Combined Effects of Metals, PCBs, Dioxins, and Furans on Cardiovascular Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Collection
2.3. Study Population
2.4. Exposure Variables
Exposure Assessment
2.5. Cardiovascular Disease Outcome Variables
Calculating the Framingham Risk Score (FRS)
2.6. Statistical Analytics
2.6.1. Descriptive Analysis
2.6.2. Linear Regression Methods
2.6.3. Bayesian Kernel Machine Regression BKMR
3. Results
3.1. Characteristics of the Sample Population
3.2. Summary Statistics of Outcome Variables by Gender
3.3. Summary Statistics Mean (S.D) of Exposure Variables by Gender
3.4. Spearman’s Correlation Analysis
3.5. Linear Regression Analysis
3.6. BKMR Analysis
3.6.1. Posterior Inclusion Probability (PIP)
3.6.2. Univariate Exposure–Response Relationship
3.6.3. Overall Exposure Effect
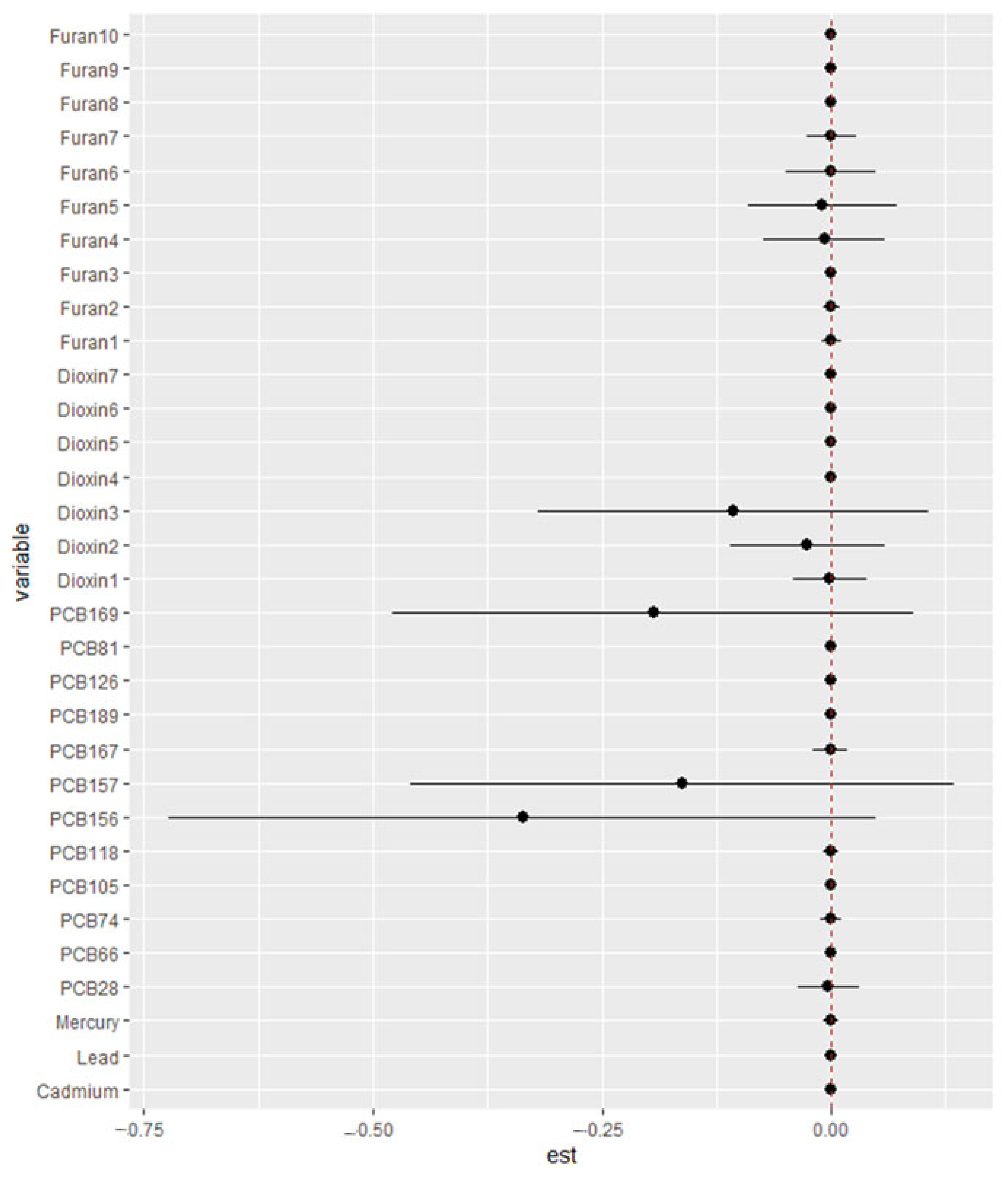
3.6.4. Single-Variable Effect
3.6.5. Bivariate Exposure–Response Relationship
4. Discussion
4.1. Overview and Descriptive Statistics Results
4.2. Associations Between Pollutant Exposures and Cardiovascular Outcomes
BKMR Results
4.3. Comparison with the Previous Literature
4.4. Broader Health Implications
4.5. Strengths and Limitations
4.5.1. Strengths
4.5.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Chemical Group | Chemical Code/Name | Study Key | Description |
|---|---|---|---|
| PCBs | PCB28 Lipid Adj (ng/g) | PCB28 | Polychlorinated biphenyl congener 28; persistent organic pollutant, bioaccumulative. |
| PCB66 Lipid Adj (ng/g) | PCB66 | PCB congener 66; moderately chlorinated PCB, bioaccumulative, persistent pollutant. | |
| PCB74 Lipid Adj (ng/g) | PCB74 | PCB congener 74; persistent, bioaccumulative, potentially toxic pollutant. | |
| PCB105 Lipid Adj (ng/g) | PCB105 | PCB congener 105; dioxin-like, bioaccumulative, toxic pollutant. | |
| PCB118 Lipid Adj (ng/g) | PCB118 | PCB congener 118; dioxin-like PCB, persistent, bioaccumulative, toxic. | |
| PCB156 Lipid Adj (ng/g) | PCB156 | PCB congener 156; highly chlorinated, dioxin-like, persistent pollutant. | |
| PCB157 Lipid Adj (ng/g) | PCB157 | PCB congener 157; persistent organic pollutant, moderate toxicity. | |
| PCB167 Lipid Adj (ng/g) | PCB167 | PCB congener 167; environmentally persistent, potential toxicological concern. | |
| PCB189 Lipid Adj (ng/g) | PCB189 | PCB congener 189; highly chlorinated, very persistent, known for bioaccumulation. | |
| PCB126-3,3′,4,4′,5-pncb Lipid Adj (pg/g) | PCB126 | Highly toxic, dioxin-like PCB congener; potent bioaccumulative chemical. | |
| PCB81-3,4,4′,5-tcb Lipid Adj (pg/g) | PCB81 | Dioxin-like PCB congener; highly toxic, persistent pollutant. | |
| PCB169-3,3′,4,4′,5,5′-hxcb Lipid Adj (pg/g) | PCB169 | Highly chlorinated dioxin-like PCB, significant bioaccumulation, toxicity. | |
| Dioxins | LBXD01LA– 1,2,3,7,8-Pentachlorodibenzo-p-dioxin Lipid Adj (pg/g) | Dioxin1- 1,2,3,7,8-PeCDD | Pentachlorodibenzo-p-dioxin; highly toxic, persistent environmental contaminant. |
| LBXD02LA– 1,2,3,4,7,8-Hexachlorinated dioxin Lipid Adj (pg/g) | Dioxin2- 1,2,3,4,7,8-HxCDD | Hexachlorinated dioxin; highly toxic, persistent, carcinogenic contaminant. | |
| LBXD03LA– 1,2,3,6,7,8-Hexachlorinated dioxin Lipid Adj (pg/g) | Dioxin3- 1,2,3,6,7,8-HxCDD | Hexachlorinated dioxin; persistent, bioaccumulative, highly toxic. | |
| LBXD04LA– 1,2,3,7,8,9-Hexachlorinated dioxin Lipid Adj (pg/g) | Dioxin4- 1,2,3,7,8,9-HxCDD | Hexachlorinated dioxin; persistent organic pollutant, toxicological concern. | |
| LBXD05LA– 1,2,3,4,6,7,8-Heptachlorinated dioxin Lipid Adj (pg/g) | Dioxin5- 1,2,3,4,6,7,8-HpCDD | Heptachlorinated dioxin; persistent, bioaccumulative, toxic contaminant. | |
| LBXD07LA– 1,2,3,4,6,7,8,9-Octachlorinated dioxin Lipid Adj (pg/g) | Dioxin6-1,2,3,4,6,7,8,9-OCDD | Octachlorinated dioxin (OCDD); highly persistent, bioaccumulative, toxic environmental pollutant. | |
| LBXTCDLA– 2,3,7,8-Octachlorinated dioxin Lipid Adj (pg/g) | Dioxin7- 2,3,7,8-TCDD | Tetrachlorodibenzo-p-dioxin (TCDD); extremely toxic, carcinogenic, persistent pollutant. | |
| Furans (PCDFs) | LBXF01LA– 2,3,7,8-Tetrachloro dibenzofuran Lipid Adj (pg/g) | Furan1- 2,3,7,8-TCDF | Tetrachlorodibenzofuran (TCDF); toxic, bioaccumulative, environmentally persistent. |
| LBXF02LA– 1,2,3,7,8-Pentachlorinated dibenzofuran Lipid Adj (pg/g) | Furan2- 1,2,3,7,8-PeCDF | Pentachlorinated dibenzofuran; highly toxic, persistent contaminant. | |
| LBXF03LA– 2,3,4,7,8-Pentachlorinated dibenzofuran Lipid Adj (pg/g) | Furan3- 2,3,4,7,8-PeCDF | Pentachlorinated dibenzofuran; toxic, persistent environmental pollutant. | |
| LBXF04LA– 1,2,3,4,7,8-Hexachlorinated dibenzofuran Lipid Adj (pg/g) | Furan4- 1,2,3,4,7,8-HxCDF | Hexachlorinated dibenzofuran; persistent, bioaccumulative, toxic pollutant. | |
| LBXF05LA– 1,2,3,6,7,8-Hexachlorinated dibenzofuran Lipid Adj (pg/g) | Furan5- 1,2,3,6,7,8-HxCDF | Hexachlorinated dibenzofuran; toxic, persistent, bioaccumulative contaminant. | |
| LBXF06LA– 1,2,3,7,8,9-Hexachlorinated dibenzofuran Lipid Adj (pg/g) | Furan6- 1,2,3,7,8,9-HxCDF | Hexachlorinated dibenzofuran; persistent pollutant with significant toxicity. | |
| LBXF07LA– 2,3,4,6,7,8-Hexachlorinated dibenzofuran Lipid Adj (pg/g) | Furan7- 2,3,4,6,7,8-HxCDF | Hexachlorinated dibenzofuran; toxic, persistent, bioaccumulative chemical. | |
| LBXF08LA– 1,2,3,4,6,7,8-Heptachlorinated dibenzofuran Lipid Adj (pg/g) | Furan8- 1,2,3,4,6,7,8-HpCDF | Heptachlorinated dibenzofuran; persistent, bioaccumulative, toxic contaminant. | |
| LBXF09LA– 1,2,3,4,7,8,9-Heptachlorinated dibenzofuran Lipid Adj (pg/g) | Furan9- 1,2,3,4,7,8,9-HpCDF | Heptachlorinated dibenzofuran; persistent, toxic environmental pollutant. | |
| LBXF10LA– 1,2,3,4,6,7,8,9-Octachlorinated dibenzofuran Lipid Adj (pg/g) | Furan10-1,2,3,4,6,7,8,9-OCDF | Octachlorinated dibenzofuran (OCDF); highly chlorinated, persistent pollutant. |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation 2024, 149, 8. [Google Scholar] [CrossRef]
- Greenfield, D.M.; Snowden, J.A. Cardiovascular Diseases and Metabolic Syndrome. In The EBMT Handbook; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. (Eds.) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Springer International Publishing: Cham, Switzerland, 2019; p. 702. [Google Scholar]
- Al-Masaoodi, R.A.; Saleh, J.M.; Al-Shemery, M.K. Determination of Cardiovascular Diseases (CVD) with Importance Biomarkers: Review; Semantic Scholar: Seattle, WA, USA, 2021. [Google Scholar]
- Hajar, R. Framingham Contribution to Cardiovascular Disease. Heart Views Off. J. Gulf Heart Assoc. 2016, 17, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Poursafa, P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, K.; Levey, A.S.; Sarnak, M.J. The clinical epidemiology of cardiovascular diseases in chronic kidney disease: Traditional Cardiac Risk Factors in Individuals with Chronic Kidney Disease. Semin. Dial. 2003, 16, 118–127. [Google Scholar] [CrossRef]
- Everson-Rose, S.A.; Lewis, T.T. Psychosocial factors and cardiovascular diseases. Annu. Rev. Public Health 2005, 26, 469–500. [Google Scholar] [CrossRef]
- Okereke, O.I.; Manson, J.E. Psychosocial Factors and Cardiovascular Disease Risk. Circ. Res. 2017, 120, 1855–1856. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef]
- Dupre, M.E.; Nelson, A.; Lynch, S.M.; Granger, B.B.; Xu, H.; Churchill, E.; Willis, J.M.; Curtis, L.H.; Peterson, E.D. Socioeconomic, Psychosocial and Behavioral Characteristics of Patients Hospitalized With Cardiovascular Disease. Am. J. Med. Sci. 2017, 354, 565–572. [Google Scholar] [CrossRef]
- Papageorgiou, N. Cardiovascular Diseases: Genetic Susceptibility, Environmental Factors and Their Interaction; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Khera, A.V.; Kathiresan, S. Genetics of coronary artery disease: Discovery, biology and clinical translation. Nat. Rev. Genet. 2017, 18, 331–344. [Google Scholar] [CrossRef]
