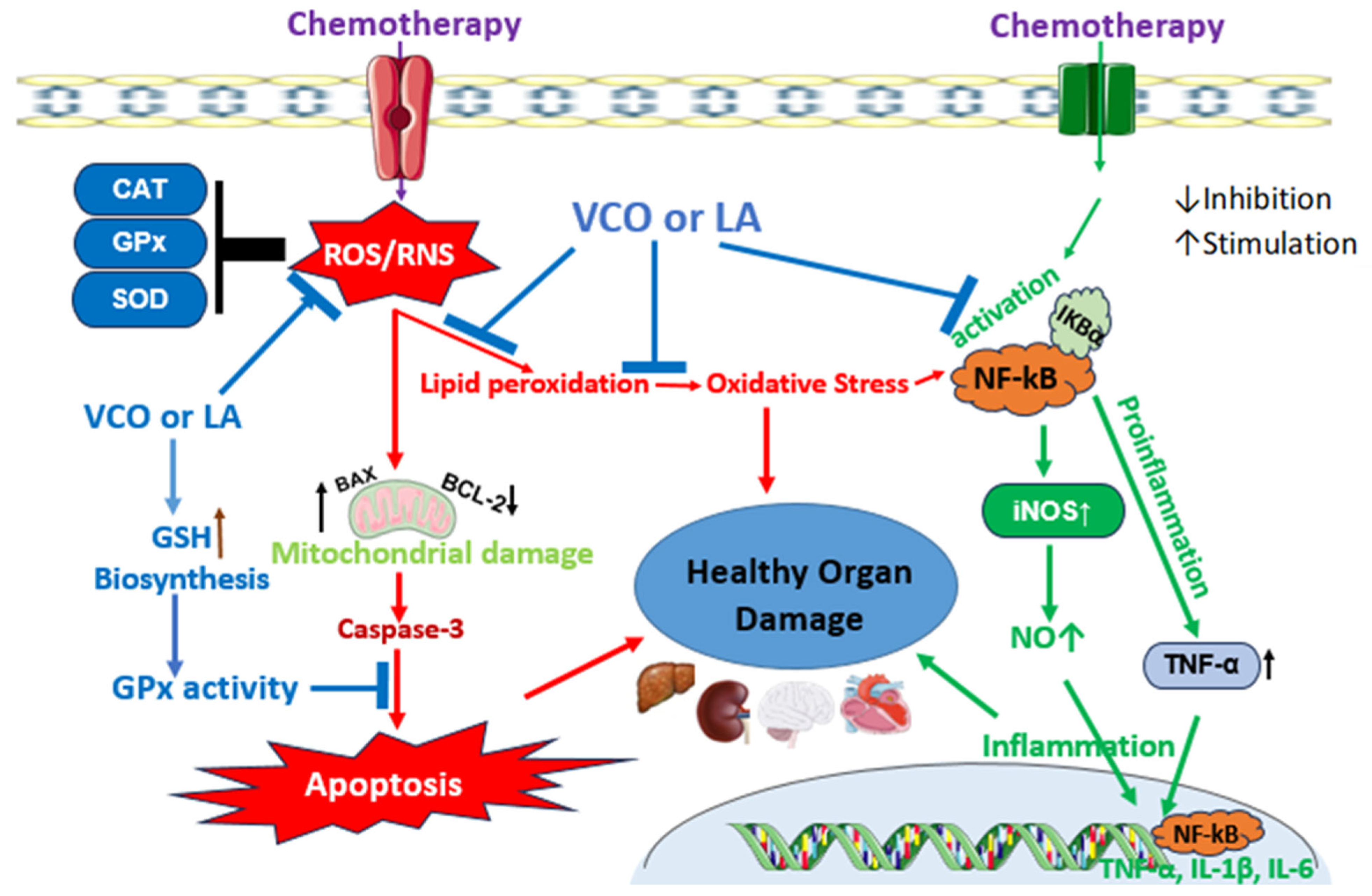
5. VCO and LA: Protection Against Chemotherapy Toxicity
5.1. VCO Protects Against Chemotherapy Toxicity
5.2. LA Protects Against Chemotherapy Toxicity
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. A to Z List of Cancer Drugs. 2023. Available online: https://www.cancer.gov/about-cancer/treatment/drugs (accessed on 16 July 2025).
- Vijayakumar, S.; Dhakshanamoorthy, R.; Baskaran, A.; Krishnan, B.S.; Maddaly, R. Drug resistance in human cancers—Mechanisms and implications. Life Sci. 2024, 352, 122907. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Famurewa, A.C.; Akash, A.; Othman, E.M. The therapeutic mechanisms of honey in mitigating toxicity from anticancer chemotherapy toxicity: A Review. J. Xenobiotics 2024, 14, 1109–1129. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, G.; Quintas-Granados, L.I.; Reyes-Hernández, O.D.; Caballero-Florán, I.H.; Peña-Corona, S.I.; Cortés, H.; Leyva-Gómez, G.; Habtemariam, S.; Sharifi-Rad, J. Review of the anticancer properties of 6-shogaol: Mechanisms of action in cancer cells and future research opportunities. Food Sci. Nutr. 2024, 12, 4513–4533. [Google Scholar] [CrossRef] [PubMed]
- Cheon, C.; Ko, S.-G. Synergistic effects of natural products in combination with anticancer agents in prostate cancer: A scoping review. Front. Pharmacol. 2022, 13, 963317. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Ramya, V.; Shyam, K.P.; Kowsalya, E.; Balavigneswaran, C.K.; Kadalmani, B. Dual roles of coconut oil and its major component lauric acid on redox nexus: Focus on cytoprotection and cancer cell death. Front. Neurosci. 2022, 16, 833630. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Aja, P.M.; Nwankwo, O.E.; Awoke, J.N.; Maduagwuna, E.K.; Aloke, K. Moringa oleifera seed oil or virgin coconut oil supplementation abrogates cerebral neurotoxicity induced by antineoplastic agent methotrexate by suppression of oxidative stress and neuroinflammation in rats. J. Food Biochem. 2019, 43, e12748. [Google Scholar]
- Boemeke, L.; Marcadenti, A.; Busnello, F.M.; Gottschall, C.B. Effects of Coconut Oil on Human Health. Open J. Endocr. Metab. Dis. 2015, 5, 84–87. [Google Scholar] [CrossRef]
- Dumancas, D.D.; Viswanath, L.C.; de Leon, A.R.; Ramasahayam, S.; Maples, R.; Koralege, R.H.; Perera, U.D.; Langford, J.; Shakir, A.; Castles, S. Health benefits of virgin coconut oil. In Vegetable Oil: Properties, Uses, and Benefits; Holt, B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016. [Google Scholar]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef]
- GopalaKrishna, A.G.; Gaurav, R.; Bhatnagar, A.S.; Prasanth Kumar, P.K.; Chandrashekar, P. Coconut Oil: Chemistry, Production and Its Applications—A Review. Indian Coconut J. 2010, 15–27. [Google Scholar]
- Kardinasari, E.; Devriany, A. Phytochemical identification of bangka origin virgin green coconut oil: Anti-inflammatory and anti-bacterial potential. Enferm. Clin. 2020, 30, 171–174. [Google Scholar] [CrossRef]
- Tham, Y.Y.; Choo, Q.C.; Muhammad, T.S.; Chew, C.H. Lauric acid alleviates insulin resistance by improving mitochondrial biogenesis in THP-1 macrophages. Mol. Biol. Rep. 2020, 47, 9595–9607. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Ugwu-Ejezie, C.S.; Iyare, E.E.; Folawiyo, A.M.; Maduagwuna, E.K.; Ejezie, F.E. Hepatoprotective effect of polyphenols isolated from virgin coconut oil against sub-chronic cadmium hepatotoxicity in rats is associated with improvement in antioxidant defense system. Drug Chem. Toxicol. 2021, 44, 418–426. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Maduagwuna, E.K.; Folawiyo, A.M.; Besong, E.E.; Eteudo, A.N.; Famurewa, O.A.; Ejezie, F.E. Antioxidant, anti-inflammatory and antiapoptotic effects of virgin coconut oil against antibiotic drug gentamicin-induced nephrotoxicity via suppression of oxidative stress and modulation of iNOS/NF-ĸB/caspase-3 signalling pathway in Wistar rats. J. Food Biochem. 2020, 44, e13100. [Google Scholar] [CrossRef]
- Nair, S.S.; Manalil, J.J.; Ramavarma, S.K.; Suseela, I.M.; Thekkepatt, A.; Raghavamenon, A.C. Virgin coconut oil supplementation ameliorates cyclophosphamide-induced systemic toxicity in mice. Hum. Exp. Toxicol. 2016, 35, 205–212. [Google Scholar] [CrossRef]
- Otuechere, C.A.; Madarikan, G.; Simisola, T.; Bankole, O.; Osho, A. Virgin coconut oil protects against liver damage in albino rats challenged with anti-folate combination, trimethoprim-sulfamethoxazole. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 249–253. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, A.; Wu, Y.; Wu, L.; Zhang, L. Silymarin in cancer therapy: Mechanisms of action, protective roles in chemotherapy-induced toxicity, and nanoformulations. J. Funct. Food 2023, 100, 105384. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Jia, K.; Kong, L. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Aja, P.M.; Maduagwuna, E.K.; Ekeleme-Egedigwe, C.A.; Ufebe, O.G.; Azubuike-Osu, S.O. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed. Pharmacother. 2017, 96, 905–911. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Ufebe, O.G.; Egedigwe, C.A.; Nwankwo, O.E.; Obaje, G.S. Virgin coconut oil supplementation attenuates acute chemotherapy hepatotoxicity induced by anticancer drug methotrexate via inhibition of oxidative stress in rats. Biomed. Pharmacother. 2017, 87, 437–442. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Akhigbe, R.E.; George, M.Y.; Adekunle, Y.A.; Oyedokun, P.A.; Akhigbe, T.M.; Fatokun, A.A. Mechanisms of ferroptotic and non-ferroptotic organ toxicity of chemotherapy: Protective and therapeutic effects of ginger, 6-gingerol and zingerone in preclinical studies. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 398, 4747–4778. [Google Scholar] [CrossRef]
- Ezzat, A.; Salem, F.E.; Kassaba, R.B.; Abdel Moneim, A.E.; El-Yamany, N.A. Evaluating the potential protective effect of virgin coconut oil against doxorubicin-mediated hepatotoxicity in rats. Adv. Basic Appl. Sci. 2023, 1, 46–54. [Google Scholar]
- Chiamaka, E.A.; Uchechukwu, E.J.; Chioma, D.S.; Asonye, R.; Chijioke, O.K.; Chiemela, D.C. Impact of virgin coconut oil and carvedilol on neurobehavior, apoptotic and inflammatory brain markers in doxorubicin treated mice. Path Sci. 2023, 9, 4020–4049. [Google Scholar] [CrossRef]
- Jayathilaka, N.; Seneviratne, K.N. Phenolic antioxidants in coconut oil: Factors affecting the quantity and quality. A review. Grasas Aceites 2022, 73, e466. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Z.; Xu, Z.; Ye, B.; Li, M.; Zheng, Q.; Ma, X.; Shi, P. Antiproliferative and apoptotic activity of gemcitabine-lauric acid conjugate on human bladder cancer cells. Iran. J. Basic Med. Sci. 2022, 25, 536–542. [Google Scholar]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Jean, N.S.; Mandal, D.; Kumar, A.; Parang, K. Fatty acyl amide derivatives of doxorubicin: Synthesis and in vitro anticancer activities. Eur. J. Med. Chem. 2011, 46, 2037–2042. [Google Scholar] [CrossRef]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
- Villarino, B.; Dy, L.; Lizada, C. Descriptive sensory evaluation of virgin coconut oil and refined, bleached and deodorized coconut oil. LWT-Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Sharma, G.K. Virgin coconut oil as functional oil. In Therapeutic, Probiotic, and Unconventional Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 291–301. [Google Scholar]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.; Steingrimsson, O.; Chormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [CrossRef]
- Zeng, Y.-Q.; He, J.-T.; Hu, B.-Y.; Li, W.; Deng, J.; Lin, Q.-L.; Fang, Y. Virgin coconut oil: A comprehensive review of antioxidant activity and mechanisms contributed by phenolic compounds. Crit. Rev. Food Sci. Nutr. 2024, 64, 1052–1075. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin coconut oil: Emerging functional food oil. Trend Food Sci. Technol. 2009, 20, 481–487. [Google Scholar] [CrossRef]
- Pooi-Pooi, S.; Ali, Y.; Lai, O.M.; Kuan, C.H.; Tang, T.K.; Lee, Y.Y.; Phuah, E.T. Enzymatic and mechanical extraction of virgin coconut oil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900220. [Google Scholar] [CrossRef]
- Oseni, N.T.; Fernando, W.M.; Coorey, R.; Gold, I.; Jayasena, V. Effect of extraction techniques on the quality of coconut oil. Afr. J. Food Sci. 2017, 11, 58–66. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Raghavarao, K.S.M.S. Effect of different treatments for the destabilization of coconut milk emulsion. J Food Eng. 2010, 97, 341–347. [Google Scholar] [CrossRef]
- Bawalan, D.D. Processing Manual for Virgin Coconut Oil, Its Products and By-Products for Pacific Island Countries and Territories; Secretariat of the Pacific Community: Noumea, New Caledonia, 2011. [Google Scholar]
- Srivastava, Y.; Semwal, A.D.; Majumdar, A. Quantitative and qualitative analysis of bioactive components present in virgin coconut oil. Cogent Food Agric. 2016, 2, 1164929. [Google Scholar] [CrossRef]
- Nevin, K.G.; Rajamohan, T. Wet and dry extraction of coconut oil: Impact on lipid metabolic and antioxidant status in cholesterol co-administered rats. Can. J. Physiol. Pharmacol. 2009, 87, 610–616. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Swamy, M.S.L. Hypocholesterimic effects of cold and hot extracted virgin coconut oil (vco) in comparison to commercial coconut oil: Evidence from a male wistar albino rat model. Food Sci. Biotechnol. 2013, 22, 1501–1508. [Google Scholar] [CrossRef]
- Dayrit, F.; Buenafe, O.E.; Chainani, E.; de Vera, I.M.; Dimzon, I.K.; Gonzales, E.; Santos, J.E. Standards for essential composition and quality factors of commercial virgin coconut oil and its differentiation from RBD coconut oil and copra oil. Philipp. J. Sci. 2007, 136, 121–131. [Google Scholar]
- Chatturong, U.; Palang, I.; To-on, K.; Deetud, W.; Chaiwong, S.; Sakulsak, N.; Sonthi, P.; Chanasong, R.; Chulikorn, E.; Kanprakobkit, W.; et al. Reduction of lauric acid content in virgin coconut oil improved plasma lipid profile in high-fat diet-induced hypercholesterolemic mice. J. Food Sci. 2023, 88, 4305–4315. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 2009, 60, 114–123. [Google Scholar] [CrossRef] [PubMed]
- German, G.B.; Dillard, C.J. Saturated fats: What dietary intake? Am. J. Clin. Nutr. 2004, 80, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Garcia, V.V.; Mabesa, R.C.; Tecson-Mendoza, E.M. Comparative physicochemical characteristics of virgin coconut oil produced by different methods. Philipp. Agric. Sci. 2005, 88, 462–475. [Google Scholar]
- Somade, O.T.; Oyinloye, B.E.; Ajiboye, B.O.; Osukoya, O.A. Methyl cellosolve-induced hepatic oxidative stress: The modulatory effect of syringic acid on Nrf2-Keap1-Hmox1-NQO1 signaling pathway in rats. Phytomedicine Plus 2023, 3, 100434. [Google Scholar] [CrossRef]
- Cordeiro, M.L.; Martins, V.G.; Silva, A.P.; Rocha, H.A.; Rachetti, V.P.; Scortecci, K.C. Phenolic acids as antidepressant agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018, 8, e020167. [Google Scholar] [CrossRef]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef]
- Gomes, H.M.; Silveira, A.K.; Gasparotto, J.; Bortolin, R.C.; Terra, S.R.; Brum, P.O.; Gelain, D.P.; Moreira, J.C. Effects of coconut oil long-term supplementation in Wistar rats during metabolic syndrome-regulation of metabolic conditions involving glucose homeostasis, inflammatory signals, and oxidative stress. J. Nutr. Biochem. 2023, 114, 109272. [Google Scholar]