- Forman, D.; Bulwer, B.E. Cardiovascular disease: Optimal approaches to risk factor modification of diet and lifestyle. Curr. Treat. Options Cardiovasc. Med. 2006, 8, 47–57. [Google Scholar] [CrossRef]
- Kashuba, R.; Menzie, C.; Martin, L. Risk of cardiovascular disease is driven by different combinations of environmental, medical and behavioral factors: Building a conceptual model for cumulative risk assessment. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 1902–1925. [Google Scholar] [CrossRef]
- Reddy, K.S.; Katan, M.B. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.O.; Edo, G.I.; Emakpor, O.L.; Oloni, G.O.; Ezekiel, G.O.; Essaghah, A.E.A.; Agoh, E.; Agbo, J.J. Lifestyle modifications for preventing and managing cardiovascular diseases. Sport Sci. Health 2024, 20, 23–36. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Gratas-Delamarche, A.; Derbré, F.; Vincent, S.; Cillard, J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop. Free Radic. Res. 2014, 48, 93–108. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Hu, X.; Chen, F.; Li, D. A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients 2023, 15, 4898. [Google Scholar] [CrossRef]
- Russo, P.; Milani, F.; De Iure, A.; Proietti, S.; Limongi, D.; Prezioso, C.; Checconi, P.; Zagà, V.; Novazzi, F.; Maggi, F. Effect of cigarette smoking on clinical and molecular endpoints in COPD patients. Int. J. Mol. Sci. 2024, 25, 5834. [Google Scholar] [CrossRef]
- Xu, L.; Zimmermann, M.; Forkey, H.; Griffin, J.; Wilds, C.; Morgan, W.S.; Byatt, N.; McNeal, C.J. How to mitigate risk of premature cardiovascular disease among children and adolescents with mental health conditions. Curr. Atheroscler. Rep. 2022, 24, 253–264. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Hayat, M.; Kerr, R.; Bentley, A.R.; Rotimi, C.N.; Raal, F.J.; Ramsay, M. Genetic associations between serum low LDL-cholesterol levels and variants in LDLR, APOB, PCSK9 and LDLRAP1 in African populations. PLoS ONE 2020, 15, e0229098. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Srivastava, D. Genetics of Human Cardiovascular Disease. Cell 2012, 148, 1242–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Zheng, Y.; Lin, W.-H.; Zhang, H.; Zhou, X.-L. Challenges and Opportunities in Cardiovascular Health Informatics. IEEE Trans. Biomed. Eng. 2013, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- DeVon, H.A.; Vuckovic, K.M.; Ryan, C.J.; Barnason, S.; Zerwic, J.J.; Pozehl, B.J.; Schulz, P.S.; Seo, Y.; Zimmerman, L. Systematic review of symptom clusters in cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2017, 16, 6–17. [Google Scholar] [CrossRef]
- de Lacerda, D.A.; Honorato, P.F.; Lima, L.L.L.; Oliveira, A.V.L.; de Lira, E.A.; de Lira, F.E.A.; de Sousa Neto, J.F.; de Lacerda, M.A.; Siqueira, T.d.O.; Braga, T.R.O. Challenges in the early diagnosis of cardiovascular diseases in primary care in Brazil: Analysis and proposals for solutions. In Proceedings of the VI Seven International Multidisciplinary Congress, Online, 24–25 June 2024. [Google Scholar]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Timothy, N.A.; Williams, E.T. Environmental Pollution by Heavy Metal: An Overview. Int. J. Environ. Chem. 2019, 3, 72–82. [Google Scholar] [CrossRef]
- Ibadi, E.A.; Awad, H.K.; Hussain, L.I. Environmental toxins and their effects on A comprehensive review of exposure: Human organs and their accumulation. J. Agric. Environ. Vet. Sci. 2024, 8, 10–24. [Google Scholar] [CrossRef]
- Hussain, C.M.; Keçili, R. Environmental pollution and environmental analysis. In Modern Environmental Analysis Techniques for Pollutants; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Mercury; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2022. [Google Scholar]
- Schwartz, J. Lead, blood pressure, and cardiovascular disease in men and women. Environ. Health Perspect. 1991, 91, 71–75. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Swaringen, B.F.; Gawlik, E.; Kamenov, G.D.; McTigue, N.E.; Cornwell, D.A.; Bonzongo, J.-C.J. Children’s exposure to environmental lead: A review of potential sources, blood levels, and methods used to reduce exposure. Environ. Res. 2022, 204, 112025. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-L.; Yang, W.-Y.; Hara, A.; Asayama, K.; Roels, H.A.; Nawrot, T.S.; Staessen, J.A. Public and occupational health risks related to lead exposure updated according to present-day blood lead levels. Hypertens. Res. 2023, 46, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Okpogba, A.N.; Ogbodo, E.C.; Amah, U.K.; Mounmbegna, E.P.; Obi-Ezeani, C.N.; Iwuji, J.C. Evaluation of some heavy metal levels in blood of lead acid battery manufacturing factory workers in Nnewi, Nigeria. Indian J. Pharm. Pharmacol. 2020, 7, 82–94. [Google Scholar]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Jyothi, N.R.; Farook, N.A.M. Mercury toxicity in public health. In Heavy Metal Toxicity in Public Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Zafar, A.; Javed, S.; Akram, N.; Naqvi, S.A.R. Health risks of mercury. In Mercury Toxicity Mitigation: Sustainable Nexus Approach; Springer: Cham, Switzerland, 2024; pp. 67–92. [Google Scholar]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2018; pp. 103–125. [Google Scholar]
- Committee, E.S. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- Jannetto, P.J.; Cowl, C.T. Elementary Overview of Heavy Metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353. [Google Scholar] [CrossRef]
- Grandjean, P.; Satoh, H.; Murata, K.; Eto, K. Adverse effects of methylmercury: Environmental health research implications. Environ. Health Perspect. 2010, 118, 1137–1145. [Google Scholar] [CrossRef]
- Lawal, K.K.; Ekeleme, I.K.; Onuigbo, C.M.; Ikpeazu, V.O.; Obiekezie, S.O. A review on the public health implications of heavy metals. World J. Adv. Res. Rev. 2021, 10, 255–265. [Google Scholar] [CrossRef]
- Penteado, J.C.P.; Vaz, J.M. O legado das bifenilas policloradas (PCBs) (The legacy of polychlorinated biphenyls (PCBs)). Química Nova 2001, 24, 390–398. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polychlorinated Biphenyls (PCBs); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2000. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp17.pdf (accessed on 11 June 2025).
- Biziuk, M.; Beyer, A. Polychlorinated Biphenyls (PCBs). In Managing Global Resources and Universal Processes; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ododo, M.M.; Wabalo, B.K. Polychlorinated Biphenyls (PCBs) and Their Impacts on Human Health: A Review. J. Environ. Pollut. Hum. Health 2019, 7, 73–77. [Google Scholar]
- Rathoure, A.K. Dioxins: Source, origin and toxicity assessment. Biodivers. Int. J. 2018, 2, 310–314. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chlorinated Dibenzo-p-Dioxins; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2024. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp104.pdf (accessed on 11 June 2025).
- Kamrin, M.A.; Rodgers, P.W. Dioxins in the Environment; Hemisphere Publishing Corp.: Washington, DC, USA, 1985. [Google Scholar]
- Marinković, N.; Pašalić, D.; Ferenčak, G.; Gršković, B.; Rukavina, A.S. Dioxins and Human Toxicity. Arh. Za Hig. Rada Toksikol. 2010, 61, 445–453. [Google Scholar] [CrossRef]
- Banerjee, R.; Banerjee, M. Medicinal significance of furan derivatives: A Review. Int. J. Rev. Life Sci. 2020, 2, 7–16. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Chlorodibenzofurans (CDFs); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2023. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp32.pdf (accessed on 11 June 2025).