- Jack, K.S.; Asaruddin, M.R.; Bhawani, S.A. Pharmacophore study, molecular docking and molecular dynamic simulation of virgin coconut oil derivatives as anti-inflammatory agent against COX-2. Chem. Biol. Technol. Agric. 2022, 9, 73. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; Dissanayake, M.S. Variation of phenolic content in coconut oil extracted by two conventional methods. Int. J. Food Sci. Technol. 2008, 43, 597–602. [Google Scholar] [CrossRef]
- Vogel, C.É.; Crovesy, L.; Rosado, E.L.; Soares-Mota, M. Effect of coconut oil on weight loss and metabolic parameters in men with obesity: A randomized controlled clinical trial. Food Funct. 2020, 11, 6588–6594. [Google Scholar] [CrossRef]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Aldred, S. Fat supplementation, health, and endurance performance. Nutrition 2004, 20, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Silalahi, J.; Silalahi, Y.C.E. Virgin coconut oil in ketogenic diet. Ind. J. Pharm. Clin. Res. 2022, 5, 36–46. [Google Scholar] [CrossRef]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.B.C.; Manaf, M.A. Medium-chain triacylglycerols. In Nutraceutical and Specialty Lipids and Their Co-Products; Shahidi, F., Ed.; Taylor & Francis Group, LLC: New York, NY, USA, 2006; pp. 27–56. [Google Scholar]
- Jaarin, K.; Norliana, M.; Kamisah, Y.; Nursyafoza, M.; Qodriyah, M.S. Potential role of virgin coconut oil in reducing cardiovascular risk factors. Exp. Clin. Cardiol. 2014, 20, 3399–3410. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Sowah, S.A.; Koulman, A.; Sharp, S.J.; Imamura, F.; Khaw, K.-T.; Forouhi, N.G. Effects of coconut oil, olive oil, and butter on plasma fatty acids and metabolic risk factors: A randomized trial. J. Lipid Res. 2024, 65, 100681. [Google Scholar] [CrossRef]
- Suryani, S.; Sariani, S.; Earnestly, F.; Marganof, M.; Rahmawati, R.; Sevindrajuta, S.; Mahlia, T.M.I.; Fudholi, A. A comparative study of virgin coconut oil, coconut oil and palm oil in terms of their active ingredients. Processes 2020, 8, 402. [Google Scholar] [CrossRef]
- Babu, S.; Arena, R.; Veluswamy, S.K.; Lavie, C. Virgin coconut oil and its potential cardioprotective effects. Postgrad. Med. 2014, 126, 76–83. [Google Scholar] [CrossRef]
- Metin, Z.E.; Bilgic, P.; Tengilimoğlu Metin, M.M.; Akkoca, M. Comparing acute effects of extra virgin coconut oil and extra virgin olive oil consumption on appetite and food intake in normal-weight and obese male subjects. PLoS ONE 2022, 17, e0274663. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Han, X.; Zhou, F.; Guo, J.; Huang, W.; Zhan, J.; You, Y. Coconut oil and medium-chain fatty acids attenuate high-fat diet-induced obesity in mice through increased thermogenesis by activating brown adipose tissue. Front. Nutr. 2022, 9, 896021. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Kasar, G.; Rasal, P.; Mahajan, M.; Upaganlawar, A.; Upasani, C. Virgin coconut oil solubilised curcumin protects nephropathy in diabetic rats. J. Pharm. Res. 2023, 22, 87–92. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Insights into coconut oil’s anti-obesity potential: In vitro modulation of lipidic accumulation, adipolysis, and immune response. Eur. J. Lipid Sci. Technol. 2023, 125, 230003. [Google Scholar] [CrossRef]
- Shetty, S.S.; Roopashree, P.G.; Ramesh, S.V.; Singh, A.; Arivalagan, M.; Manikantan, M.R. Virgin coconut oil (VCO) ameliorates high fat diet (HFD)-induced obesity, dyslipidemia and bestows cardiovascular protection in rats. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 249–259. [Google Scholar] [CrossRef]
- Bhojraj, N.; Shankarguru, G.M.; Bhaskaran, R.M.; Devraj, I.M. Evaluation of antimicrobial efficacy of coconut oil and low-fluoride mouthwashes against Streptococcus mutans in children: A comparative clinicomicrobiological study. World J. Dent. 2022, 13, 562–567. [Google Scholar] [CrossRef]
- de Vasconcelos, M.H.; Tavares, R.L.; Torres Junior, E.U.; Dorand, V.A.; Batista, K.S.; Toscano, L.T.; Silva, A.S.; Cordeiro, A.M.T.d.M.; Meireles, B.R.L.d.A.; Araujo, R.d.S.; et al. Extra virgin coconut oil (Cocos nucifera L.) exerts anti-obesity effect by modulating adiposity and improves hepatic lipid metabolism, leptin and insulin resistance in diet-induced obese rats. J. Funct. Foods 2022, 94, 105122. [Google Scholar] [CrossRef]
- Van, A.; Thi, N. Antibacterial activity of hydrolyzed virgin coconut oil by immobilized lipase. Vietnam J. Sci. Technol. 2018, 54, 227. [Google Scholar] [CrossRef]
- Shino, B.; Peedikayil, F.C.; Jaiprakash, S.R.; Ahmed Bijapur, G.; Kottayi, S.; Jose, D. Comparison of antimicrobial activity of chlorhexidine, coconut oil, probiotics, and ketoconazole on candida albicans isolated in children with early childhood caries: An in vitro study. Scientifica 2016, 2016, 7061587. [Google Scholar] [CrossRef]
- Iranloye, B.; Oludare, G.; Olubiyi, M.; Oludare, G.; Olubiyi, M. Anti-Diabetic and antioxidant effects of virgin coconut oil in alloxan induced diabetic male Sprague Dawley rats. J. Diabetes Mellit. 2013, 3, 221–226. [Google Scholar] [CrossRef][Green Version]
- Vasconcelos, L.H.; Silva, M.C.; Costa, A.C.; de Oliveira, G.A.; de Souza, I.L.; Araujo, R.D.; Alves, A.F.; Cavalcante, F.D.; da Silva, B.A. Virgin coconut oil prevents airway remodeling and recovers tracheal relaxing reactivity by reducing transforming growth factor β expression on asthmatic guinea pig. J. Funct. Foods 2024, 122, 106544. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chuenbarn, T.; Limmatvapirat, S.; Tubtimsri, S. Nanoemulsion-based orodispersible film formulation of guava leaf oil for inhibition of oral cancer cells. Pharmaceutics 2023, 15, 2631. [Google Scholar] [CrossRef] [PubMed]
- Sabra, M.S.; Allam, E.A.; Abd El-Aal, M.; Hassan, N.H.; Mostafa, A.M.; Ahmed, A.A. A novel pharmacological strategy using nanoparticles with glutathione and virgin coconut oil to treat gentamicin-induced acute renal failure in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2025, 398, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Arman, E.; Almahdy, A.; Dafriani, P.; Almasdy, D. Combined effect of topical application of virgin coconut oil (VCO) and black cumin oil (Nigella sativa) on the upregulation of VEGF gene expression and wound healing in diabetic ulcerated rats. Int. J. Appl. Pharm. 2024, 16, 35–40. [Google Scholar] [CrossRef]
- Mansouri, E.; Asghari, S.; Nikooei, P.; Yaseri, M.; Vasheghani-Farahani, A.; Hosseinzadeh-Attar, M.J. Effects of virgin coconut oil consumption on serum brain-derived neurotrophic factor levels and oxidative stress biomarkers in adults with metabolic syndrome: A randomized clinical trial. Nutr. Neurosci. 2024, 27, 487–498. [Google Scholar] [CrossRef]
- Akintoye, O.O.; Ajibare, A.J.; Omotuyi, I.O. irgin coconut oil reverses behavioral phenotypes of letrozole-model of PCOS in Wistar rats via modulation of Nrf2 upregulation. J. Taibah Univ. Med. Sci. 2023, 18, 831–841. [Google Scholar]
- Santos, H.O.; Howell, S.; Teixeira, F.J. Coconut oil as a vehicle for lipophilic drug administration. J. Diabetes Obes. 2019, 6, 8–12. [Google Scholar]
- Tripathi, A.; Kar, S.K.; Shukla, R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 2018, 16, 7–17. [Google Scholar] [CrossRef]
- Aldemh, O.; Al-Nefeiy, F.A.; Bawazir, E. A comparative Study of the Effects of virgin coconut oil and vitamin d on Alzheimer’s disease induced by aluminium chloride in male albino rats hippocampus. Egypt. J. Vet. Sci. 2025, 56, 559–573. [Google Scholar] [CrossRef]
- Thawkar, S.; Kaur, G. Betanin combined with virgin coconut oil inhibits neuroinflammation in aluminum chloride-induced toxicity in rats by regulating NLRP3 inflammasome. J. Tradit. Compliment. Med. 2024, 14, 287–299. [Google Scholar] [CrossRef]
- Deepika, N.P.; Kondengadan, M.S.; Sweilam, S.H.; Rahman, H.; Muhasina, K.M.; Ghosh, P.; Bhargavi, D.; Palati, D.J.; Maiz, F.; Duraiswamy, B. Neuroprotective role of coconut oil for the prevention and treatment of Parkinson’s disease: Potential mechanisms of action. Biotechnol. Genet. Eng. Rev. 2024, 40, 3346–3378. [Google Scholar]
- Rahim, N.S.; Lim, S.M.; Mani, V.; Abdul Majeed, A.B.; Ramasamy, K. Enhanced memory in Wistar rats by virgin coconut oil is associated with increased antioxidative, cholinergic activities and reduced oxidative stress. Pharm. Biol. 2017, 55, 825–832. [Google Scholar] [CrossRef]
- Vasconcelos, M.H.; Tavares, R.L.; Dutra, M.L.; Batista, K.S.; D’Oliveira, A.B.; Pinheiro, R.O.; Pereira, R.D.; Lima, M.D.; Salvadori, S.M.; de Souza, E.L.; et al. Extra virgin coconut oil (Cocos nucifera L.) intake shows neurobehavioural and intestinal health effects in obesity-induced rats. Food Funct. 2023, 14, 6455–6469. [Google Scholar] [CrossRef] [PubMed]
- Pruseth, B.; Banerjee, S.; Ghosh, A. Integration of In Silico and In Vitro approach to reveal the anticancer efficacy of virgin coconut oil. Cord 2020, 36, 1–9. [Google Scholar] [CrossRef]
- Illam, S.P.; Kandiyil, S.P.; Narayanankutty, A.; Veetil, S.V.; Babu, T.D.; Uppu, R.M.; Raghavamenon, A.C. Virgin coconut oil complements with its polyphenol components mitigate sodium fluoride toxicity in vitro and in vivo. Drug Chem. Toxicol. 2022, 45, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, O.O.; Ajibare, A.J.; Folawiyo, M.A.; Asuku, A.O.; Jimoh-Abdulghaffar, H.O.; Akintoye, A.O.; Babalola, K.T.; Idowu, O.O.; Oyiza, Y.G. Virgin coconut oil-supplemented diet reverses behavioural phenotypes of sodium benzoate-model of acetylcholinesterase dysfunction and cognitive impairment: Role of Nrf2/NfKb signaling pathway. Niger. J. Physiol. Sci. 2022, 37, 225–233. [Google Scholar]
- Akintunde, O.W.; Joseph, O.O.; Afolabi, H.O.; Alamu, O.A.; Abdulrahaman, A. Protective and ameliorative effects of virgin coconut oil on cadmium-damaged wistar rats’ testes. World J. Surg. Surg. Res. 2023, 6, 1440. [Google Scholar]
- Allam, E.A.; Darwish, M.H.; Abou Khalil, N.S.; Abd El-Baset, S.H.; Abd El-Aal, M.; Elrawy, A.; Ahmed, A.A.; Sabra, M.S. Evaluation of the therapeutic potential of novel nanoparticle formulations of glutathione and virgin coconut oil in an experimental model of carbon tetrachloride-induced liver failure. BMC Pharmacol. Toxicol. 2024, 25, 74. [Google Scholar] [CrossRef]
- Ajibare, A.J.; Akintoye, O.O.; Folawiyo, M.A.; Babalola, K.T.; Omotuyi, O.I.; Oladun, B.T.; Aransi-ola, K.T.; Odetayo, A.F.; Olayaki, L.A. Therapeutic potential of virgin coconut oil in mitigating sodium benzoate-model of male infertility: Role of Nrf2/Hmox-1/NF-kB signaling pathway. Iran. J. Basic Med. Sci. 2024, 27, 543–551. [Google Scholar]
- Ajeigbe, K.O.; Oladokun, O.O. Exploring the trichloroacetic acid-induced toxicity on the hepato-renal system and intervention by virgin coconut oil-rich diet. Acta Biol. Slov. 2024, 67, 16–30. [Google Scholar] [CrossRef]
- Kamalaldin, N.A.; Yusop, M.R.; Sulaiman, S.A.; Yahaya, B.H. Apoptosis in lung cancer cells induced by virgin coconut oil. Regen. Res. 2015, 4, 30–36. [Google Scholar]
- Enos, R.T.; Velázquez, K.T.; McClellan, J.L.; Cranford, T.L.; Nagarkatti, M.; Nagarkatti, P.S.; Davis, J.M.; Murphy, E.A. High-fat diets rich in saturated fat protect against azoxymethane/dextran sulfate sodium-induced colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G906–G919. [Google Scholar] [CrossRef] [PubMed]