- De, B.; Sen, S.; Easwari, T.S. Chemistry and Therapeutic Aspect of Furan: A Short Review. Asian J. Res. Chem. 2015, 8, 428–438. [Google Scholar] [CrossRef]
- Seok, Y.-J.; Her, J.-Y.; Kim, Y.-G.; Kim, M.Y.; Jeong, S.Y.; Kim, M.K.; Lee, J.Y.; Kim, C.-I.; Yoon, H.-J.; Lee, K.-G. Furan in Thermally Processed Foods—A Review. Toxicol. Res. 2015, 31, 241–253. [Google Scholar] [CrossRef]
- Maga, J.A. Furans in foods. CRC Crit. Rev. Food Sci. Nutr. 1979, 11, 355–400. [Google Scholar] [CrossRef]
- Petrosino, V.; Motta, G.; Tenore, G.C.; Coletta, M.A.; Guariglia, A.; Testa, D. The role of heavy metals and polychlorinated biphenyls (PCBs) in the oncogenesis of head and neck tumors and thyroid diseases: A pilot study. Biometals 2018, 31, 285–295. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic Effects of Heavy Metals and Pesticides in Living Systems. Front. Chem. 2017, 5, 70. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Barouki, R.; Brack, W.; Dalla Costa, S.; Dorne, J.C.M.; Drakvik, P.E.; Faust, M.; Karjalainen, T.K.; Kephalopoulos, S.; van Klaveren, J.; et al. Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 2018, 120, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, J.; Zhang, R.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Obeng-Gyasi, E. Interaction of per-and polyfluoroalkyl substances and allostatic load among adults in various occupations. Diseases 2022, 10, 26. [Google Scholar] [CrossRef]
- Zarerad, E.; Niksalehi, K.; Armandeh, M.; Sani, M.A.; Ataei, M.; Mousavi, T.; Maghsoudi, A.S.; Hassani, S. Polychlorinated Biphenyls: A Review of Recent Updates on Food Safety and Environmental Monitoring, Health and Toxicological Implications, and Analysis. Mini Rev. Med. Chem. 2023, 23, 1390–1411. [Google Scholar] [CrossRef]
- Lee, H.K.; Pak, Y.K. Persistent Organic Pollutants, Mitochondrial Dysfunction, and Metabolic Syndrome. In Mitochondrial Dysfunction Caused by Drugs and Environmental Toxicants; Wiley: Hoboken, NJ, USA, 2018; pp. 691–707. [Google Scholar]
- Igwe, E.; Onoja, S.; Nwodo, P.; Baharane, V.; Diakite, S.; Saquee, F.; Ugwu, B.; Amechi, O.; Niambe, O.; Shaibu, O. Identification of Sources of Some Priority Heavy Metallic Pollutants Caus-ing Environmental Degradation and It’s Health Implications. J. Ind. Pollut. Control 2023, 39, 1–13. [Google Scholar]
- Pant, A.B. Nanotoxicology. In Dictionary of Toxicology; Springer: Singapore, 2024; p. 682. [Google Scholar] [CrossRef]
- Lind, P.M.; Lind, L. Are persistent organic pollutants linked to lipid abnormalities, atherosclerosis and cardiovascular disease? A review. J. Lipid Atheroscler. 2020, 9, 334. [Google Scholar] [CrossRef]
- Raghavan, A.; Pirruccello, J.P.; Ellinor, P.T.; Lindsay, M.E. Using genomics to identify novel therapeutic targets for aortic disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 334–351. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. NHANES 2003/2004. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2003 (accessed on 11 June 2025).
- Caussy, D.; Gochfeld, M.; Gurzau, E.; Neagu, C.; Ruedel, H. Lessons from case studies of metals: Investigating exposure, bioavailability, and risk. Ecotoxicol. Environ. Saf. 2003, 56, 45–51. [Google Scholar] [CrossRef]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Rice, C.P.; O’Keefe, P.; Kubiak, T. Sources, pathways, and effects of PCBs, dioxins, and dibenzofurans. In Handbook of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2002; pp. 525–598. [Google Scholar]
- Evans, R.M.; Martin, O.V.; Faust, M.; Kortenkamp, A. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci. Total Environ. 2016, 543, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Yip, F.; Christensen, B.; Sircar, K.; Naeher, L.; Bruce, N.; Pennise, D.; Lozier, M.; Pilishvili, T.; Loo Farrar, J.; Stanistreet, D.; et al. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ. Int. 2017, 99, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R. Fish and shellfish as dietary sources of methylmercury and the ω-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: Risks and benefits. Environ. Res. 2004, 95, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Downward, G.S.; Hu, W.; Large, D.; Veld, H.; Xu, J.; Reiss, B.; Wu, G.; Wei, F.; Chapman, R.S.; Rothman, N.; et al. Heterogeneity in coal composition and implications for lung cancer risk in Xuanwei and Fuyuan counties, China. Environ. Int. 2014, 68, 94–104. [Google Scholar] [CrossRef]
- Akagi, H.; Grandjean, P.; Takizawa, Y.; Weihe, P. Methylmercury Dose Estimation from Umbilical Cord Concentrations in Patients with Minamata Disease. Environ. Res. 1998, 77, 98–103. [Google Scholar] [CrossRef]
- Baishaw, S.; Edwards, J.; Daughtry, B.; Ross, K. Mercury in seafood: Mechanisms of accumulation and consequences for consumer health. Rev. Environ. Health 2007, 22, 91–114. [Google Scholar] [CrossRef]
- Abballe, A.; Barbieri, P.G.; di Domenico, A.; Garattini, S.; Iacovella, N.; Ingelido, A.M.; Marra, V.; Miniero, R.; Valentini, S.; De Felip, E. Occupational exposure to PCDDs, PCDFs, and PCBs of metallurgical workers in some industrial plants of the Brescia area, northern Italy. Chemosphere 2013, 90, 49–56. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated biphenyls (PCBs) in the environment: Occupational and exposure events, effects on human health and fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- Jacobs, D.E.; Clickner, R.P.; Zhou, J.Y.; Viet, S.M.; Marker, D.A.; Rogers, J.W.; Zeldin, D.C.; Broene, P.; Friedman, W. The prevalence of lead-based paint hazards in U.S. housing. Environ. Health Perspect. 2002, 110, A599–A606. [Google Scholar] [CrossRef]
- David, O. Carpenter. Polychlorinated Biphenyls (PCBs): Routes of Exposure and Effects on Human Health. Rev. Environ. Health 2006, 21, 1–24. [Google Scholar] [CrossRef]