- Vysakh, A.; Ratheesh, M.; Rajmohanan, T.P.; Pramod, C.; Premlal, S.; Girishkumar, B.; Sibi, P.I. Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action. Int. Immunopharmacol. 2014, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Widhiarta, K.D. Virgin coconut oil for HIV-positive people. Cord 2016, 32, 50–57. [Google Scholar]
- Angeles-Agdeppa, I.; Nacis, J.S.; Dayrit, F.M.; Tanda, K.V. Virgin coconut oil (VCO) supplementation relieves symptoms and inflammation among COVID-19 positive adults: A single-blind randomized trial. J. Nutr. Sci. 2024, 13, e5. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2018, 9, 5–14. [Google Scholar] [CrossRef]
- Zainodin, E.L.; Rashidi, N.F.; Mutalib, H.A.; Ishak, B.; Ghazali, A.R. Antioxidant properties of virgin coconut oil and its cytotoxicity towards human keratinocytes. Nat. Prod. Commun. 2024, 19, 1–9. [Google Scholar] [CrossRef]
- Silalahi, J. Nutritional values and health protective properties of coconut oil. Indones. J. Pharm. Clin. Res. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic acid, but not lauric acid, induces metabolic inflammation, mitochondrial fragmentation, and a drop in mitochondrial membrane potential in human primary myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Alves, N.F.B.; de Queiroz, T.M.; de Almeida Travassos, R.; Magnani, M.; de Andrade Braga, V. Acute treatment with lauric acid reduces blood pressure and oxidative stress in spontaneously hypertensive rats. Basic Clin. Pharmacol. Toxicol. 2017, 120, 348–353. [Google Scholar] [CrossRef]
- Ong, M.H.L.; Wong, H.K.; Tengku-Muhammad, T.S.; Choo, Q.-C.; Chew, C.-H. Proatherogenic proteoglycanase ADAMTS-1 is down-regulated by lauric acid through PI3K and JNK signaling pathways in THP-1 derived macrophages. Mol. Biol. Rep. 2019, 46, 2631–2641. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Gopal, T.; Bhatt, S.; Ai, W.; Ma, C.; Talmon, G.A.; Desouza, C. Lauric acid versus palmitic acid: Effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology 2020, 9, 346. [Google Scholar] [CrossRef]
- Alex, E.A.; Dubo, A.B.; Ejiogu, D.C.; Iyomo, K.W.; Jerome, K.V.; Aisha, N.D.; Daikwo, A.; Yahaya, J.; Osiyemi, R.; Yaro, J.D. Evaluation of oral administration of lauric acid supplement on fasting blood glucose level and pancreatic histomorphological studies in high fat diet/streptozotocin-induced type 2 diabetic male wistar rats. J. Diabetes Metab. 2020, 11, 849. [Google Scholar]
- Zhan, W.; Peng, H.; Xie, S.; Deng, Y.; Zhu, T.; Cui, Y.; Cao, H.; Tang, Z.; Jin, M.; Zhou, Q. Dietary lauric acid promoted antioxidant and immune capacity by improving intestinal structure and microbial population of swimming crab (Portunus trituberculatus). Fish Shellfish Immunol. 2024, 151, 109739. [Google Scholar] [CrossRef] [PubMed]
- Fontinha, F.; Martins, N.; Magalhães, R.; Peres, H.; Oliva-Teles, A. Dietary lauric acid supplementation positively affects growth performance, oxidative and immune status of European seabass juveniles. Fishes 2025, 10, 190. [Google Scholar] [CrossRef]
- Tayag, E.A.; Santiago, E.G.; Manado, M.A.; Alban, P.N.; Agdamag, D.M.; Lazo, S.; Adel, A.; Tactacan, R.; Caspellan, A.O.; Dayrit, C.; et al. A study on the safety and efficacy of monoglyceride of lauric acid as adjuvant treatment in persons living with HIV infection. Cord 2000, 16, 34. [Google Scholar] [CrossRef]
- Namachivayam, A.; Gopalakrishnan, A.V. Effect of lauric acid against ethanol-induced hepatotoxicity by modulating oxidative stress/apoptosis signalling and HNF4α in Wistar albino rats. Heliyon 2023, 9, e21267. [Google Scholar] [CrossRef]
- Olubiyi, M.V.; Kawu, M.U.; Magaji, M.G.; Salahdeen, H.M.; Magaji, R.A. Influence of lauric acid on the relaxation of corpus cavernosum in streptozotocin-induced diabetic male Wistar rats. Future J. Pharm. Sci. 2022, 8, 60–67. [Google Scholar] [CrossRef]
- Shaheryar, Z.A.; Khan, M.A.; Hameed, H.; Zaidi, S.A.; Anjum, I.; Rahman, M.S. Lauric acid provides neuroprotection against oxidative stress in mouse model of hyperglycaemic stroke. Eur. J. Pharmacol. 2023, 956, 175990. [Google Scholar] [CrossRef]
- Babu, S.V.; Veeresh, B.; Patil, A.A.; Warke, Y.B. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur. J. Pharmacol. 2010, 626, 262–265. [Google Scholar] [CrossRef]
- Kisioglu, B.; Onal, E.; Karabulut, D.; Onbasilar, I.; Akyol, A. Neuroprotective roles of lauric acid and resveratrol: Shared benefits in neuroinflammation and anxiety, distinct effects on memory enhancement. Food Sci. Nutr. 2024, 12, 9735–9748. [Google Scholar] [CrossRef] [PubMed]
- Dobri, A.M.; Dudău, M.; Enciu, A.M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; Díaz, A.; González-López, G.; Guevara, J. Differential biochemical-inflammatory patterns in the astrocyte-neuron axis of the hippocampus and frontal cortex in wistar rats with metabolic syndrome induced by high fat or carbohydrate diets. J. Chem. Neuroanat. 2022, 126, 102186. [Google Scholar] [CrossRef]
- Hamsi, M.A.; Othman, F.; Das, S.; Kamisah, Y.; Thent, Z.C.; Qodriyah, H.M.; Zakaria, Z.; Emran, A.; Subermaniam, K.; Jaarin, K. Effect of consumption of fresh and heated virgin coconut oil on the blood pressure and inflammatory biomarkers: An experimental study in Sprague Dawley rats. Alex. J. Med. 2015, 51, 53–63. [Google Scholar] [CrossRef]
- Ameena, M.; Arumugham, M.; Ramalingam, K.; Shanmugam, R. Biomedical applications of lauric acid: A narrative review. Cureus 2024, 16, e62770. [Google Scholar] [CrossRef]
- Verma, P.; Naik, S.; Nanda, P.; Banerjee, S.; Naik, S.; Ghosh, A. In vitro anticancer activity of virgin coconut oil and its fractions in liver and oral cancer cells. Anticancer Agents Med. Chem. 2019, 19, 2223–2230. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Alyamani, S.H.; Abdu, F. Incorporation of methotrexate into coconut oil nanoemulsion potentiates its antiproliferation activity and attenuates its oxidative stress. Drug Deliv. 2020, 27, 422–430. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Folawiyo, A.M.; Enohnyaket, E.B.; Azubuike-Osu, S.O.; Abi, I.; Obaje, S.G.; Famurewa, O.A. Beneficial role of virgin coconut oil supplementation against acute methotrexate chemotherapy-induced oxidative toxicity and inflammation in rats. Integr. Med. Res. 2018, 7, 257–263. [Google Scholar] [CrossRef]
- Smith, D.E.; Salerno, J.W. Selective growth inhibition of a human malignant melanoma cell line by sesame oil in vitro. Prostaglandins Leukot. Essent. Fat. Acids 1992, 46, 145–150. [Google Scholar] [CrossRef]
- Salerno, J.W.; Smith, D.E. The use of sesame oil and other vegetable oils in the inhibition of human colon cancer growth in vitro. Anticancer Res. 1991, 11, 209–215. [Google Scholar]
- Calderon, J.; Brillantes, J.; Buenafe, M.; Cabrera, N.; Campos, E.; Canoy, I.; Capili, C.; Carasco, M.; Cielo, P.; Co, M.; et al. Virgin Coconut Oil Inhibits skbr-3 breast cancer cell proliferation and synergistically enhances the growth inhibitory effects of trastuzumab (herceptin™). Eur. J. Med. Res. 2009, 14 (Suppl. II), I–XXII, 1–208. [Google Scholar]
- Roopashree, P.G.; Shetty, S.S.; Kumari, N.S. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Weng, W.-H.; Leung, W.-H.; Pang, Y.-J.; Hsu, H.-H. Lauric acid can improve the sensitization of Cetuximab in KRAS/BRAF mutated colorectal cancer cells by retrievable microRNA-378 expression. Oncol. Rep. 2016, 35, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 2014, 59, 214–224. [Google Scholar] [CrossRef]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.C.; Galli, G.R.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.R.; Maggiolini, M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discov. 2017, 3, 17063. [Google Scholar] [CrossRef]
- Matteis, V.D.; Cascione, M.; De Giorgi, M.L.; Leporatti, S.; Rinaldi, R. Encapsulation of thermo-sensitive lauric acid in silica shell: A green derivate for chemo-thermal therapy in breast cancer cell. Molecules 2019, 24, 2034. [Google Scholar] [CrossRef]
- Xin, Y.T.; Wang, L.Y.; Chang, H.H.; Ma, F.H.; Sun, M.L.; Chen, L.; Gao, H. Construction of PAMAM-based nanocomplex conjugated with Pt(IV)-complex and lauric acid exerting both anti-tumor and antibacterial effects. Chin. J. Polym. Sci. 2023, 41, 887–896. [Google Scholar] [CrossRef]
- Su, M.; Wen, X.; Yu, Y.; Li, N.; Li, X.; Qu, X.; Elsabahy, M.; Gao, H. Engineering lauric acid-based nanodrug delivery systems for restoring chemosensitivity and improving biocompatibility of 5-FU and OxPt against Fn-associated colorectal tumor. Mater. Chem. B 2024, 12, 3947. [Google Scholar] [CrossRef]
- Mishra, B.; Acharya, P.C.; De, U.C. Synthesis and antineoplastic efficacy of anthraquinone and saturated fatty acid conjugates. ChemistrySelect 2023, 8, e202301502. [Google Scholar] [CrossRef]
- Assiri, M.A.; Ali, A.; Ibrahim, M.; Khan, M.U.; Ahmed, K.; Akash, M.S.; Abbas, M.A.; Javed, A.; Suleman, M.; Khalid, M.; et al. Potential anticancer and antioxidant lauric acid-based hydrazone synthesis and computational study toward the electronic properties. RSC Adv. 2023, 13, 21793. [Google Scholar] [CrossRef]
- Mustafa, A.; Indiran, M.A.; Ramalingam, K.; Perumal, E.; Shanmugham, R.; Karobari, M.I. Anticancer potential of thiocolchicoside and lauric acid loaded chitosan nanogel against oral cancer cell lines: A comprehensive study. Sci. Rep. 2024, 14, 9270. [Google Scholar] [CrossRef]
- Verma, P.; Ghosh, A.; Ray, M.; Sarkar, S. Lauric acid modulates cancer-associated microRNA expression and inhibits the growth of the cancer cell. Anticancer Agents Med. Chem. 2020, 20, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Henson, E.S.; Xiao, W.; Huang, D.; McMillan-Ward, E.M.; Israels, S.J.; Gibson, S.B. Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 2016, 12, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.; Upadhyay, A.; Ido, L.J.; Hanna-Rose, W. Epidermal growth factor receptor cell survival signaling requires phosphatidylcholine biosynthesis. G3 2016, 6, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef]
- Amado, R.G.; Wolf, M.; Peeters, M.; Van Cutsem, E.; Siena, S.; Freeman, D.J.; Juan, T.; Sikorski, R.; Suggs, S.; Radinsky, R.; et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 1626–1634. [Google Scholar] [CrossRef]
- Bergman, A.M.; Adema, A.D.; Balzarini, J.; Bruheim, S.; Fichtner, I.; Noordhuis, P.; Fodstad, Ø.; Myhren, F.; Sandvold, M.L.; Hendriks, H.R.; et al. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Investig. New Drugs 2011, 29, 456–466. [Google Scholar] [CrossRef]
- Guo, F.; Li, H.; Wang, J.; Wang, J.; Zhang, J.; Kong, F.; Zhang, Z.; Zong, J. MicroRNAs in hepatocellular carcinoma: Insights into regulatory mechanisms, clinical significance, and therapeutic potential. Cancer Manag. Res. 2024, 16, 1491–1507. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Fischer, C.L.; Blanchette, D.R.; Brogden, K.A.; Dawson, D.V.; Drake, D.R.; Hill, J.R.; Wertz, P.W. The roles of cutaneous lipids in host defense. Biochim. Biophys. Acta 2014, 1841, 319–322. [Google Scholar] [CrossRef]
- Lieberman, S.; Enig, M.G.; Preuss, H.G. A review of monolaurin and lauric acid: Natural virucidal and bactericidal agents. Focus Altern. Complement. Ther. 2006, 12, 310–314. [Google Scholar] [CrossRef]
- Ferah Okkay, I.; Famurewa, A.C.; Bayram, C.; Okkay, U.; Mendil, A.S.; Sezen, S.; Ayaz, T.; Gecili, I.; Ozkaraca, M.; Senyayla, S.; et al. Arbutin abrogates cisplatin-induced hepatotoxicity via upregulating Nrf2/HO-1 and suppressing genotoxicity, NF-κB/iNOS/TNF-α and caspase-3/Bax/Bcl2 signaling pathways in rats. Toxicol. Res. 2024, 13, tfae075. [Google Scholar] [CrossRef] [PubMed]