- Asadollahi-Baboli, M. Exploring QSTR analysis of the toxicity of phenols and thiophenols using machine learning methods. Environ. Toxicol. Pharmacol. 2012, 34, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Luben, T.J.; Buckley, B.J.; Patel, M.M.; Stevens, T.; Coffman, E.; Rappazzo, K.M.; Owens, E.O.; Hines, E.P.; Moore, D.; Painter, K. A cross-disciplinary evaluation of evidence for multipollutant effects on cardiovascular disease. Environ. Res. 2018, 161, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Seubert, J.M.; Kennedy, C.J. Benzo[a]pyrene Toxicokinetics in Rainbow Trout (Oncorhynchus mykiss) Acclimated to Different Salinities. Arch. Environ. Contam. Toxicol. 2000, 38, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R.; Clickner, R.P.; Jeffries, R.A. Adult Women’s Blood Mercury Concentrations Vary Regionally in the United States: Association with Patterns of Fish Consumption (NHANES 1999–2004). Environ. Health Perspect. 2009, 117, 47–53. [Google Scholar] [CrossRef]
- Vaye, O.; Ngumbu, R.S.; Xia, D. A review of the application of comprehensive two-dimensional gas chromatography MS-based techniques for the analysis of persistent organic pollutants and ultra-trace level of organic pollutants in environmental samples. Rev. Anal. Chem. 2022, 41, 63–73. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Whitcomb, B.W.; Louis, G.M.B.; Louis, T.A. Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environ. Health Perspect. 2005, 113, 853–857. [Google Scholar] [CrossRef]
- Lee, E.-S.Y.; Chen, H.; Soliman, K.F.A.; Charlton, C.G. Effects of Homocysteine on the Dopaminergic System and Behavior in Rodents. NeuroToxicology 2005, 26, 361–371. [Google Scholar] [CrossRef]
- Baratta, M.; Jian, W.; Hengel, S.; Kaur, S.; Cunliffe, J.; Boer, J.; Hughes, N.; Kar, S.; Kellie, J.; Kim, Y.J. 2023 White Paper on Recent Issues in Bioanalysis: Deuterated Drugs; LNP; Tumor/FFPE Biopsy; Targeted Proteomics; Small Molecule Covalent Inhibitors; Chiral Bioanalysis; Remote Regulatory Assessments; Sample Reconciliation/Chain of Custody (PART 1A—Recommendations on Mass Spectrometry, Chromatography, Sample Preparation Latest Developments, Challenges, and Solutions and BMV/Regulated Bioanalysis PART 1B—Regulatory Agencies’ Inputs on Regulated Bioanalysis/BMV, Biomarkers/IVD/CDx/BAV, Immunogenicity, Gene & Cell Therapy and Vaccine). Bioanalysis 2024, 16, 307–364. [Google Scholar]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. JACC 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendaño, M.; Muennig, P.; Guida, F.; Ricceri, F.; d’Errico, A.; Barros, H.; Bochud, M.; et al. Socioeconomic status and the 25 x 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017, 389, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Boafo, Y.S.; Mostafa, S.; Obeng-Gyasi, E. Association of Combined Metals and PFAS with Cardiovascular Disease Risk. Toxics 2023, 11, 979. [Google Scholar] [CrossRef]
- Chung, M.K.; House, J.S.; Akhtari, F.S.; Makris, K.C.; Langston, M.A.; Islam, K.T.; Holmes, P.; Chadeau-Hyam, M.; Smirnov, A.I.; Du, X.; et al. Decoding the exposome: Data science methodologies and implications in exposome-wide association studies (ExWASs). Exposome 2024, 4, osae001. [Google Scholar] [CrossRef]
- Yu, H.; Hutson, A.D. A robust Spearman correlation coefficient permutation test. Communications in Statistics Theory Methods 2022, 53, 2141–2153. [Google Scholar] [CrossRef]
- Akinbode, O.L.; Obeng-Gyasi, E. Combined Effects of Arsenic, Cadmium, and Mercury with Cardiovascular Disease Risk: Insights from the All of Us Research Program. Int. J. Environ. Res. Public Health 2025, 22, 239. [Google Scholar] [CrossRef]
- Zar, J.H. Spearman rank correlation. In Encyclopedia of Biostatistics; Wiley: Hoboken, NJ, USA, 2005; Volume 7. [Google Scholar]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Silveyra, P.; Al Housseiny, H.; Rebuli, M.E. Sex and gender differences in the susceptibility to environmental exposures. In Sex-Based Differences in Lung Physiology; Springer Nature: New York, NY, USA, 2021; pp. 251–290. [Google Scholar]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The Importance of Gender to Understand Sex Differences in Cardiovascular Disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef]
- Pearce, N. Epidemiology in Latin America: An opportunity for a global dialogue. J. Epidemiol. Community Health 2008, 62, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Avilés-Santa, M.L.; Parrinello, C.M.; Hanna, D.B.; Jung, M.; Castañeda, S.F.; Hankinson, A.L.; Isasi, C.R.; Birnbaum-Weitzman, O.; Kim, R.S.; et al. Body Mass Index, Sex, and Cardiovascular Disease Risk Factors Among Hispanic/Latino Adults: Hispanic Community Health Study/Study of Latinos. J. Am. Heart Assoc. 2014, 3, e000923. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B.; Cornwell, C.R.; Courtney, J.G.; Ettinger, A.S. Blood Lead Levels in U.S. Children Ages 1–11 Years, 1976–2016. Environ. Health Perspect. 2021, 129, 037003. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Shenassa, E.D. The Environmental “Riskscape” and Social Inequality: Implications for Explaining Maternal and Child Health Disparities. Environ. Health Perspect. 2006, 114, 1150–1153. [Google Scholar] [CrossRef]
- Smith, M.W.; Patterson, N.; Lautenberger, J.A.; Truelove, A.L.; McDonald, G.J.; Waliszewska, A.; Kessing, B.D.; Malasky, M.J.; Scafe, C.; Le, E.; et al. A High-Density Admixture Map for Disease Gene Discovery in African Americans. Am. J. Hum. Genet. 2004, 74, 1001–1013. [Google Scholar] [CrossRef]
- Wigle, D.T. Child Health and the Environment; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Kitayama, S.; Varnum, M.E.; Salvador, C.E. Cultural neuroscience. In Handbook of Cultural Psychology; The Guilford Press: New York, NY, USA, 2019; pp. 79–118. [Google Scholar]
- Aminov, Z.; Haase, R.F.; Pavuk, M.; Carpenter, D.O.; Anniston Environmental Health Research Consortium. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. Environ. Health 2013, 12, 108. [Google Scholar] [CrossRef]
- Goncharov, A.; Haase, R.F.; Santiago-Rivera, A.; Morse, G.; McCaffrey, R.J.; Rej, R.; Carpenter, D.O. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ. Res. 2008, 106, 226–239. [Google Scholar] [CrossRef]
- Lind, P.M.; van Bavel, B.; Salihovic, S.; Lind, L. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environ. Health Perspect. 2012, 120, 38–43. [Google Scholar] [CrossRef]
- Everett, C.J.; Mainous, A.G., 3rd; Frithsen, I.L.; Player, M.S.; Matheson, E.M. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2008, 108, 94–97. [Google Scholar] [CrossRef]
- O’Neill, M.S.; Diez-Roux, A.V.; Auchincloss, A.H.; Shen, M.; Lima, J.A.; Polak, J.F.; Barr, R.G.; Kaufman, J.; Jacobs, D.R. Long-Term Exposure to Airborne Particles and Arterial Stiffness: The Multi-Ethnic Study of Atherosclerosis (MESA). Environ. Health Perspect. 2011, 119, 844–851. [Google Scholar] [CrossRef]
- Avila-Alejo, J.O.; González-Palomo, A.K.; Plascencia-Villa, G.; José-Yacamán, M.; Navarro-Contreras, H.R.; Pérez-Maldonado, I.N. Low cytotoxicity of anisotropic gold nanoparticles coated with lysine on peripheral blood mononuclear cells “in vitro”. Environ. Toxicol. Pharmacol. 2017, 56, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Newcomb, P.A. Urinary Cadmium as a Marker of Exposure in Epidemiological Studies. Environ. Health Perspect. 2013, 121, A296. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M.L. Protecting Human Health From Air Pollution: Shifting From a Single-pollutant to a Multipollutant Approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Kortenkamp, A. Ten Years of Mixing Cocktails: A Review of Combination Effects of Endocrine-Disrupting Chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The Dose-Response Revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Lind, L.; Lind, P.M. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J. Intern. Med. 2012, 271, 537–553. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.L.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Lettmeier, B.; Gothe, R.M.; Beinhoff, C.; Siebert, U.; Drasch, G. Mercury as a serious health hazard for children in gold mining areas. Environ. Res. 2008, 107, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Bechi, N.; Ietta, F.; Romagnoli, R.; Focardi, S.; Corsi, I.; Buffi, C.; Paulesu, L. Estrogen-Like Response to p-Nonylphenol in Human First Trimester Placenta and BeWo Choriocarcinoma Cells. Toxicol. Sci. 2006, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Larkin, A.; Siddens, L.K.; Krueger, S.K.; Tilton, S.C.; Waters, K.M.; Williams, D.E.; Baird, W.M. Application of a fuzzy neural network model in predicting polycyclic aromatic hydrocarbon-mediated perturbations of the Cyp1b1 transcriptional regulatory network in mouse skin. Toxicol. Appl. Pharmacol. 2013, 267, 192–199. [Google Scholar] [CrossRef]
- Merrill, M.L.; Emond, C.; Kim, M.J.; Antignac, J.-P.; Bizec, B.L.; Clément, K.; Birnbaum, L.S.; Barouki, R. Toxicological Function of Adipose Tissue: Focus on Persistent Organic Pollutants. Environ. Health Perspect. 2013, 121, 162–169. [Google Scholar] [CrossRef]
- Vaziri, N.D. Mechanisms of lead-induced hypertension and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H454–H465. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease--a systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Lamas, G.A.; Bhatnagar, A.; Jones, M.R.; Mann, K.K.; Nasir, K.; Tellez-Plaza, M.; Ujueta, F.; Navas-Acien, A. Contaminant Metals as Cardiovascular Risk Factors: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2023, 12, e029852. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Jones, M.R.; Dominguez-Lucas, A.; Guallar, E.; Navas-Acien, A. Cadmium exposure and clinical cardiovascular disease: A systematic review. Curr. Atheroscler. Rep. 2013, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Pavuk, M.; Serio, T.C.; Cusack, C.; Cave, M.; Rosenbaum, P.F.; Birnbaum, L.S. Hypertension in Relation to Dioxins and Polychlorinated Biphenyls from the Anniston Community Health Survey Follow-Up. Environ. Health Perspect. 2019, 127, 127007. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Arisawa, K.; Hiyoshi, M.; Satoh, H.; Sumiyoshi, Y.; Morinaga, K.; Kodama, K.; Suzuki, T.; Nagai, M.; Suzuki, T. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environ. Res. 2008, 108, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Melymuk, L.; Blumenthal, J.; Sáňka, O.; Shu-Yin, A.; Singla, V.; Šebková, K.; Pullen Fedinick, K.; Diamond, M.L. Persistent Problem: Global Challenges to Managing PCBs. Environ. Sci. Technol. 2022, 56, 9029–9040. [Google Scholar] [CrossRef]
- Singh, K.; Chan, H.M. Association of blood polychlorinated biphenyls and cholesterol levels among Canadian Inuit. Environ. Res. 2018, 160, 298–305. [Google Scholar] [CrossRef]
- Bertazzi, P.A.; Bernucci, I.; Brambilla, G.; Consonni, D.; Pesatori, A.C. The Seveso studies on early and long-term effects of dioxin exposure: A review. Environ. Health Perspect. 1998, 106, 625–633. [Google Scholar] [CrossRef]
- Lind, P.M.; Penell, J.; Salihovic, S.; van Bavel, B.; Lind, L. Circulating levels of p,p′-DDE are related to prevalent hypertension in the elderly. Environ. Res. 2014, 129, 27–31. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Faroon, O.; Ruiz, P. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health 2016, 32, 1825–1847. [Google Scholar] [CrossRef]
- Czarnota, J.; Gennings, C.; Wheeler, D.C. Assessment of Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. Cancer Inform. 2015, 14 (Suppl. 2), 159–171. [Google Scholar] [CrossRef]
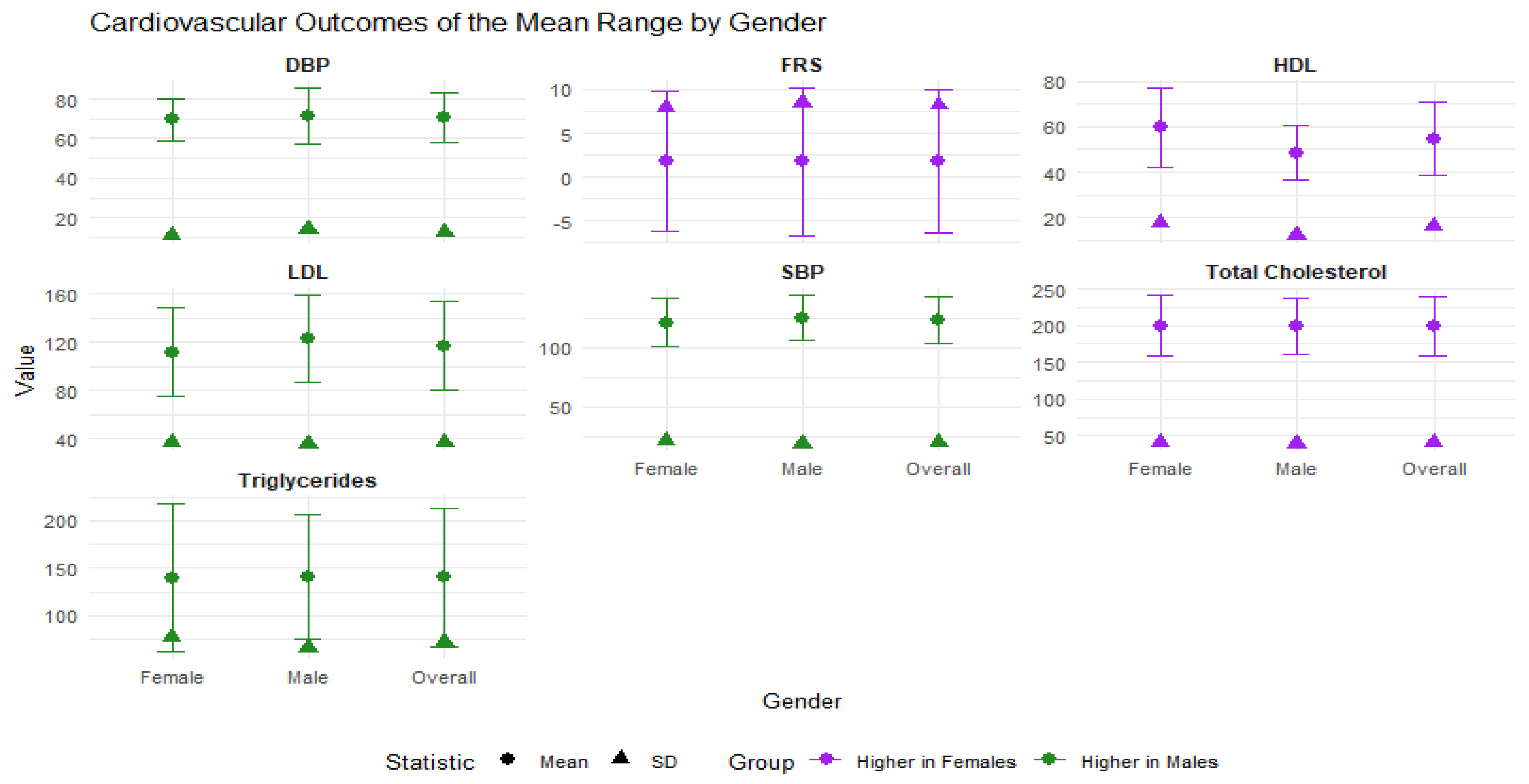
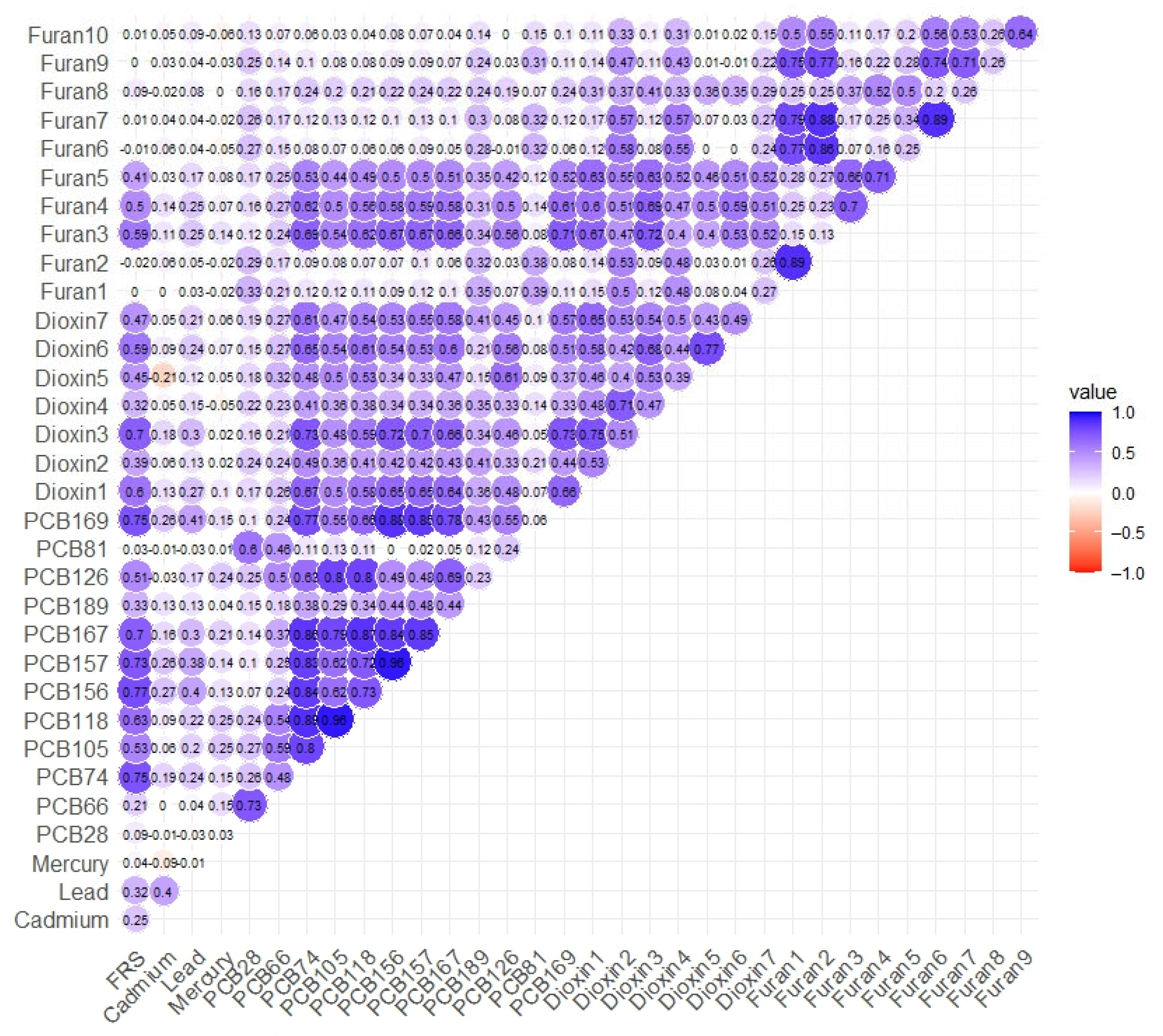
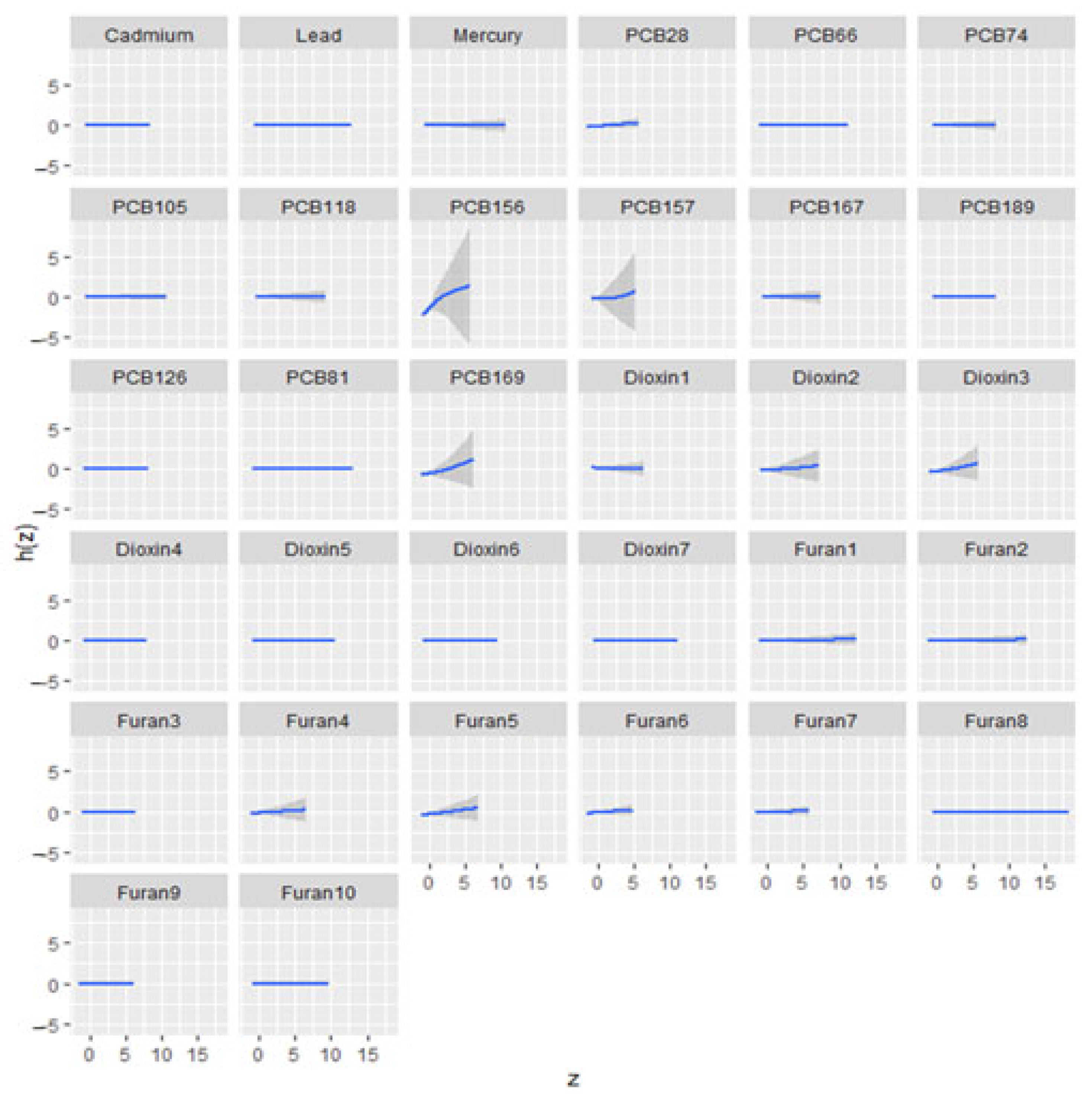

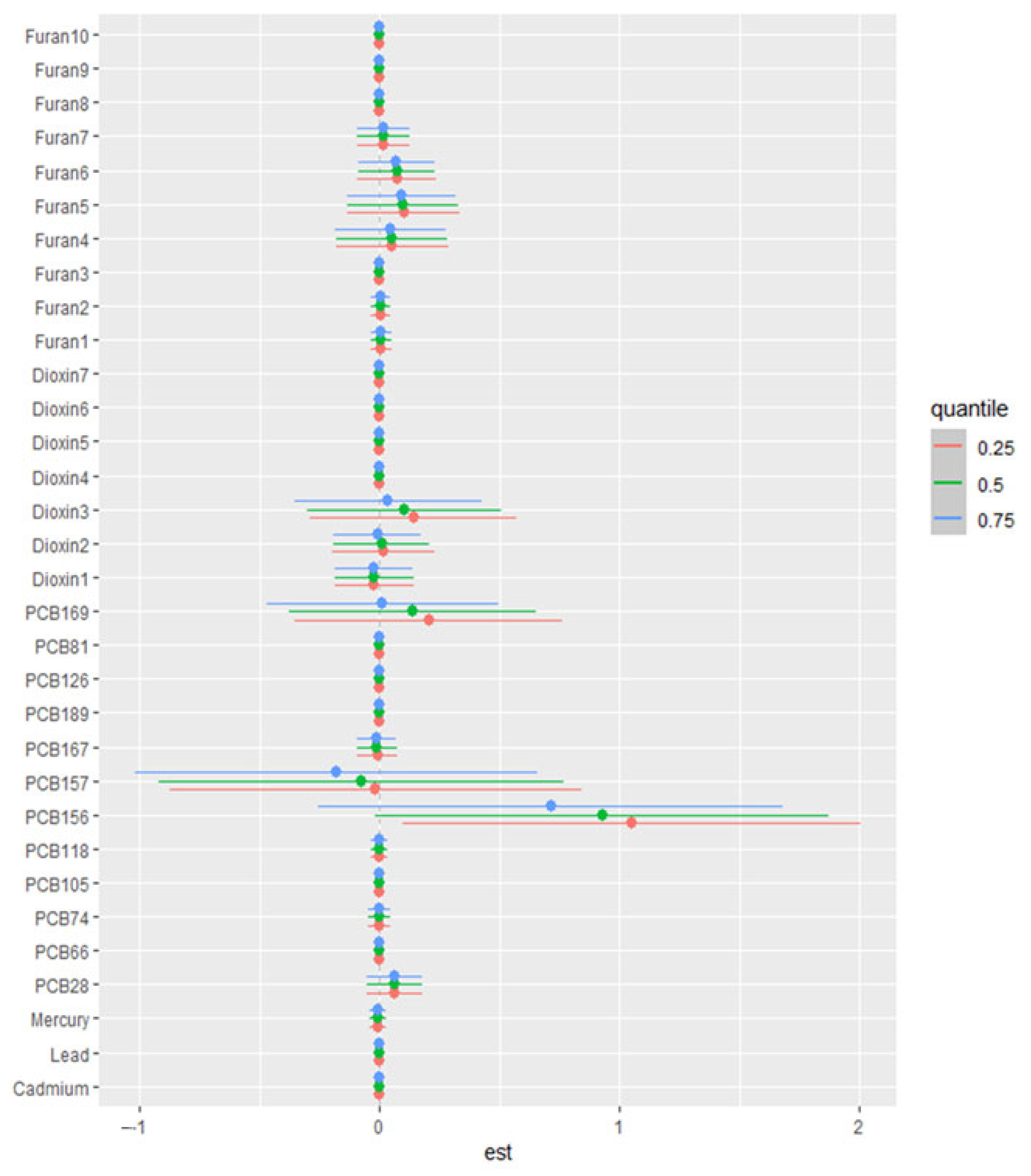

| n = 432 | Overall Mean (S.D) | Male Mean (S.D) | Female Mean (S.D) | |
|---|---|---|---|---|
| AGE (years) | Age | 46.26 (17.17) | 47.56 (17.54) | 45.16 (16.82) |