- Arunima, S.; Rajamohan, T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats—A comparative study. Food Funct. 2013, 4, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J. Amelioration of doxorubicin-induced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J. Pharm. Pharmacol. 2005, 57, 1043–1052. [Google Scholar] [CrossRef]
- Silalahi, J.; Yuandani, R.; Satria, D. Virgin coconut oil modulates TCD4+ AND TCD8+ cell profile of doxorubicin-induced immune-suppressed rats. Asian J. Pharm. Clin. Res. 2018, 11, 37–38. [Google Scholar] [CrossRef][Green Version]
- Girsang, Y.E.; Nasution, A.N.; Lister, I.N. Comparison Hepatoprotective effect of virgin coconut oil and Curcuma longa linn against doxorubicin induced hepatotoxicity in Wistar rats. In Proceedings of the 2020 3rd International Conference on Mechanical, Electronics, Computer, and Industrial Technology (MECnIT), Medan, Indonesia, 25–27 June 2020; pp. 99–103. [Google Scholar]
- Utari, A.U.; Djabir1, Y.Y.; Palinggi, B.P. Combination of virgin coconut oil and extra virgin olive oil elicits superior protection against doxorubicin cardiotoxicity in rats. Turk. J. Pharm. Sci. 2022, 19, 138–144. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Illam, S.P.; Rao, V.; Shehabudheen, S.; Raghavamenon, A.C. Hot-processed virgin coconut oil abrogates cisplatin-induced nephrotoxicity by restoring redox balance in rats compared to fermentation-processed virgin coconut oil. Drug Chem. Toxicol. 2022, 45, 1373–1382. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Mukherjee, A.G.; Wanjari, U.R.; Sukumar, A.; Murali, R.; Renu, K.; Vellingiri, B.; Dey, A.; Gopalakrishnan, A.V. Repurposing FDA-approved drugs against the toxicity of platinum-based anticancer drugs. Life Sci. 2022, 305, 120789. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Wang, C.; Olatunji, O.J.; Famurewa, A.C. Tiliacora triandra attenuates cisplatin triggered hepatorenal and testicular toxicity in rats by modulating oxidative inflammation, apoptosis and endocrine deficit. Front. Biosci. 2022, 27, 44–53. [Google Scholar] [CrossRef]
- Senin, M.M.; Al-Ani, I.M.; Mahmud, A.M.; Muhammad, N.; Kasmuri, H.M. Protective effect of virgin coconut oil on cyclophosphamide-induced histological changes in lymphoid tissues. Int. Med. J. Malays. 2018, 17, 65–74. [Google Scholar] [CrossRef]
- Law, K.S.; Azman, N.; Omar, E.A.; Musa, M.Y.; Yusoff, N.M.; Sulaiman, S.A.; Hussain, N.H. The effects of virgin coconut oil (VCO) as supplementation on quality of life (QOL) among breast cancer patients. Lipids Health Dis. 2014, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Tangco, E.; Habana, M.A.; Cordero, C.; Mantaring, J.; Banuelos, G.; Sarmiento, T.; Olvina, M.; Aguilar, C.; Tan-Pusag, C. PO-0659: Prevention of mucositis in nasopharyngeal carcinoma using virgin coconut oil and salt and soda mouthwash. Radiother. Oncol. 2014, 111, 4–5. [Google Scholar] [CrossRef]
- Nukaga, S.; Mori, T.; Miyagawa, Y.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Nakashima, C.; et al. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci. 2020, 111, 4605–4615. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54–79. [Google Scholar] [CrossRef]
| Bioactive Class | Bioactive Constituents | Reported Effect | References |
|---|---|---|---|
| Medium-chain fatty | LA (C12), capric acid (C10), caprylic acid (C8), caproic acid (C6) | Antimicrobial, immunomodulatory, antiviral | [40,44,45,46] |
| Polyphenol | Ferulic acid, catechins, p-coumaric acid, | Antioxidant, anti-inflammatory | [42] |
| Tocopherols | α-Tocopherol | Antioxidant | [45,47] |
| Phytosterols | β-Sitosterol | Hypocholesterolemia | [43] |
| Phenolic acids | Caffeic acid, gallic acid | Free radical scavenging, anti-inflammatory, blood glucose regulation, chemoprevention, and immunomodulation | [48,49] |
| Flavonoids | Quercetin | Antioxidant, vascular protection | [40] |
| Component/Feature | VCO | Butter | Hydrogenated Oil |
|---|---|---|---|
| Medium-chain triglycerides | High | Low | Absent |
| Polyphenols | Moderate | Low | Absent |
| Trans-fats | Absent | Absent | High |
| Cholesterol | Absent | High | Variable |
| Absorption rate | Fast | Slow | Slow |
| Metabolic oxidation | Fast | Slow | Slow |
| LDL/HDL effect | Increases HDL, decreases LDL/HDL ratio | Increase LDL | Highly increase LDL |
| VCO Nature | Cancer Type | Model/Cell Line | Effect/Activity | Dose | Timing | References |
|---|---|---|---|---|---|---|
| VCO | Liver and oral cancers | HepG2, KB | Antiproliferation | 50–100 µg/mL | 72 h | [121] |
| VCO nanoemulsion | Lung cancer | A549, EAC | Antiproliferation | Not specified | 48 h | [122] |
| VCO | Skin cancer | Melanoma cell line | Antiproliferation | 10–300 µg/mL | 48 h | [124] |
| VCO | Colon cancer | Colon adenocarcinoma cell line | Antiproliferation | 30 µg/mL | 72 h | [125] |
| CO | Colon cancer | In vivo | ↑ Colonic mucin 2 protein | High-fat SFA-rich diet | 28 days | [97] |
| VCO | Lung cancer | NCI-H1299, A549 | Antiproliferation ↑ Apoptosis | 25–100 µg/mL | 24–72 h | [96] |
| CO | Neuroblastoma | SH-SY5Y | Antiproliferation ↑ Apoptosis Mitochondrial damage | 50–150 µg/mL | 24–72 h | [7] |
| VCO-trastuzumab | Breast cancer | SKBR-3 | Antiproliferation | Not specified | 48 h | [126] |
| LA/Derivative | Cancer Type | Model/Cell Line | Effect/Activity | Dose | Timing | Normal Cell Control Used | References |
|---|---|---|---|---|---|---|---|
| LA | Neuroblastoma | ISH-SY5Y | ↑ ROS, apoptosis, mitochondrial damage | 20–100 µg/mL | 24–72 h | No | [7] |
| LA | Liver and colorectal cancers | HepG2, HT-29 | Antiproliferation, ↓ epidermal growth factor receptor (EGFR), and ↑ apoptosis, anti-metastatic effect | 30–50 µg/mL | 24–48 h | No | [30] |
| LA-cetuximab or LA alone | KRAS/BRAF-mutated colorectal cancer | HCT116 | Antiproliferation, ↑ microRNA-378, ↓ MAP, ↓ ERK-2 | 25 µM | 48 h | No | [128] |
| LA | Colon cancer | HCT116 | Antiproliferation, ↑ ROS, ↓ GSH, ↑ apoptosis, G2/M cell cycle arrest | 20–60 µM | 24–48 h | Yes | [129] |
| LA | Endometrial and breast cancers | Ishikawa, MCF-7 | Antiproliferation, ↑ apoptosis, ↑ EGFR/ERK | 25–100 µM | 24–48 h | Yes | [130] |
| LA | Colon cancer | HT-29 | Antiproliferation | 30 µM | 72 h | Yes | [125] |
| LA-Gemcitabine or LA alone | Bladder cancer | T24 | Antiproliferation, ↑ apoptosis, ↓ PPARG and ↓ COX2 genes, cell cycle arrest | 10–40 µM | 48 h | No | [27] |
| LA nanocapsule (SiO2@LA) | Breast cancer | MCF-7 | ↓ Cell viability, ↑ ROS, ↑ apoptosis | Not specified | 24 h | Yes | [131] |
| LA-platinum nanocomplex | Colorectal cancer | HT-29 | ↓ Cell viability, ↑ ROS | 5 µM | 24 h | No | [132] |
| LA-nanodrug systems (5-FU-LA@PPL) | Colorectal cancer | Xenograft (BALB/c nude mice) | ↓ Cell viability ↓ autophagy | 5 µM | 48 h | No | [133] |
| LA-conjugated 2-hydroxyanthraquinone | Breast, colon and leukemia cancers | HL-60, HCT116, | ↓ Cell viability | 10–25 µM | 24–48 h | No | [134] |
| LA-based hydrazones | Liver cancer | HepG2 | Antiproliferation | 50 µM | 48 h | No | [135] |
| LA-chitosan-based nanogel | Oral cancer | Ga9-22 | Antiproliferation, ↓ cell viability, ↑ ROS, G2/M, cell cycle arrest, and ↑ Bad/Bax/p53 apoptosis | 20 µg/mL | 24 h | Yes | [136] |
| LA | Oral and liver cancers | HepG2, SCC-15 | ↓ Oncogenic microRNA, ↑ tumor-suppressor microRNA, cell cycle arrest, ↑ apoptosis | 20 µg/mL | 24–48 h | No | [137] |
| VCO or LA | Anticancer Drug | Type of Toxicity | Animal Model Details | Mechanistic Outcomes (% Improvement) | Dose and Timing | References |
|---|---|---|---|---|---|---|
| VCO | Doxorubicin (DOX) | Neurobehavioral toxicity | Swiss albino mice, DOX-induced, post-treatment, behavior assays | ↓ AchE, TNF-α, iNOS; improved behavior; ↓ oxidative stress (approx. 35–50% change in markers) | 5 mL/kg/day VCO × 28 days, DOX 5 mg/kg i.p. | [25] |
| VCO | Doxorubicin (DOX) | Immunosuppression | Balb/c mice, DOX-induced, pre-treatment, immune assays | ↑ CD4+, CD8+, lymphocyte proliferation, macrophage activity (up to 45%) | 10 mL/kg/day VCO × 14 days, DOX 10 mg/kg i.p. | [150] |
| VCO | Doxorubicin (DOX) | Hepatic and cardiac toxicity | Wistar rats, DOX-induced, post-treatment, histopathological evaluation | ↓ ALT, AST, LDH, CK-MB (~30–40%); restored histology | 5 mL/kg/day VCO × 21 days, DOX 5 mg/kg i.p. | [151,152] |
| VCO | Doxorubicin (DOX) | Liver toxicity | Wistar rats, DOX-induced, post-treatment, cytokine, and antioxidant evaluation | ↓ AST, ALT, MDA, inflammatory cytokines (30–60%), ↑ Bcl-2, antioxidant enzymes | 5 mL/kg/day VCO × 21 days, DOX 5 mg/kg i.p. | [24] |
| VCO | Cisplatin (CP) | Nephrotoxicity | Wistar rats, CP-induced nephrotoxicity, comparison of hot/cold VCO | ↓ Creatinine, urea, ↑ SOD, CAT; higher in hot-processed VCO (20–50% difference vs. CP-only) | 5 mL/kg/day VCO × 10 days, CP 5 mg/kg i.p. | [153] |
| VCO | Cyclophosphamide (CYP) | Hepatorenal toxicity | Swiss albino mice, CYP-induced, pre-treatment, hepatorenal function assessed | ↑ GSH, ↓ MDA, restored organ function (30–45%) | 10 mL/kg/day VCO × 20 days, CYP 200 mg/kg i.p. | [17] |
| VCO | Cyclophosphamide (CYP) | Lymphoid toxicity (altered hematological indices, histological spleen/thymus changes) | Swiss mice, CYP-induced, spleen/thymus histology, hematological changes | Improved spleen/thymus histology and hematology (qualitative only) | 5 mL/kg/day VCO × 10 days, CYP 100 mg/kg | [156] |
| VCO | Methotrexate (MTX) | Oxidative stress & inflammation | Wistar rats, MTX-induced, pre-treatment with VCO nanoemulsion, antioxidant evaluation | ↑ SOD, CAT, ↓ lipid peroxidation (35–60%) | 5 mL/kg/day VCO nanoemulsion × 7 days, MTX 20 mg/kg | [122] |
| Methotrexate (MTX) | Hepatotoxicity, nephrotoxicity, neurotoxicity, systemic toxicity | Wistar rats, MTX-induced, systemic toxicity markers assessed | ↓ ALT, creatinine, TNF-α, IL-6; ↑ SOD, CAT (~40–60%) | 5 mL/kg/day VCO × 10 days, MTX 20 mg/kg | [8,21,22,123] | |
| LA | LA and glucose | Cachexia (muscle atrophy, weight loss) | C57BL/6 mice, cancer cachexia model, LA + glucose supplementation | ↑ Muscle mass, ↑ MYL1 gene (approx. 30%) | 250 mg/kg LA + 1 g/kg glucose × 14 days | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bose, D.; Olorunlana, A.; Abdel-Latif, R.; Famurewa, A.C.; Othman, E.M. Virgin Coconut Oil and Its Lauric Acid, Between Anticancer Activity and Modulation of Chemotherapy Toxicity: A Review. J. Xenobiot. 2025, 15, 126. https://doi.org/10.3390/jox15040126
Bose D, Olorunlana A, Abdel-Latif R, Famurewa AC, Othman EM. Virgin Coconut Oil and Its Lauric Acid, Between Anticancer Activity and Modulation of Chemotherapy Toxicity: A Review. Journal of Xenobiotics. 2025; 15(4):126. https://doi.org/10.3390/jox15040126
Chicago/Turabian StyleBose, Debalina, Adetayo Olorunlana, Rania Abdel-Latif, Ademola C. Famurewa, and Eman M. Othman. 2025. "Virgin Coconut Oil and Its Lauric Acid, Between Anticancer Activity and Modulation of Chemotherapy Toxicity: A Review" Journal of Xenobiotics 15, no. 4: 126. https://doi.org/10.3390/jox15040126
APA StyleBose, D., Olorunlana, A., Abdel-Latif, R., Famurewa, A. C., & Othman, E. M. (2025). Virgin Coconut Oil and Its Lauric Acid, Between Anticancer Activity and Modulation of Chemotherapy Toxicity: A Review. Journal of Xenobiotics, 15(4), 126. https://doi.org/10.3390/jox15040126