| BMI | BMI | 28.31 (6.07) | 27.98 (5.26) | 28.60 (6.68) |
| INCOME | $0 to $14,999 | 13.19% (57) | 12.6% (25) | 13.6% (32) |
| $15,000 to $24,999 | 14.81% (64) | 13.2% (26) | 16.2% (38) | |
| $25,000 to $44,999 | 24.31% (105) | 25.2% (50) | 23.5% (55) | |
| $45,000 to $64,999 | 9.49% (41) | 14.1% (28) | 14.1% (33) | |
| $65,000 to $74,999 | 6.25% (27) | 7.6% (15) | 5.1% (12) | |
| $75,000 and over | 22.22% (96) | 22.7% (45) | 21.8% (51) | |
| (%, n) | Unavailable | 5.09% (22) | 4.5% (9) | 5.6% (13) |
| ETHNICITY (%, n) | Mexican American | 57.17% (247) | 57.1% (113) | 57.3% (134) |
| Other Hispanic | 16.9% (73) | 17.2% (34) | 16.7% (39) | |
| Non-Hispanic White | 19.21% (83) | 17.2% (34) | 20.9% (49) | |
| Non-Hispanic Black | 3.24% (14) | 3.5% (7) | 3.0% (7) | |
| Other Race | 3.47% (15) | 5.1% (10) | 2.1% (5) | |
| SMOKERS (%, n) | No | 29.40% (127) | 33.8% (67) | 25.6% (60) |
| Yes | 18.98% (82) | 25.8% (51) | 13.2% (31) | |
| NA | 51.62% (223) | 40.4% (80) | 61.1% (143) | |
| ALCOHOL | No | 25.7% (111) | 17.7% (35) | 32.5% (76) |
| Yes | 68.5% (296) | 80.3% (159) | 58.5% (137) | |
| (%, n) | NA | 5.8% (25) | 2.0% (4) | 9.0% (21) |
| Overall Mean (S.D) | Male Mean (S.D) | Female Mean (S.D) | p_Value | |
|---|---|---|---|---|
| Cadmium | 0.58 (0.76) | 0.60 (0.81) | 0.56 (0.72) | 0.6613 |
| Lead | 2.07 (2.51) | 2.68 (3.40) | 1.55 (1.13) | <0.0001 |
| Mercury | 1.86 (2.91) | 1.84 (2.66) | 1.87 (3.12) | 0.927 |
| PCB28 | 5.41 (2.79) | 5.30 (2.66) | 5.50 (2.90) | 0.4558 |
| PCB66 | 1.64 (1.33) | 1.45 (0.85) | 1.80 (1.61) | 0.0034 |
| PCB74 | 7.97 (10.34) | 6.88 (9.56) | 8.90 (10.90) | 0.0408 |
| PCB105 | 2.18 (3.86) | 1.84 (3.57) | 2.47 (4.08) | 0.0854 |
| PCB118 | 10.92 (18.13) | 9.33 (16.43) | 12.27 (19.37) | 0.0882 |
| PCB156 | 5.69 (6.34) | 6.37 (6.58) | 5.11 (6.08) | 0.0408 |
| PCB157 | 1.36 (1.57) | 1.49 (1.63) | 1.25 (1.52) | 0.1113 |
| PCB167 | 1.38 (1.98) | 1.36 (2.05) | 1.40 (1.92) | 0.8553 |
| PCB189 | 0.39 (0.66) | 0.46 (0.73) | 0.33 (0.58) | 0.048 |
| PCB126 | 26.11 (32.02) | 23.13 (28.01) | 28.64 (34.91) | 0.0694 |
| PCB81 | 6.04 (4.73) | 6.28 (5.82) | 5.83 (3.57) | 0.3365 |
| PCB169 | 15.94 (14.81) | 18.96 (16.97) | 13.39 (12.16) | 0.0001 |
| 1,2,3,7,8-PeCDD | 4.49 (4.23) | 4.72 (4.10) | 4.30 (4.33) | 0.3012 |
| 1,2,3,4,7,8-HxCDD | 4.20 (3.45) | 4.37 (3.61) | 4.05 (3.31) | 0.3279 |
| 1,2,3,6,7,8-HxCDD | 28.35 (24.48) | 30.21 (25.76) | 26.77 (23.28) | 0.1494 |
| 1,2,3,7,8,9-HxCDD | 4.44 (3.68) | 4.37 (3.30) | 4.50 (3.97) | 0.7103 |
| 1,2,3,4,6,7,8-HpCDD | 36.81 (35.32) | 32.19 (26.33) | 40.72 (41.08) | 0.0095 |
| 1,2,3,4,6,7,8,9-OCDD | 297.51 (309.91) | 250.82 (274.35) | 337.01 (332.58) | 0.0033 |
| 2,3,7,8-TCDD | 1.99 (2.24) | 1.89 (2.36) | 2.07 (2.13) | 0.3863 |
| 2,3,7,8-TCDF | 1.61 (0.81) | 1.69 (0.88) | 1.53 (0.73) | 0.0417 |
| 1,2,3,7,8-PeCDF | 1.67 (0.66) | 1.78 (0.76) | 1.58 (0.55) | 0.0019 |
| 2,3,4,7,8-PeCDF | 5.20 (3.92) | 5.40 (4.03) | 5.03 (3.81) | 0.3298 |
| 1,2,3,4,7,8-HxCDF | 4.19 (2.90) | 4.41 (2.92) | 4.00 (2.87) | 0.1421 |
| 1,2,3,6,7,8-HxCDF | 3.74 (2.61) | 3.96 (2.76) | 3.56 (2.46) | 0.1181 |
| 1,2,3,7,8,9-HxCDF | 1.91 (0.74) | 2.02 (0.71) | 1.82 (0.75) | 0.0049 |
| 2,3,4,6,7,8-HxCDF | 1.90 (0.74) | 2.02 (0.71) | 1.80 (0.74) | 0.0019 |
| 1,2,3,4,6,7,8-HpCDF | 9.48 (16.74) | 10.96 (23.27) | 8.23 (7.57) | 0.1139 |
| 1,2,3,4,7,8,9-HpCDF | 2.03 (0.84) | 2.23 (0.92) | 1.87 (0.73) | <0.0001 |
| 1,2,3,4,6,7,8,9-OCDF | 3.60 (2.99) | 4.06 (3.35) | 3.20 (2.58) | 0.0033 |
| FRS | DBP | SBP | HDL Cholesterol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | 2.50% | 97.50% | Estimate | 2.50% | 97.50% | Estimate | 2.50% | 97.50% | Estimate | 2.50% | 97.50% |
| Cadmium | 0.0524 | −0.2425 | 0.3473 | 0.2543 | −2.0124 | 2.5211 | 0.3376 | −2.6814 | 3.3567 | 0.8949 | −1.6259 | 3.4157 |
| Lead | 0.0326 | −0.0847 | 0.1498 | −0.3343 | −1.2356 | 0.567 | −0.4529 | −1.6534 | 0.7476 | 1.6352 | 0.6328 | 2.6375 |
| Mercury | −0.1294 | −0.2366 | −0.0221 | −0.3496 | −1.1742 | 0.4749 | −0.7925 | −1.8907 | 0.3056 | 0.5281 | −0.3889 | 1.445 |
| PCB28 | 0.0781 | −0.0789 | 0.2351 | −0.3706 | −1.5774 | 0.8361 | 0.1664 | −1.4409 | 1.7737 | 0.078 | −1.264 | 1.42 |
| PCB66 | 0 × 10⁰ | −0.5203 | 0.5204 | −1.787 | −5.7868 | 2.2131 | −0.3795 | −5.7069 | 4.948 | −1.517 | −5.9652 | 2.9311 |
| PCB74 | −0.0362 | −0.1646 | 0.0922 | −0.242 | −1.2288 | 0.7449 | −0.0446 | −1.359 | 1.2699 | −0.3141 | −1.4115 | 0.7834 |
| PCB105 | 0.4967 | −0.2493 | 1.2427 | 2.955 | −2.7797 | 8.6892 | −2.4813 | −10.1189 | 5.1564 | 7.2819 | 0.9049 | 13.6589 |
| PCB118 | −0.1188 | −0.296 | 0.0584 | −0.6759 | −2.0379 | 0.6861 | −0.0098 | −1.8238 | 1.8043 | −1.5648 | −3.0794 | −0.0502 |
| PCB156 | 0.0589 | −0.2569 | 0.3747 | 1.441 | −0.9861 | 3.8687 | −0.244 | −3.4769 | 2.9891 | −2.5911 | −5.2905 | 0.1083 |
| PCB157 | 0.0641 | −1.3694 | 1.4977 | −4.796 | −15.8161 | 6.2233 | −0.6909 | −15.3679 | 13.9862 | 11.845 | −0.4095 | 24.0995 |
| PCB167 | 0.4992 | −0.3142 | 1.3126 | 3.43 | −2.8221 | 9.6824 | 8.4078 | 0.0805 | 16.7351 | 2.3399 | −4.6129 | 9.2928 |
| PCB189 | −0.2927 | −0.8616 | 0.2762 | 0.1178 | −4.2552 | 4.4908 | −0.0504 | −5.8747 | 5.7739 | −3.419 | −8.2819 | 1.444 |
| PCB126 | −0.0216 | −0.0463 | 0.003 | −0.0461 | −0.2358 | 0.1436 | 0.1438 | −0.1089 | 0.3964 | −0.0226 | −0.2335 | 0.1884 |
| PCB81 | 0.0363 | −0.0515 | 0.1241 | 0.5052 | −0.17 | 1.1804 | −0.6219 | −1.5212 | 0.2774 | −0.2184 | −0.9693 | 0.5325 |
| PCB169 | −0.0186 | −0.0729 | 0.0356 | −0.0857 | −0.5025 | 0.3311 | −0.2218 | −0.7769 | 0.3333 | −0.2154 | −0.6789 | 0.2481 |
| 1,2,3,7,8-PeCDD | 0.0232 | −0.1216 | 0.168 | −0.1989 | −1.312 | 0.9142 | 1.2638 | −0.2187 | 2.7463 | 1.1602 | −0.0776 | 2.3981 |
| 1,2,3,4,7,8-HxCDD | −0.0947 | −0.2586 | 0.0693 | −0.2817 | −1.5423 | 0.9789 | −0.063 | −1.742 | 1.616 | 1.8077 | 0.4058 | 3.2096 |
| 1,2,3,6,7,8-HxCDD | −0.0076 | −0.0343 | 0.0192 | −0.0011 | −0.2065 | 0.2043 | 0.1795 | −0.0941 | 0.4531 | −0.2212 | −0.4496 | 0.0072 |
| 1,2,3,7,8,9-HxCDD | −0.0133 | −0.1441 | 0.1174 | −0.2434 | −1.2486 | 0.7618 | −1.0308 | −2.3696 | 0.308 | −0.5812 | −1.6991 | 0.5366 |
| 1,2,3,4,6,7,8-HpCDD | 0.0047 | −0.016 | 0.0254 | −0.0683 | −0.2274 | 0.0909 | −0.1302 | −0.3421 | 0.0818 | −0.1826 | −0.3595 | −0.0056 |
| 1,2,3,4,6,7,8,9-OCDD | −0.0009 | −0.0031 | 0.0014 | 0.0182 | 0.0008 | 0.0356 | 0.0359 | 0.0128 | 0.0591 | 0.0126 | −0.0068 | 0.0319 |
| 2,3,7,8-TCDD | 0.1505 | −0.1338 | 0.4348 | 0.7044 | −1.4809 | 2.8898 | 0.2198 | −2.6909 | 3.1304 | 0.1668 | −2.2635 | 2.597 |
| 2,3,7,8-TCDF | −0.2456 | −1.1107 | 0.6194 | 2.056 | −4.5931 | 8.7058 | −1.1339 | −9.9902 | 7.7224 | 9.3056 | 1.911 | 16.7002 |
| 1,2,3,7,8-PeCDF | 0.5568 | −0.6099 | 1.7235 | −3.498 | −12.4659 | 5.4708 | 0.0152 | −11.9296 | 11.96 | −12.5138 | −22.4871 | −2.5405 |
| 2,3,4,7,8-PeCDF | 0.0213 | −0.1563 | 0.1989 | −0.0248 | −1.3901 | 1.3405 | −2.1838 | −4.0022 | −0.3654 | 0.2497 | −1.2686 | 1.768 |
| 1,2,3,4,7,8-HxCDF | 0.0002 | −0.2087 | 0.2091 | −0.7256 | −2.3316 | 0.8803 | 0.4267 | −1.7122 | 2.5657 | −0.9021 | −2.688 | 0.8838 |
| 1,2,3,6,7,8-HxCDF | 0.0025 | −0.1992 | 0.2042 | 0 × 10⁰ | −1.5503 | 1.5504 | −0.9673 | −3.0322 | 1.0976 | 0.5732 | −1.1508 | 2.2973 |
| 1,2,3,7,8,9-HxCDF | −0.1117 | −0.891 | 0.6675 | −2.92 | −8.9105 | 3.0702 | −7.9312 | −15.9097 | 0.0472 | 0.1835 | −6.4781 | 6.8451 |
| 2,3,4,6,7,8-HxCDF | 0.2336 | −0.6677 | 1.1349 | 6.342 | −0.5864 | 13.2707 | 7.4923 | −1.7358 | 16.7204 | 2.4686 | −5.2364 | 10.1735 |
| 1,2,3,4,6,7,8-HpCDF | 0.0283 | −0.0085 | 0.0651 | 0.0772 | −0.2056 | 0.3599 | −0.1359 | −0.5124 | 0.2407 | 0.2335 | −0.0809 | 0.548 |
| 1,2,3,4,7,8,9-HpCDF | −0.0298 | −0.3969 | 0.3374 | 0.9836 | −1.8387 | 3.8059 | 4.6609 | 0.902 | 8.4199 | 0.2411 | −2.8974 | 3.3796 |
| 1,2,3,4,6,7,8,9-OCDF | −0.0112 | −0.1118 | 0.0894 | −0.618 | −1.3916 | 0.1557 | 0.4179 | −0.6126 | 1.4483 | −0.759 | −1.6193 | 0.1014 |
| LDL Cholesterol | Total Cholesterol | Triglycerides | ||||||||||
| Variable | Estimate | 2.50% | 97.50% | Estimate | 2.50% | 97.50% | Estimate | 2.50% | 97.50% | |||
| Cadmium | −6.0506 | −12.1415 | 0.0402 | −3.7856 | −9.9776 | 2.4065 | 6.8229 | −4.225 | 17.8708 | |||
| Lead | 1.2753 | −1.1466 | 3.6972 | 2.3144 | −0.1477 | 3.6972 | −3.01 | −7.4029 | 1.383 | |||
| Mercury | 0.1087 | −2.1068 | 2.3242 | 0.1087 | −1.8907 | 2.3242 | −0.3652 | −4.225 | 3.9022 | |||
| PCB28 | −0.9122 | −4.1548 | 2.3305 | 0.1664 | −1.4409 | 1.7737 | −2.2123 | −2.9085 | 2.7421 | |||
| PCB66 | 1.6917 | −9.0562 | 12.4397 | −0.5855 | −5.7069 | 10.341 | −3.8406 | −11.512 | 10.341 | |||
| PCB74 | 0.3296 | −2.3222 | 2.9814 | 0.4055 | −2.3222 | 3.1013 | 0.4055 | −2.2904 | 6.8105 | |||
| PCB105 | 10.7004 | −4.7082 | 26.109 | 16.702 | 1.0374 | 32.3666 | −4.641 | −33.72 | 22.178 | |||
| PCB118 | −3.5709 | −7.2307 | 0.0888 | −4.641 | −8.3615 | −0.9205 | 2.3313 | −4.3069 | 8.9695 | |||
| PCB156 | 5.4245 | −1.098 | 11.9469 | 5.4245 | −3.7879 | 9.4737 | 0.0808 | −11.7499 | 11.9116 | |||
| PCB157 | −16.7059 | −46.3162 | 12.9044 | −16.7059 | −46.3162 | 13.9862 | −29.8984 | −15.3679 | 13.9862 | |||
| PCB167 | 15.0296 | −1.7705 | 31.8296 | 13.2651 | −1.7705 | 31.8296 | −20.0693 | −51.7845 | 11.2449 | |||
| PCB189 | −13.2181 | −24.9684 | −1.4678 | −12.376 | −24.3219 | −1.4678 | 21.1604 | −5.1529 | 30.3442 | |||
| PCB126 | −0.3785 | −0.8882 | 0.1312 | −0.1211 | −0.8882 | 0.1312 | 1.3933 | 0.4679 | 4.3539 | |||
| PCB81 | 0.7397 | −1.0746 | 2.554 | 0.7397 | −1.0746 | 2.554 | −2.8892 | −4.5673 | 2.4178 | |||
| PCB169 | −1.7456 | −2.8655 | −0.6256 | −1.522 | −2.6606 | −0.3835 | 2.1635 | 0.1321 | 4.1949 | |||
| 1,2,3,7,8-PeCDD | −0.4382 | −3.4291 | 2.5528 | 1.1698 | −1.8708 | 4.2104 | 1.1698 | −3.1078 | 7.7425 | |||
| 1,2,3,4,7,8-HxCDD | 0.5033 | −2.8841 | 3.8906 | 1.9035 | −1.5401 | 3.8906 | −2.0164 | −8.1605 | 4.9059 | |||
| 1,2,3,6,7,8-HxCDD | −0.5101 | −1.062 | 0.0419 | −0.5101 | −1.062 | 0.0419 | −1.0918 | −2.092 | 0.4731 | |||
| 1,2,3,7,8,9-HxCDD | 1.6823 | −1.0187 | 4.3833 | −1.0308 | −1.2517 | 0.308 | 1.8724 | −1.8708 | 7.7425 | |||
| 1,2,3,4,6,7,8-HpCDD | 0.2382 | −0.1894 | 0.6658 | 0.033 | −0.1894 | 0.6658 | −0.1302 | −0.4017 | 0.6734 | |||
| 1,2,3,4,6,7,8,9-OCDD | −0.0371 | −0.0838 | 0.0096 | −0.0188 | −0.0663 | 0.0287 | 0.029 | −0.0557 | 0.1137 | |||
| 2,3,7,8-TCDD | 0.309 | −5.5632 | 6.1812 | 0.3048 | −5.6649 | 6.2745 | −0.9104 | −11.5616 | 9.7409 | |||
| 2,3,7,8-TCDF | −0.5556 | −18.423 | 17.3117 | 4.2134 | −13.9507 | 22.3776 | −23.0568 | −55.4654 | 9.3519 | |||
| 1,2,3,7,8-PeCDF | −0.4972 | −24.5954 | 23.6011 | −12.5138 | −34.5954 | 23.6011 | 0.0152 | −34.2253 | 60.3987 | |||
| 2,3,4,7,8-PeCDF | 2.6819 | −0.9866 | 6.3505 | 3.1156 | −0.9866 | 6.8451 | 3.1156 | −0.6139 | 6.8452 | |||
| 1,2,3,4,7,8-HxCDF | 2.7534 | −1.5619 | 7.0686 | 2.4715 | −1.9159 | 7.0686 | 2.9631 | −1.9154 | 6.8584 | |||
| 1,2,3,6,7,8-HxCDF | −0.0623 | −4.2281 | 4.1036 | 0.6035 | −4.2281 | 4.1036 | 0.5045 | −3.6316 | 4.8386 | |||
| 1,2,3,7,8,9-HxCDF | −4.5552 | −20.6514 | 11.541 | −4.5552 | −20.6514 | 11.541 | −7.9312 | −63.7826 | −5.3902 | |||
| 2,3,4,6,7,8-HxCDF | −2.6908 | −21.3081 | 15.9265 | −2.6908 | −17.7634 | 20.0898 | 1.1632 | −26.8828 | 40.6553 | |||
| 1,2,3,4,6,7,8-HpCDF | −0.1103 | −0.87 | 0.6495 | −0.2424 | −0.87 | 0.6495 | −1.8075 | −3.1859 | −0.4299 | |||
| 1,2,3,4,7,8,9-HpCDF | −11.0937 | −18.6773 | −3.5102 | −12.3215 | −20.0315 | −3.5102 | −7.3934 | −21.1488 | 6.9623 | |||
| 1,2,3,4,6,7,8,9-OCDF | 0.5159 | −1.563 | 2.5947 | 0.5159 | −1.563 | 2.5947 | 2.7165 | −0.6126 | 1.4483 | |||
| DBP | SBP | FRS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | Group PIP | Conditional PIP | Group PIP | Conditional PIP | Group PIP | Conditional PIP | ||
| Cadmium | 1 | 0.1662 | 0.0173 | 0.0054 | 0.2593 | 0.0006 | 0 | ||
| Lead | 1 | 0.1662 | 0.0327 | 0.0054 | 0.3704 | 0.0006 | 0 | ||
| Mercury | 1 | 0.1662 | 0.95 | 0.0054 | 0.3704 | 0.0006 | 1 | ||
| PCB28 | 2 | 0.0703 | 0.0381 | 0.9019 | 0.0008 | 0.9336 | 0.0018 | ||
| PCB66 | 2 | 0.0703 | 0.0188 | 0.9019 | 0.0412 | 0.9336 | 0 | ||
| PCB74 | 2 | 0.0703 | 0.1507 | 0.9019 | 0.0267 | 0.9336 | 0.0006 | ||
| PCB105 | 2 | 0.0703 | 0.0148 | 0.9019 | 0.0169 | 0.9336 | 0.0001 | ||
| PCB118 | 2 | 0.0703 | 0.0205 | 0.9019 | 0.0608 | 0.9336 | 0.0007 | ||
| PCB156 | 2 | 0.0703 | 0.0939 | 0.9019 | 0 | 0.9336 | 0.6638 | ||
| PCB157 | 2 | 0.0703 | 0.0825 | 0.9019 | 0 | 0.9336 | 0.1381 | ||
| PCB167 | 2 | 0.0703 | 0.0324 | 0.9019 | 0.0024 | 0.9336 | 0.0001 | ||
| PCB189 | 2 | 0.0703 | 0.0324 | 0.9019 | 0.0003 | 0.9336 | 0 | ||
| PCB126 | 2 | 0.0703 | 0.0267 | 0.9019 | 0.8508 | 0.9336 | 0 | ||
| PCB81 | 2 | 0.0703 | 0.06 | 0.9019 | 0 | 0.9336 | 0 | ||
| PCB169 | 2 | 0.0703 | 0.4295 | 0.9019 | 0 | 0.9336 | 0.2073 | ||
| 1,2,3,7,8-PeCDD | 3 | 0.0978 | 0.2834 | 0.2299 | 0.0642 | 0.066 | 0.2121 | ||
| 1,2,3,4,7,8-HxCDD | 3 | 0.0978 | 0.2036 | 0.2299 | 0.003 | 0.066 | 0.3861 | ||
| 1,2,3,6,7,8-HxCDD | 3 | 0.0978 | 0.2931 | 0.2299 | 0.0046 | 0.066 | 0.5927 | ||
| 1,2,3,7,8,9-HxCDD | 3 | 0.0978 | 0.0961 | 0.2299 | 0.0863 | 0.066 | 0 | ||
| 1,2,3,4,6,7,8-HpCDD | 3 | 0.0978 | 0.0446 | 0.2299 | 0.3689 | 0.066 | 0 | ||
| 1,2,3,4,6,7,8,9-OCDD | 3 | 0.0978 | 0.0209 | 0.2299 | 0.0268 | 0.066 | 0 | ||
| 2,3,7,8-TCDD | 3 | 0.0978 | 0.0585 | 0.2299 | 0.0002 | 0.066 | 0 | ||
| 2,3,7,8-TCDF | 4 | 0.1164 | 0.0144 | 0.183 | 0.0136 | 0.0239 | 0.0151 | ||
| 1,2,3,7,8-PeCDF | 4 | 0.1164 | 0.0491 | 0.183 | 0.0052 | 0.0239 | 0.0084 | ||
| 2,3,4,7,8-PeCDF | 4 | 0.1164 | 0.1391 | 0.183 | 0.92 | 0.0239 | 0 | ||
| 1,2,3,4,7,8-HxCDF | 4 | 0.1164 | 0.0325 | 0.183 | 0.0466 | 0.0239 | 0.2993 | ||
| 1,2,3,6,7,8-HxCDF | 4 | 0.1164 | 0.0306 | 0.183 | 0.0037 | 0.0239 | 0.51 | ||
| 1,2,3,7,8,9-HxCDF | 4 | 0.1164 | 0.1404 | 0.183 | 0.0015 | 0.0239 | 0.0124 | ||
| 2,3,4,6,7,8-HxCDF | 4 | 0.1164 | 0.1431 | 0.183 | 0.0033 | 0.0239 | 0.0435 | ||
| 1,2,3,4,6,7,8-HpCDF | 4 | 0.1164 | 0 | 0.183 | 0 | 0.0239 | 0 | ||
| 1,2,3,4,7,8,9-HpCDF | 4 | 0.1164 | 0.0908 | 0.183 | 0.061 | 0.0239 | 0 | ||
| 1,2,3,4,6,7,8,9-OCDF | 4 | 0.1164 | 0.0745 | 0.183 | 0 | 0.0239 | 0 | ||
| HDL | LDL Cholesterol | Total Cholesterol | Triglycerides | ||||||
| Variable | Group | Group PIP | Conditional PIP | Group PIP | Conditional PIP | Group PIP | Conditional PIP | Group PIP | Conditional PIP |
| Cadmium | 1 | 0.0882 | 0 | 0.2946 | 0.9293 | 0.0052 | 0.9845 | 0.8211 | 0.0002 |
| Lead | 1 | 0.0882 | 0.0163 | 0.2946 | 0.0648 | 0.0052 | 0.0155 | 0.8211 | 0.9208 |
| Mercury | 1 | 0.0882 | 0.9837 | 0.2946 | 0.0058 | 0.0052 | 0 | 0.8211 | 0.079 |
| PCB28 | 2 | 0.0491 | 0.1079 | 0.9383 | 0 | 0.0395 | 0 | 0.4136 | 0.0089 |
| PCB66 | 2 | 0.0491 | 0.0179 | 0.9383 | 0.001 | 0.0395 | 0 | 0.4136 | 0.0037 |
| PCB74 | 2 | 0.0491 | 0.0204 | 0.9383 | 0.0017 | 0.0395 | 0.1266 | 0.4136 | 0.0147 |
| PCB105 | 2 | 0.0491 | 0.048 | 0.9383 | 0.0014 | 0.0395 | 0 | 0.4136 | 0.0112 |
| PCB118 | 2 | 0.0491 | 0.0798 | 0.9383 | 0.0037 | 0.0395 | 0.0162 | 0.4136 | 0.0074 |
| PCB156 | 2 | 0.0491 | 0.0953 | 0.9383 | 0.004 | 0.0395 | 0.2452 | 0.4136 | 0.0181 |
| PCB157 | 2 | 0.0491 | 0.1743 | 0.9383 | 0.0084 | 0.0395 | 0.2817 | 0.4136 | 0.027 |
| PCB167 | 2 | 0.0491 | 0.0497 | 0.9383 | 0.006 | 0.0395 | 0.0794 | 0.4136 | 0.0155 |
| PCB189 | 2 | 0.0491 | 0.0204 | 0.9383 | 0.0028 | 0.0395 | 0.0993 | 0.4136 | 0.017 |
| PCB126 | 2 | 0.0491 | 0.0497 | 0.9383 | 0.002 | 0.0395 | 0 | 0.4136 | 0.8508 |
| PCB81 | 2 | 0.0491 | 0 | 0.9383 | 0 | 0.0395 | 0 | 0.4136 | 0.0147 |
| PCB169 | 2 | 0.0491 | 0.1531 | 0.9383 | 0.9698 | 0.0395 | 0.157 | 0.4136 | 0.0109 |
| 1,2,3,7,8-PeCDD | 3 | 0.0733 | 0.4037 | 0.3343 | 0.5271 | 0.0112 | 0.0573 | 0.3652 | 0.0194 |
| 1,2,3,4,7,8-HxCDD | 3 | 0.0733 | 0.0327 | 0.3343 | 0.0125 | 0.0112 | 0.0072 | 0.3652 | 0.0194 |
| 1,2,3,6,7,8-HxCDD | 3 | 0.0733 | 0.2714 | 0.3343 | 0.1351 | 0.0112 | 0.8172 | 0.3652 | 0.5877 |
| 1,2,3,7,8,9-HxCDD | 3 | 0.0733 | 0.1304 | 0.3343 | 0.1811 | 0.0112 | 0.0358 | 0.3652 | 0.1518 |
| 1,2,3,4,6,7,8-HpCDD | 3 | 0.0733 | 0.1335 | 0.3343 | 0.0869 | 0.0112 | 0.0287 | 0.3652 | 0.0157 |
| 1,2,3,4,6,7,8,9-OCDD | 3 | 0.0733 | 0.0245 | 0.3343 | 0.0141 | 0.0112 | 0.0287 | 0.3652 | 0.1952 |
| 2,3,7,8-TCDD | 3 | 0.0733 | 0.0001 | 0.3343 | 0.0432 | 0.0112 | 0.0251 | 0.3652 | 0.0194 |
| 2,3,7,8-TCDF | 4 | 0.0536 | 0 | 1 | 0.1706 | 1 | 1 | 1 | 0 |
| 1,2,3,7,8-PeCDF | 4 | 0.0536 | 0.0105 | 1 | 0.8294 | 1 | 0 | 1 | 1 |
| 2,3,4,7,8-PeCDF | 4 | 0.0536 | 0.4892 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,4,7,8-HxCDF | 4 | 0.0536 | 0.0635 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,6,7,8-HxCDF | 4 | 0.0536 | 0.0152 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,7,8,9-HxCDF | 4 | 0.0536 | 0.0916 | 1 | 0 | 1 | 0 | 1 | 0 |
| 2,3,4,6,7,8-HxCDF | 4 | 0.0536 | 0.0672 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,4,6,7,8-HpCDF | 4 | 0.0536 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,4,7,8,9-HpCDF | 4 | 0.0536 | 0.0497 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1,2,3,4,6,7,8,9-OCDF | 4 | 0.0536 | 0.0695 | 1 | 0 | 1 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyemi, B.; Obeng-Gyasi, E. Combined Effects of Metals, PCBs, Dioxins, and Furans on Cardiovascular Dysfunction. J. Xenobiot. 2025, 15, 94. https://doi.org/10.3390/jox15030094
Akinyemi B, Obeng-Gyasi E. Combined Effects of Metals, PCBs, Dioxins, and Furans on Cardiovascular Dysfunction. Journal of Xenobiotics. 2025; 15(3):94. https://doi.org/10.3390/jox15030094
Chicago/Turabian StyleAkinyemi, Bolanle, and Emmanuel Obeng-Gyasi. 2025. "Combined Effects of Metals, PCBs, Dioxins, and Furans on Cardiovascular Dysfunction" Journal of Xenobiotics 15, no. 3: 94. https://doi.org/10.3390/jox15030094
APA StyleAkinyemi, B., & Obeng-Gyasi, E. (2025). Combined Effects of Metals, PCBs, Dioxins, and Furans on Cardiovascular Dysfunction. Journal of Xenobiotics, 15(3), 94. https://doi.org/10.3390/jox15030094








